Abstract
A coprecipitation method using sample constituents as carrier precipitants was developed that can remove molybdenum, which interferes with the determination of cadmium in grain samples via isotope dilution inductively coupled plasma mass spectrometry (ID-ICPMS). Samples were digested with HNO3, HF, and HClO4, and then purified 6 M sodium hydroxide solution was added to generate colloidal hydrolysis compounds, mainly magnesium hydroxide. Cadmium can be effectively separated from molybdenum because the cadmium forms hydroxides and adsorbs onto and/or is occluded in the colloid, while the molybdenum does not form hydroxides or adsorb onto the hydrolysis colloid. The colloid was separated by centrifugation and then dissolved with 0.2 M HNO3 solution to recover the cadmium. The recovery of Cd achieved using the coprecipitation was >97%, and the removal efficiency of Mo was approximately 99.9%. An extremely low procedural blank (below the detection limit of ICPMS) was achieved by purifying the 6 M sodium hydroxide solution via Mg coprecipitation using Mg(NO3)2 solution. The proposed method was applied to two certified reference materials (NIST SRM 1567a wheat flour and SRM 1568a rice flour) and CCQM-P64 soybean powder. Good analytical results with small uncertainties were obtained for all samples. This method is simple and reliable for the determination of Cd in grain samples by ID-ICPMS.

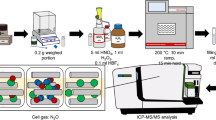
Overview of a coprecipitation method using sample constituents
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium is one of the most toxic heavy metal pollutants in the environment, and it accumulates in the food chain. Cadmium has a long biological half-life (10–30 years) in the human body, and the intake of small amounts of it can have extreme toxicological effects on the human organism [1]. Recently, the CODEX Alimentarius Commission of the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) proposed a new international standard for Cd concentrations in a variety of staple foodstuffs [2]. Hence, in most countries, surveillance measures are currently taken regarding the presence of Cd in staple foods such as rice, wheat and bean grains in order to protect public health.
Inductively coupled plasma mass spectrometry (ICPMS) is one of the powerful techniques used to determine trace elements in foodstuffs [3–8]. When used in combination with an isotope dilution (ID) technique, it can provide highly accurate and precise determinations [5–10]. In particular, the adoption of a multicollector (MC)-ICPMS system yields excellent accuracy and precision for isotope ratio measurements [8–10]. However, ID-ICPMS measurements of Cd are subject to spectral interferences from concomitant elements in the sample [10–16]. Potential interferences in ICPMS measurements of cadmium isotopes can arise from Pd+, Sn+, ZrO+, ZrOH+, and MoO+. In the case of grains such as rice, wheat and bean, interference from MoO+ seriously affects the Cd isotope ratio measurement. The concentration of Mo in grains is commonly ∼100–200-fold higher than that of Cd [12, 19–21], though the formation ratio of MoO+ in ICPMS is typically 0.1–0.4% [12, 17]. Therefore, a correction for this interference must be made or a selective separation of Cd from Mo must be performed when determining Cd in grain samples by ID-ICPMS.
Several correction and separation methods for MoO+ have been investigated, such as mathematical correction [12], organic solvent addition [13], electrothermal vaporization [14], and chemical separation [6, 7, 15, 16]. While the mathematical correction methods are relatively simple, the error in the interference correction is prone to increase when the interference is fairly intense, and thus it is not suitable for obtaining highly accurate analytical results. On the other hand, the chemical separation methods reported have been complicated and time-consuming, although the analytical results obtained should be more reliable than those obtained using mathematical correction. Recently, an ICPMS equipped with a reaction cell was applied to reduce the interference of ZrO+ and MoO+ on Cd+, where O2 gas [17] and a mixture of He and H2 gases [18] were used as the reaction gas. Although these gases can only react with oxide ions, the use of the reaction cell system resulted in a collisional loss of sensitivity to Cd+.
