Abstract
A novel analytical procedure was developed for the rapid determination of disease biomarkers of maple syrup urine disease (MSUD),L-valine,L-leucine,L-isoleucine, andL-phenylalanine in dried blood spots. Amino acids extracted from neonatal dried blood spots were rapidly derivatized with bis-(trimethylsilyl)trifluoroacetamide (BSTFA) and then analyzed by gas chromatography-mass spectrometry (GC-MS). Derivatization conditions and the method validation were studied: optimal derivatization conditions were acetonitrile as reaction solvent, a temperature of 100°C, and a reaction time of 30 min. The proposed method provided a detection limit lower than 2.0 μM, recovery between 92% and 106%, and relative standard deviation less than 8.0%. The method was further tested in screening for neonatal MSUD by determination ofL-valine,L-leucineL-isoleucine, andL-phenylalanine in blood samples. The experimental results show that GC-MS following BSTFA derivatization is a rapid, simple, and sensitive method for the determination of amino acid disease biomarkers in blood samples, and is a potential tool for fast screening of MSUD.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maple syrup urine disease (MSUD) is an autosomal recessive disorder of the metabolism of the branched-chain amino acids (BCAA) ofL-leucine,L-isoleucine, andL-valine and their corresponding branched-chain keto acids (BCKAS). The disorder is caused by a severe deficiency in the activity of the branched chain α-keto acid dehydrogenase complex (BCKD; EC 1.2.4.1) [1]. Tavares et al. found that extracellular glutamate levels may be increased in MSUD and that excitotoxicity may be involved in the neuropathology of this disorder [2]. It was also found that cytoskeletal disorganization might be one of the factors associated with the neurodegeneration characteristic of MSUD disease [3]. A marked increase of serum and urine concentrations of BCAAs and BCKAs is the biochemical hallmark of the disorder. The patients with MSUD predominantly present severe neurological symptoms, including psychomotor delay or mental retardation, hypotonia, lethargy, coma, and generalized convulsions. Although the pathophysiology of the neurologic dysfunctions of MSUD is poorly known, there is a large body of evidence associating defectiveL-leucine metabolism and the neurologic symptoms of these patients [4, 5]. MSUD can be diagnosed by the determination ofL-leucine,L-isoleucine, andL-valine in neonatal blood samples. HPLC, enantioselective multidimensional capillary gas chromatography-mass spectrometry (enantio-MDGC-MS), and tandem mass spectrometry (MS-MS) have been applied to the diagnosis of MSUD [6–10]. MS-MS can simultaneously measureL-leucine,L-isoleucine,L-valine, andL-phenylalanine in blood samples, and the diagnosis of MUSD was ascertained from the ratio of the concentrations ofL-leucine,L-isoleucine, andL-valine to that ofL-phenylalanine. This leads to the reduction of false positive MUSD diagnoses [6]. Since MS-MS has the advantages of high throughput and accuracy, it is the most powerful tool for the diagnosis of MSUD and furthermore has become the gold standard for screening of MSUD in developed countries.
Compared with other inherited metabolic disease such as phenylketonuria (PKU), MSUD is a rare genetic disorder with an incidence of about 1 in 80,000 in China. In general, diagnosis of MSUD is performed by the analysis of amino acid in neonatal blood spots by using HPLC. However, the HPLC method requires complex sample preparation and is time-consuming.
