Abstract
A multilayer film composed of ferrocene(Fc)-appended poly(allylamine hydrochloride) (Fc-PAH) and poly(potassium vinylsulfate) (PVS) has been prepared on the surface of a gold(Au) electrode by using a layer-by-layer self-assembly technique. Fc-containing polyelectrolyte multilayer (PEM) film-modified electrodes can electrochemically catalyze the oxidation of ascorbic acid successfully. For a 2 (Fc-PAH/PVS) bilayer-covered electrode the catalytic current increased linearly with increasing concentration of ascorbic acid over the concentration range 6 μmol L–1–3 mmol L–1. To extend the dynamic range for ascorbic acid, the surface of the Au electrode was first covered with a (PAH/PVS)2 film on which an additional (Fc-PAH/PVS)5 film was coated. This strategy successfully extended the dynamic range of the electrode up to 25 mmol L–1 ascorbic acid, because the (PAH/PVS)2 layer blocked access of ascorbic acid to the electrode surface. The upper detection limit of the (PAH/PVS)2 (Fc-PAH/PVS)5 film-modified electrode is much higher than those of Fc-based ascorbic acid sensors reported so far. Electron transfer is diffusion-controlled within the (PAH/PVS)2(Fc-PAH/PVS)5 film.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The layer-by-layer adsorption strategy has been widely applied for the construction of a variety of ultrathin polyelectrolyte multilayer (PEM) films [1]. PEM films are prepared by sequentially immersing a substrate such as a silicon, glass, or plastic slide, or electrode in positively and then negatively charged polyelectrolyte solutions in a cyclic procedure. In particular, if one controls the spatial arrangement of the functionality in the multimaterials films one can engineer the properties of devices. Thus, PEM films have, to date, been studied extensively for developing a novel class of materials applicable to chemical sensors such as gas sensors [2], humidity sensors [3], and biosensors [4, 5]. Recently, PEM films consisting of redox-polymer and enzyme, for example, ferrocene-appended poly(allylamine hydrochloride) (Fc-PAH) and glucose oxidase (GOx) [6], or osmium complex-attached polymer and GOx [7, 8], have been successfully prepared on the electrode surface. For these bioelectrodes, GOx molecules can be electrically wired to the electrode surface by using the redox polymer as mediator. Thus, these redox center-containing PEM film-covered electrodes have been employed to construct amperometric glucose biosensors [6–8].
We have found that the polymer type and the Fc content of the PEM films significantly affected the redox properties [9]. We also found that the redox behavior of the Fc-PAH/poly(potassium vinylsulfate) (PVS) film was sensitive to the polarity of the electric charges of electrochemically inactive films prepared on the outermost surface; the redox current of the Fc-PAH/PVS film-coated electrodes increased on deposition of polycation on the surface and decreased on deposition of a polyanion surface layer. The apparent potentials also shifted positively and negatively on addition of cationic and anionic polymers, respectively, to the surface. A possible application of this phenomenon to a new kind of electrochemical ion sensor has been suggested [10].
Ascorbic acid (vitamin C, abbreviation AA) plays vital role in processes of oxidation and reduction in the human organism by participating in many metabolic reactions. AA has also been used to prevent and treat the common cold, mental illness, infertility, cancer, and AIDS [11]. Additionally, AA is generally used as an antioxidant in food and pharmaceutical preparations. Therefore, it is of great importance to detect AA.
Many methods including UV spectrometry [12], enzymatic analysis [13], and electrochemical methods [14–21], have been proposed to measure AA. Among these, electrochemical approaches have proved to be advantageous because of their simplicity, high sensitivity, and rapidness. Redox mediator-modified electrodes have attracted most attention, because redox mediators can facilitate oxidation of AA at lower electrode potentials on a chemically-modified electrode surface [17–21]. However, most of these Fc-containing membrane-modified electrodes reported for AA sensing are not sensitive and have a narrow dynamic detection range. Therefore, it is challenging work to improve the sensitivity and extend the dynamic detection range.