In the present work, we describe a coprecipitation method that uses sample constituents, mainly magnesium, as carrier precipitants. Grain samples contain an adequate amount of Mg (100–1000 mg/kg in grains [19–21]) for use as the carrier precipitant, because the solubility product constant of Mg(OH)2 ([Mg][OH]2) at 25 °C is 10−10.4, and the Mg(OH)2 colloid is bulky. Upon the addition of NaOH solution to the digested sample solution, a colloidal suspension, mainly magnesium hydroxide, is generated by hydrolysis. Cadmium also forms hydroxides and adsorbs onto and/or is occluded in the colloid, while Mo neither forms hydroxides nor absorbs onto the hydrolysis colloid. As a result, Cd can be effectively separated from Mo. This method does not require the addition of any carrier precipitants or complexing reagents. In addition, Cd, Zr and Sn impurities in the NaOH solution can easily be removed through the addition of magnesium nitrate solution prior to the coprecipitation. Therefore, an extremely low procedural blank level can be achieved. We have applied this method to the determination of Cd in two certified reference materials, NIST SRM1568a rice flour and SRM1567a wheat flour, as well as a soybean powder sample used in International Comparison Pilot Study 64 of the Comite Consultatif pour la Quantite de Matiere (CCQM-P64).
Experimental
Instrumentation
The ICPQMS instrument used was an Agilent 7500c (Agilent Technologies, Tokyo, Japan). The instrumental operating conditions are summarized in Table 1. A naturally aspirated sample uptake was adopted to obtain a stable signal intensity. The oxide ion formation ratio of Mo (MoO+/Mo+) was 0.1% throughout the experiment.
Chemicals and samples
The standard solution of Cd (∼1000 μg ml −1) used in this experiment was purchased from Kanto Chemicals (Tokyo, Japan). The enriched isotope 111Cd (96.44% enriched) in oxide form was purchased from Oak Ridge National Laboratory (Oak Ridge, TN, USA). It was dissolved in diluted HNO3 solution and stored in a clean PFA bottle. The nitric acid, perchloric acid, and hydrofluoric acid used were all of Ultrapur grade and were purchased from Kanto Chemicals. Magnesium nitrate solution (10000 mg kg−1), of “matrix modifier for atomic absorption spectroscopy grade,” was obtained from Kanto Chemicals. 6 M NaOH solution was prepared from NaOH pellets of analytical grade (Wako Pure Chemical Industries, Osaka, Japan). Pure water prepared by a Milli-Q water purification system (resistivity 18 MΩ cm, Nihon Millipore Kogyo, Tokyo, Japan) was used throughout the experiments.
The NIST SRM 1567a wheat flour and SRM 1568a rice flour were obtained from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA). The CCQM-P64 soybean powder sample was provided by the National Institute of Metrology China (NIM, Beijing, China). Although no information on the Cd concentration in the CCQM-P64 sample was provided, the sample homogeneity should be sufficient to validate the method we develop here.
Purification of 6 M NaOH solution
Cadmium, Zr and Sn impurities in 6 M NaOH solution was removed via magnesium coprecipitation. The procedure was as follows. Twelve milliliters of 6 M NaOH solution were placed into a polypropylene centrifuge tube, which was prewashed with HNO3. One milliliter of 10,000 mg L−1 Mg (NO3)2 solution was added to the tube, and then the tube was shaken for 1 min. After centrifugation at 3500 rpm for 5 min, the supernatant was transferred to another polypropylene centrifuge tube, and the magnesium coprecipitation procedure was repeated twice. The final supernatant was used for the coprecipitation procedure.
Sample digestion procedure
The grain samples were digested using a microwave digestion system (ETHOS 1, Milestone, Bergamo, Italy) as follows. The sample (∼0.5 g) was placed in a Teflon vessel, and an appropriate amount of 111Cd spike solution, 7.5 ml of HNO3, and 0.5 ml of HClO4 were added to it. Microwave irradiation (ramp: 150 °C for 30 min, hold for 5 min) was then performed. After cooling the vessels, 0.5 ml of HF and 0.5 ml of HClO4 were added to the vessel, and the microwave irradiation (ramp: 220 °C for 25 min, hold for 20 min) was performed again. The solution in the vessel was evaporated to one drop in an evaporation chamber at 200 °C on a hot plate [22]. Finally, the evaporated residue was dissolved in 20 ml of 0.2 M HNO3 at 80 °C. All of the PTFE vessels used in the digestion procedure were prewashed with aqua regia and HF and the the same microwave irradiation procedure was applied to prevent any potential Zr contamination.