Although MS-MS is the most powerful tool for diagnosis of MSUD, it is very expensive and few hospitals in developing countries can afford it. Gas chromatography-mass spectrometry (GC-MS) is a relative simple and inexpensive instrument. Recently it was applied to screening for inborn errors of metabolism (IEM) by determination of amino acids and organic acids in blood and urine [11–18]. In our previous study,n-butanol and trifluoroacetic anhydride were applied to derivatization of amino acids, prior to GC-MS analysis [19]. Diagnosis of neonatal MSUD by the determinationL-leucine,L-isoleucine, andL- valine by GC-MS has also been shown to be feasible [19]; however, the method required two-step derivatization (esterification and acylation), which led to a time-consuming screening procedure. Rapid screening of neonatal MSUD therefore demands development of a faster GC-MS method for determination of the four amino acids. The key problem to is to improve the derivatization of amino acids. Recently, silylating agents such as bis-(trimethylsilyl)trifluoroacetamide (BSTFA) were introduced to allow modification of amino acids, specifically the silylation of amino and carboxyl groups of amino acids in a single step. Fast analysis of amino acids in body fluids could be carried out by GC-MS with BSTFA. BSTFA has been applied to the derivatization of amino acids and organic acids in urine in clinical diagnosis [20–22]. Molnar-Perl and Katona studied derivatization reactions of protein amino acids with BSTFA andN-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (MTBSTFA), and the fragmentation patterns of the derivatives were obtained. However, the optimum derivatization conditions of amino acids with BSTFA were not found [13].
In this work, BSTFA derivatization followed by GC-MS analysis was developed for fast determination of the MSUD biomarkers,L-valine,L-leucine,L-isoleucine, andL-phenlalanine in blood spot samples, and further applied to screening for neonatal MSUD.
Experimental
Chemicals, standards, and blood samples
Bis-(trimethylsilyl)trifluoroacetamide (BSTFA) was purchased from Merck.L-Phenylalanine,L-valine,L-leucine, andL-isoleucine were purchased from Sigma. Ethyl acetate (EAT), acetonitrile (ACN), pyridine (PRD), and methanol were from Chemical Agent Company, Shanghai, China. Standard and GC calibration solutions spanning the concentration range for the four amino acids from 10.0 to 1,000.0 μmol L−1 were prepared by appropriate dissolution of the amino acid in water. A standard aqueous of each amino acid was prepared with a concentration of 50 μmol L−1 and stored at −10°C until being used for investigation of the derivatization reactions of the four amino acids with BSTFA. The dried blood spots collected from eight newborns in the Fuzhou Hospital (China) were used for screening for the metabolism disorder diseases (PKU and MSUD). The samples were obtained and used in the study with the permission of the hospital.
Investigation of derivatization reactions of amino acids with BSTFA
At first, the reaction was studied by using EAT, ACN or PRD as solvent. A volume of 100 μL of the four amino acid solutions (50.0 μmol L−1) were added to 1-mL vials and the solvent was evaporated under a stream of N2 at 40°C. The residue was reacted with 100 μL BSTFA/EAT (1:1, v/v), 100 μL BSTFA/ACN(1:1, v/v), and 100 μL BSTFA/PRD(1:1, v/v), respectively. The reactions were carried out at 120°C for 30 min. The derivatives were evaporated to dryness under nitrogen at 40°C and redissolved with 100 μL ACN.
Next, investigation of derivatization temperature and time was performed. A volume of 100 μL solution of the four amino acids (50.0 μmol L−1) was added to 1-mL vials and the solvent was evaporated under a stream of N2 at 40°C. The residue was reacted with 100 μL BSTFA/acetonitrile (ACN) mixture (1:1, v/v) at 60, 80, 100, 120, and 150°C with reaction times of 10,20, 30, 40, and 60 min at each temperature. The derivatives were evaporated to dryness under nitrogen at 40°C and redissolved with 100 μL ACN.
Derivation of standards and samples
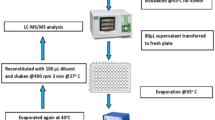
Dried blood spots on filter paper were prepared by punching out an 8.0-mm-diameter circle (corresponding to 20 μL of whole blood) into a 1-mL vial with a standard paper punch. A 200-μL volume of 0.1% HCl/methanol was added to the vial at 4°C for 60 min and then centrifuged at 15,000 g for 20 min. A 100-μL aliquot of supernatant fluid was transferred to a 1-mL vial and then evaporated to dryness under a stream of N2 at 40°C. The residue was reacted with 100 μL of BSTFA/ACN mixture at 100°C for 30 min. The derivatives were evaporated to dryness under a stream of N2 at 40°C, and then redissolved with 100 μL ACN.