With this aim in view, we prepared PEM films composed of Fc-PAH and PVS (Fig. 1) on the surface of a gold (Au) electrode (Fig. 2). Electrocatalytic determination of AA with higher sensitivity by using Fc-PAH/PVS film-modified electrodes was then carried out. Furthermore, the surface of Au electrode was coated with an electrochemically inactive (PAH/PVS) PEM film, on which Fc-containing PEM was then deposited. This method would be useful for extending the dynamic range of the electrode to higher concentrations of AA. Electron-transfer mechanism of the two kinds of FcPEM film-covered electrones were also compared.
Experimental
Reagents and chemicals
An aqueous solution (20%) of poly(allylamine hydrochloride) (PAH; Mw~10,000) and an aqueous 30% solution of poly(ethyleneimine) (PEI; Mw 60,000–80,000) were purchased from Nittobo (Tokyo, Japan). Polypotassium vinylsulfate (PVS; (Mw~242,000), sodium borohydride, and ascorbic acid were bought from Nacalai Tesque (Kyoto, Japan). Tri(hydroxymethyl)aminomethane (Tris) and ferrocenecarboxaldehyde were obtained from Aldrich (Milwaukee, WI, USA). N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) was obtained from Dojido Laboratories (Kumamoto, Japan). Sodium 3-mercapto-1-propanesulfonate (MPS) was bought from Tokyo Kasei (Tokyo, Japan).
Synthesis of ferrocene-appended poly(allylamine hydrochloride) (Fc-PAH)
Ferrocene-appended poly(allylamine hydrochloride) was synthesized as described elsewhere [9, 10]. The amount of the Fc moiety in the Fc-PAH was 4 mol% (molar ratio of Fc to amine group in the monomer), as determined by UV–visible absorbance spectroscopy at 251 nm to measure absorption originating from the Fc residue (ɛ251=4.5×103 mol–1 L cm−1) using (ferrocenylmethyl)-dimethylamine as model compound. The chemical structure of Fc-PAH is shown in Fig. 1.
Pretreatment of the Au disc electrode
Polycrystalline Au disc electrodes (diameter: 1.6 mm) were used. Before adsorption of the PEM films the electrodes were first polished with aqueous slurries of successively finer alumina paste, sonicated thoroughly in water. The electrodes were further electrochemically treated by potential sweep from −0.2 to 1.7 V (vs. Ag/AgCl) in 0.5 mol L–1 H2SO4 at scan rate of 10 V s−1 for 15 min, or until the cyclic voltammogram characteristic of a clean gold surface was obtained.
Multilayer film self-assembly on Au disc electrodes
The deposition conditions are briefly described below. A clean Au electrode was dipped overnight in a 10 mmol L–1 aqueous solution of 3-mercapto-1-propanesulfonate (MPS) to make the surface negatively-charged. The MPS-modified Au electrodes were immersed in a Fc-PAH solution and a PVS solution alternately and repeatedly for 15 min, with 5-min intermediate rinses in 10 mmol L–1 acetate buffer solution (pH 5.0). Multilayers of (Fc-PAH/PVS) PEM film-covered Au electrodes were thus prepared. For preparation of (PAH/PVS)2/(Fc-PAH/PVS)5 film-coated Au electrodes, the MPS-modified gold electrode was first modified with a (PAH/PVS)2 film which was then covered with an additional (Fc-PAH/PVS)5 film. All polyelectrolyte solutions used were at a concentration of 2 mg mL−1, prepared with 10 mmol L–1 HEPES buffer solution containing 0.1 mol L–1 NaCl (pH 5.0).