Coprecipitation using sample constituents
Ten milliliters of the digested solutions were each placed into polypropylene centrifuge tubes which had been prewashed with HNO3. After 1 mL of the purified 6 M NaOH solution was added, the tubes were shaken manually and then centrifuged at 3500 rpm for 5 min. After decanting the supernatant, the precipitant was washed with Milli-Q water. Finally, the precipitant was dissolved with 2 mL of 0.2 M HNO3.
Isotope dilution procedure
All of the analytical results reported here were quantified with a double-ID protocol [23, 24], where 111Cd was employed as a spike isotope, and 110Cd / 111Cd was selected as a measurement isotope pair. All of the analytical results were calculated using the following equation (1):
where C x is the analyte concentration in the sample (g kg−1), C z is the concentration of the standard solution (g kg−1); m x is the mass of the sample (g), m z is the mass of the Cd standard solution (g) used for reverse-isotope dilution, m y is the mass of the spike solution (g) mixed into the sample, m′ y is the mass of spike solution (g) mixed into the Cd standard solution, R b is the 110Cd/111Cd ratio measured in the spiked sample digested solution, R b′ is the 110Cd/111Cd ratio measured in the standard–spike mixture solution, R x is the 110Cd/111Cd ratio in the sample, R y is the 110Cd/111Cd ratio measured in the spike solution, R z is the 110Cd/111Cd ratio in the standard solution, w is the correction factor for dry mass, B is the the procedural blank value (n = 3), and K b , K b′, and K y are the mass discrimination correction factors for each isotope ratio measurement, which were calculated using the measured 110Cd/111Cd ratio in 20 ng g−1 of the Cd standard solution.
Results and discussion
Separation of Cd from Mo by hydrolysis colloid separation
ICP-MS spectra of the unspiked digested solution of the NIST SRM 1568a before and after coprecipitation are shown in Fig. 1a and b, respectively. The spectra were obtained over the m/z range 90–120. NIST SRM 1568a is a good example of a grain sample that contains high levels of Mo (Mo: 1.46 mg kg−1, Cd: 0.022 mg kg−1). The isotope ratio 110Cd/111Cd before the coprecipitation was 0.9163 ± 0.0063 (mean ± standard error of mean, n = 10), and was significantly different from that of the Cd standard solution (0.9565 ± 0.0012, n = 10). In this case, the interference of MoO+ on 110Cd+ and 111Cd+ was found to be ∼7% and ∼12% of the signal intensity observed, respectively. As can be seen in Fig. 1b, most of the Mo was removed by the coprecipitation. The signal intensity of 95Mo+ after the coprecipitation was found to be ∼700 CPS, while it was ∼160,000 CPS before the coprecipitation, so over 99.9% of Mo was removed using the coprecipitation procedure. In addition, matrices in the digested solutions such as K, Ca, P, Cl and residual carbonic compounds are also removed with the coprecipitation. On the other hand, over 97% of the Cd was recovered. After the coprecipitation, the ratio of 110Cd/111Cd was 0.9560 ± 0.0012 (n = 10), which was in fairly good agreement with that measured for the Cd standard solution. Furthermore, the precision of the isotope ratio measurement improved, because the Cd in the final solution was preconcentrated fivefold compared to the original digested solution. As a result, it was confirmed that coprecipitation utilizing the Mg in the sample as the carrier precipitant was effective at removing Mo from the digested grain solution and thus it enabled a reliable isotope ratio measurement for the Cd in the grain samples to be obtained.
Effect of magnesium concentration and digestion conditions on the recovery of Cd
Since the amount of carrier precipitant influences the recovery of Cd during the coprecipitation, the dependence of the recovery of Cd on the concentration of Mg in the digested solution was examined. For this experiment, test solutions were prepared by diluting two types of digested solutions of SRM1568a with 2% HNO3 solution. These digested solutions were obtained with HNO3+HF digestion and HNO3+HClO4+HF digestion. Nitric acid solutions that contain 0.0005 mg kg−1 of Cd, 0.1 mg kg−1 of Mo and 2–20 mg kg−1 of Mg were also examined as model solutions. The results obtained are summarized in Fig. 2.