GC calibration solutions of four amino acids from 10.0 to 1,000 μmol L−1 were prepared by dissolving the amino acids into water. A volume of 100 μL calibration solutions spanning the concentration range from 10.0 to 1,000 μmol L−1 was added to 1-mL vials and the solvent was then evaporated under a stream of N2 at 40°C. The same procedure for derivatization and preparation was followed as described above.
Gas chromatography-mass spectrometry
A Finnigan Voyager gas chromatograph-mass spectrometer (GC-MS) was used in 70-eV electron impact (EI) mode. Analytes were separated using an Agilent HP-5MS capillary column of 30m×0.25 mm with a phase thickness of 0.25 μm. A 1-μL volume of the sample was injected in the split mode (30:1). The oven temperature program was as follows: initial temperature 80°C for 2 min; increased to 300°C at 15°C min−1; 300°C was maintained for 10 min. Helium (99.999%) was used as carrier gas with a flow rate of 1.0 mL min−1. The quadrupole temperature was 280°C. The qualitative analysis was carried out under full-scan acquisition mode within the 41- to 500-amu range. Quantification was performed in the selected ion monitoring (SIM) mode usingm/z 144 andm/z 246 forL-valine,m/z 158 andm/z 260 for bothL-leucine andL-isoleucine, andm/z 192 and/z m 294 forL-phenylalanine.
Detection limit, recovery, and precision
The optimum derivatization conditions were used for the study of the method validation. Detection limits were performed by replicate analyses of 100 μL calibration solution with the low concentration of 10.0 μmol L−1. Detection limits were calculated on the basis of 3 of S/N. Ten-μL volumes of standard solutions (10, 50, 200 μmol L−1) were added to three blood samples, respectively. Recoveries were obtained by comparing their real values with those calculated by an external standard method. Precision of the assay was calculated by four replicate analyses of the same blood sample by the complete analytical procedure.
Results and discussion
Study of amino acid derivatization reaction conditions
To obtain the optimum derivatization conditions, investigation of reactions ofL-valine,L-leucine,L-isoleucine, andL-phenylalanine with BSTFA was carried out. At first, the effect of solvent on derivatization reactions was studied. The peak areas of amino acid derivative were 1.04 × 108, 1.52 × 108, and 0.86 × 108 for EAT, ACN, and PRD, respectively. Obviously, the amino acid derivative peak areas reached their maximum value by using ACN as the reaction solvent. Next, reaction temperature and time were studied. Derivatization reactions were performed at different temperatures (60, 80, 100, 120, and 150°C) and different times (10, 20, 30, 40, and 60 min). The peak area sum of four amino acid derivatives was used for determination of the optimum conditions. As seen from Fig. 1, the maximum peak area sum was obtained at a reaction temperature of 100°C and time of 30 min. Thus, the optimum derivatization conditions are ACN as solvent, a temperature of 100°C, and a reaction time of 30 min.
Determination of amino acids in blood spots
The optimum reaction conditions were used for the derivatization of amino acids in the blood samples. The free hydroxyl and amino groups of amino acid were simultaneously modified by BSTFA to afford and a single derivative for each amino acid. Therefore, amino acids in the samples can be determined by the measurement of their corresponding BSTFA derivatives.
The total ion chromatogram of neonatal blood spots is shown in Fig. 2. The retention times ofL-valine,L-leucine,L-isoleucine, andL-phenylalanine derivatives are 7.03, 7.61, 7.83, and 10.84 min, respectively. Figure 1 indicates that single derivatives ofL-valine,L-leucine,L-isoleucine, andL-phenylalanine were obtained under the optimum reaction conditions. Actually, selected ion monitoring (SIM) can improve sensitivity in the analysis of amino acid derivatives. Selection of the characteristic ions as quantification ion was very important.
Figure 3 shows the EI mass spectra ofL-valine,L-leucine,L-isoleucine, andL-phenalanine derivatives. As can be seen from Fig. 2,L-valine,L-leucine, andL-isoleucine BSTFA derivatives produced [M−COOSi(CH3) 3]+ fragment peaks atm/z 144 for the L-valine derivative,m/z158 for both L-leucine and L-isoleucine derivatives, and m/z 192 for the L-phenylalanine derivative. These derivatives also produced [M−CH3] + fragment ions at m/z 246 for the L-valine derivative,m/z 260 for bothL-leucine andL-isoleucine derivatives, and m/z 294 for theL-phenylalanine derivative. As can be seen from Fig. 3, mass spectral data ofL-leucine andL-isoleucine derivatives are very similar; however, Fig. 2 showed that their retention times are different. Therefore, the retention time can ensure the qualitative accuracy. The characteristic ions atm/z 144 andm/z 246 forL-valine,m/z 158 andm/z 260 for bothL-leucine andL-isoleucine, andm/z 192 andm/z 294 forL-phenylalanine were used for SIM experiments to determine concentrations of the four amino acids in neonatal blood samples. Figure 4 shows the SIM (m/z 144, 158, 192, 246, 260, and 294) chromatogram of a neonatal blood sample by GC-MS following BSTFA derivatization.
A calibration curve at concentrations of 10.0 to 1,000 μmol L−1 for each of the four amino acids was achieved (Table 1). The regression lines and the equations for each amino acid tested showed an excellent relationship between the signal (select ion peak area,y) and amino acid concentration (x, μmol L−1).
The concentrations of the four amino acids in blood samples were calculated by an external standard method. The analytical results are shown in Table 2.L-Leucine,L-isoleucine, andL-valine concentrations in the blood samples of MSUD positive patients were found to be significantly higher than those in control blood samples. According to Qu’s method [7, 8], we measured the four amino acid in the eight blood samples by using HPLC, and very close results were observed. This shows that GC-MS following BSTFA is a reliable method for analysis of amino acids in blood samples. Chace et al. proved that diagnosis of MSUD could be accurately performed from the ratio of concentration ofL-leucine andL-isoleucine toL-phenylalanine [6]. In the work, to reduce the false positive diagnosis of MSUD, the ratio ofL-leucine andL-isoleucine toL-phenylalanine was used for the diagnosis of MSUD. Table 2 shows that the ratio of total concentration ofL-leucine andL-isoleucine toL-phenylalanine in patients with MSUD was more than 8.0, while the ratio in normal blood was less than 4.0. The results were similar to those obtained by MS-MS method [6], which indicates that GC-MS is an alternative method for diagnosis of MSUD. At the same time, the ratio ofL-valine toL-phenylalanine in patients with MSUD was more than 2.0, while the ratio in normal blood was less than 1.5, which suggests that the concentration ratio ofL-valine toL-phenylalanine might be used for diagnosis of MSUD.
Detection limit, recovery, and precision
The detection limits of the four amino acids were calculated on the basis of 3 times the signal-to-noise ratio. The detection limit values ofL-valine,L-leucine,L-isoleucine, andL-phenylalanine were 0.8, 1.1, 0.9, and 1.7 μmol L−1, respectively. The detection limits were below the physiologically normal ranges forL-valine,L-leucine,L-isoleucine, andL-phenylalanine.
The analytical recoveries of added amino acids from blood were determined in triplicate at concentrations of 10, 50, and 200 μmol L−1. The respective mean values obtained were 99%, 93%, 99%, and 92% at 10 μmol L−1; 102%, 90%, 102%, and 94% at 50 μmol L−1; 100%, 98%, 106%, and 96% at 200 μmol L−1.
Precision of the assay was calculated by replicate analysis of the same blood sample by the complete analytical procedure for blood spots described in the “Experimental”. Precision was expressed by relative standard deviation (RSD) values. These RSD values represent the within-assay variation and were 7.2% forL-valine, 5.3% forL-leucine, 4.6% forL-isoleucine, 7.4% forL-phenylalanine, and 6.0% for the ratio ofL-leucine andL-isoleucine toL-phenylalanine ratio (n=4). The calibration curves forL-valine,L-leucine,L-isoleucine, andL-phenylalanine determined for the same sample on different occasions within 15 days, representing the interassay variation, were 6.2%, 5.4%, 4.8%, and 4.3%, respectively (n=4). The absolute concentrations of amino acids were 206, 198, 222, and 215 μmol L−1, respectively.