For the model solutions, almost full Cd recovery was obtained when the concentration of Mg was above 2 mg kg−1. Over 90% of the Cd was also recovered for the digested solution with HNO3+HF at 2 mg kg−1 of Mg. The removal efficiencies of Mo were over 99.9% for both the digested solutions and the model solutions. Since grain samples commonly contain ∼200–1000 mg kg−1 of Mg, the concentration range of Mg in the digested solution will be 5–25 mg kg−1. Therefore, the present coprecipitation method can be applied to various grain samples.
It is also worth noting that the recovery of Cd decreased as the concentration of Mg increased and the dilution factor of the digested solution decreased. It is well known that competitive complex formation with organic residues interferes with the recoveries of analytes in chemical separation processes [25–28], and that this competitive complex formation could strongly affect the recovery at the small dilution factor used for the sample solution. The recovery of Cd was greatly enhanced by using HClO4 in the sample digestion. Competitive complex formation with organic residues is suppressed in this case because the amount of organic residue in the digestion solution of NIST SRM1568a is decreased by approximately one order of the magnitude when HNO3+HClO4+HF digestion is used. The recovery of Cd in the coprecipitation does not actually influence the analytical results because the 111Cd spike solution was added to the sample prior to the digestion. However, increasing the recovery improves the reliability of the analytical results, and so HNO3+HClO4+HF digestion was used in subsequent experiments.
Preventing Zr and Sn contamination
The NaOH solution is a potentially major source of Cd impurities in the procedural blank of the present coprecipitation method, and a source of contamination due to interfering elements such as Zr and Sn. The level of cadmium impurities in the NaOH solution used was found to be negligible, while significant concentrations of Zr and Sn were found. Therefore, purification via magnesium coprecipitation was applied to remove these Zr and Sn impurities. The concentrations of Cd, Zr, and Sn in the 6 M NaOH before and after the Mg coprecipitation are summarized in Table 2. The Zr and Sn impurities in the NaOH solution were effectively removed using the Mg coprecipitation; the concentration levels of these elements afterwards were insignificant.
The digestion procedural blank values of Cd were evaluated using three digestion vessels that were subjected to the full digestion and evaporation procedure without the sample. The blank digested solutions were measured by ICPMS along with an external calibration method at m/z 90, 95 and 111. The blank value was below the detection limit of the ICPMS measurement (< 0.003 μg kg−1). The contamination from Zr and Sn in the digestion procedure was also evaluated. The contamination of Sn was negligible, but significant contamination of Zr was found. Zr may have leached from the PTFE vessels during the digestion. Yang et al. suggested that Zr leaches from fluoropolymers such as FEP, PTFE, and PFA [29]; the titanium and/or zirconium metallocene compound catalysts that are frequently utilized for polymer production in the chemical industry may be the source of such impurities. Therefore, all of the PTFE vessels used in the digestion procedure were pre-washed with aqua regia and HF and the same microwave irradiation program was applied to them too. After doing this, the contamination of Zr during the sample digestion was significantly reduced and thus the spectral interferences from ZrO+ and ZrOH+ were reduced to negligible levels.
Determination of Cd in the certified reference materials and the CCQM sample
Cd was determined in the certified reference materials and the CCQM sample using ID-ICPMS with and without coprecipitation. A mathematical correction achieved using the Mo oxide ion formation ratio was also carried out for comparison. The analytical results obtained are summarized in Table 3, in which the values were expressed as mean ± standard deviation. The certified Cd values for the certified reference materials are also shown in the table.
The concentrations of Mo in the NIST SRM 1568a rice flour (1.46 mg kg−1) and the CCQM-P64 soybean powder (1.38 mg kg−1) were three times higher than the Mo concentration in the NIST SRM 1567a wheat flour (0.48 mg kg−1), and so the interference from MoO+ was significant for the NIST SRM 1568a rice flour and the CCQM-P64 soybean powder. The analytical results obtained with the coprecipitation for the certified reference materials were both in good agreement with the certified values, and good precisions were observed (relative standard deviation, RSD: 0.3–0.6%). The precisions of the analytical results for the CCQM soybean sample were also good (RSD: 0.1%). On the other hand, the precisions of the analytical results obtained when the mathematical correction was used were 2.5–5.7-fold worse than those obtained using coprecipitation, although the results did agree with the certified values. The utilization of coprecipitation led to not only the elimination of the interference but also the fivefold preconcentration of Cd. Therefore, the reliability of the isotope ratio measurement was improved, so the analytical precisions obtained with the coprecipitation were better than those obtained with the mathematical correction. As a consequence, the proposed method is reliable for the determination of Cd in grain samples.
Conclusion
A coprecipitation method using Mg that reduces interference from Mo during the determination of Cd in grain samples via ID-ICPMS has been developed and tested. Excellent separation of Cd from Mo was achieved. A quite low procedural blank was obtained because there was no need to add any precipitant reagents and because the NaOH solution was easily purified via Mg coprecipitation. Furthermore, the preconcentration of Cd can also be achieved, because matrices in digested solutions such as K, Ca, P, Cl, and residual carbonic compounds are also removed by the coprecipitation. We conclude that this method is simple, easy to perform, and reliable for the determination of Cd in grain samples.
References
Nordberg GF (2004) BioMetals 17:485–489
CODEX Alimentarius Commission (2006) CODEX Alimentarius website. http://www.codexalimentarius.net/web/index_en.jsp. Cited 5th June 2007
Okamoto K (1991) Sci Total Environ 107:29–44
Cubadda F (2004) J AOAC Int 87:173–204
Vassileva E, Quetel CR (2004) Anal Chim Acta 519:79–86
Vassileva E, Quetel CR, Petrov I (2003) Spectrochim Acta B 58:1553–1565
Park CJ, Suh JK (1997) J Anal Atom Spectrom 12:573–577
Klingbeil P, Vogl J, Pritzkow W, Riebe G, Müller J (2001) Anal Chem 73:1881–1888
Fortunato G, Wunderli S (2003) Anal Bioanal Chem 377:111–116
Vogl J (2007) J Anal Atom Spectrom 22:475–492
Inagaki K, Takatsu A, Uchiumi A, Nakama A, Okamoto K (2001) J Anal Atom Spectrom 16:1370–1374
Rodushkin I (1998) Fresenius J Anal Chem 362:541–546
Karunasagar D, Arunachalam J (2001) Anal Chim Acta 441:291–296
Li PC, Jiang SJ (2003) Anal Chim Acta 495:143–150
Jiang SJ, Palmieri MD, Frits JS, Houk RS (1987) Anal Chim Acta 200:559–571
Hwang TJ, Jiang SJ (1997) J Anal Atom Spectrom 12:579–584
Chang CC, Liu HT, Jiang SJ (2003) Anal Chim Acta 493:213–218
Yip YC, Chu HS, Chan KC, Chan KK, Cheung PY, Sham WC (2007) Anal Bioanal Chem 386:1475–1487
Phuong TD, Chuong PV, Khiem DT, Kokot S (1995) Analyst 124:553–560
Wolnik KA, Fricke FL, Capar SG, Baude GL, Meyer MW, Satzger RD, Kuennen RW (1983) J Agric Food Chem 31:1244–1249
Wolnik KA, Fricke FL, Capar SG, Meyer R, Satzger MW, Bonnin D, Gaston E CM (1985) J Agric Food Chem 33:807–811
Isoyama H, Uchida T, Oguchi K, Iida C, Nakagawa G (1990) Anal Sci 6:385–388
Henrion A (1994) Fresenius J Anal Chem 350:657–658
Ellison SLR, Rosslein M, Williams A (2000) EURACHEM/CITAC guide: quantifying uncertainty in analytical measurement, 2nd edn. CITAC Secretariat, Teddington, UK, pp 89–96
Pella PA, Kingston HM, Sleber JR, Feng LY (1983) Anal Chem 55:1193–1194
Yang JY, Yang MH, Lin SM (1985) Anal Chem 57:472–474
Yang JY, Yang MH, Lin SM (1990) Anal Chem 62:146–150
Inagaki K, Haraguchi H (2000) Analyst 125:191–196
Yang XJ, Pin C (2002) Anal Chim Acta 458:375–386
Acknowledgement
This research was supported by the SME Intellectual Foundation Construction Project of the Japanese Ministry of Economy, Trade and Industry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inagaki, K., Narukawa, T., Yarita, T. et al. Determination of cadmium in grains by isotope dilution ICP–MS and coprecipitation using sample constituents as carrier precipitants. Anal Bioanal Chem 389, 691–696 (2007). https://doi.org/10.1007/s00216-007-1396-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1396-7