Conclusions
GC-MS with BSTFA was successfully developed for the determination ofL-valine,L-leucine,L-isoleucine, and L-phenylalanine in neonatal blood spots. Derivatization of the four amino acids with BSTFA at 100°C needs only 30 min, and the four amino acids were determined by measurement of their trimethylsilyl derivatives by GC-MS in the SIM mode. The present method provides low detection limits and excellent precision. It has been shown that GC-MS with BSTFA is a simple, rapid, and sensitive method for determination of amino acids in blood samples, and that fast diagnosis of MSUD can be carried out on the basis of the ratio of total concentration ofL-leucine andL-isoleucine toL-phenylalanine [9, 12, 14–17, 21]. The proposed method could allow complete screening for neonatal MSUD within 60 min.
References
Scriver AL, Beaudet WS, Sly DV (1995) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York
Tavares RG, Santos CES, Tasca AI, Wajner M, Souza DO, Dutra-Filho CS (2000) J Neurol Sci 181:44–49
Pessoa-Pureur R, Funchal C, Pelaez PD, Vivian L, Loureiro SO, Miranda RD, Wajner M (2002) Metab Brain Dis 17:65–75
Efron ML (1965) New Engl J Med 272:1058–1064
Snyderman SE, Norton PM, Roitman E (1964) Pediatrics 34:454–461
Chace DH, Hillman SL, Millngton DS, Kahler SG (1995) Clin Chem 41:62–68
Podebrad F, Heil M, Leib S, Geier B, Beck T (1997) Hrc-J High Res Chromatogr 20:355–362
Qu Y, Slocum RH, Fu J, Rasmussen WE, Rector HD, Miller JB, Coldwell JG (2001) Clin Chim Acta 312:153–162
Qu Y, Miller JB, Slocum RH (1991) Clin Chim Acta 203:191–197
Araujo P, Wassermann G.F, Tallini K, Furlanetto V, Vargas CR, Wannmacher CMD, Dutra CS, Wyse ATS, Wajner M (2001) Neurochem Int 38:529–537
Deng CH, Shang CQ, Hu YM, Zhang XM (2002) J Chromatogr B 775:115–120
Utsumomiya M, Nozaki T, Zhang C (1997) Proc J Jpn Soc Biomed Mass Spectrom 22:119–124
Molnar-Perl I, Katona ZF (2000) Chromatographia 51:S228–S234
Jellum E, Horn L, Thoresen O (1986) Scand J Clin Lab Invest 184:21–26
Kaiser FE, Gehrke CW, Zumalt RW (1974) J Chromatogr 94:113–122
Zomezely C, Marco G, Emery E (1962) Anal Chem 34:1414–1421
Zhang C, Matsumoto M, Inoue Y (1996) J Kanazawa Med Univ 21:399–406
Kuhara T, Shinka T, Inoue Y (1999) J Chromatogr B 731:141–147
Deng CH, Deng YH (2003) J Chromatogr B 792:261–268
Fu XW, Iga M, Yamaguchi S (2000) Early Hum Dev 58:41
Deng CH, Deng YH, Wang B, Yang XH (2002) J Chromatogr B 780:407–413
Deng CH, Yin XY, Zhang LJ, Zhang XM (2005) Rapid Commun Mass Spectrum 19:2227–2234
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, X., Deng, C., Wang, B. et al. Quantification of trimethylsilyl derivatives of amino acid disease biomarkers in neonatal blood samples by gas chromatography-mass spectrometry. Anal Bioanal Chem 384, 931–938 (2006). https://doi.org/10.1007/s00216-005-0241-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-0241-0