Gravimetric measurements with QCM
A quartz crystal microbalance (QCM) (QCA 917 system, Seiko EG&G, Tokyo, Japan) was employed to monitor the formation of multilayer films. A 9 MHz AT-cut quartz resonator coated with a thin platinum layer was used as a probe on which the adsorption of 1 ng of polymer induces a −0.91 Hz change in the resonance frequency [22]. The surface of the quartz resonator was rinsed thoroughly with Milli-Q water before use. The PEM films were deposited on the quartz resonator in a similar way as for deposition on the Au electrode. The probe was dried in air until the frequency showed a steady-state value. All data were obtained in air for the dry films.
Preparation of PEM films on quartz slides
Films were prepared on quartz slides to estimate the loading of the Fc moiety in the film by means of UV–visible absorption spectroscopy. The surface of a quartz slide (5×1×0.1 cm3) was cleaned with a mixture of sulfuric acid and chromic acid. The quartz slide was dipped in dichlorodimethylsilane (10% solution in toluene) overnight at room temperature to make the surface hydrophobic and sonicated successively with toluene, acetone, and distilled water before use. In order to form a stable PEM film the Fc-PAH/PVS multilayer film was deposited on the slide precoated with a (PEI/PVS) film in a similar manner. UV–visible absorption spectra were measured using a Shimadzu (Kyoto, Japan) UV3100 spectrophotometer.
Electrochemical measurements
The electrochemical response was measured in a conventional three-electrode system using a modified Au electrode as working electrode, a platinum wire as counter electrode, and an Ag/AgCl (3.3 mol L–1 KCl) electrode as reference electrode. All potentials were reported in this context with respect to this reference. An HAB-151 potentiostat/galvanostat (Hokuto Denko, Japan) was used for cyclic voltammetry (CV) and amperometry measurements. All the measurements were performed at room temperature (~20 °C).
Results and discussion
Preparation of (Fc-PAH/PVS) m films
The quartz crystal microbalance is useful for evaluation of PEM film-formation. The frequency change (−ΔF) after layer-by-layer deposition of the constituent polymer was monitored by means of a QCM in air for dry films. It is clear that the −ΔF value increased linearly after addition of each adsorption layer, suggesting that PEM film was successfully prepared (data not shown).
UV spectra can also be used to monitor the formation of the PEM film. Figure 3 shows the UV absorption spectra of different bilayers of (Fc-PAH/PVS) deposited on the quartz slide. The Fc loadings in the 2-bilayer, 5-bilayer, and 10-bilayer (Fc-PAH/PVS) films were 0.30, 1.35, and 2.30 nmol cm−2, respectively, estimated from the absorbance at 251 nm originating from the Fc moieties in the films [10].
The amount of Fc in the film can also be calculated by integration of the area under the anodic peak in CV as 0.21, 0.54, 1.3 nmol cm−2 for 2-bilayer, 5-bilayer and 10-bilayer (Fc-PAH/PVS), respectively, at a potential scan rate of 50 mV s−1. Apparently, not all of the Fc residues in the film are electrochemically active [10].
Electrocatalytic oxidation of AA at the Fc-containing PEM film-covered electrode
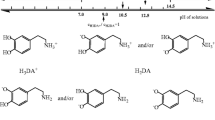
A previously described procedure was used to prepare the Fc-PAH film-covered Au electrodes under the optimal conditions [10]. Figure 4 shows results from CV of an (Fc-PAH/PVS)2 film-modified Au electrode in the absence (a) and in the presence (b, c, d, and e) of ascorbic acid. The anodic peak current (ipa) was greatly enhanced in the presence of AA, whilst the corresponding cathodic peak disappeared, suggesting electrocatalytic oxidation of AA occurred [17, 18]. The ipa value increased with increasing concentration of AA. This is because the Fc moiety in the PEM film mediates electron transfer between the ascorbic acid and electrode on the basis of following electrochemical reactions:
where H2A and A represent ascorbic acid and dehydroascorbic acid, respectively, and Fc-PAH and Fc+-PAH denote the reduced and oxidized forms of the Fc-appended redox polymer.
The electrocatalytic current (icat) of an (Fc-PAH/PVS)2-film-modified Au electrode is shown in Fig. 5. The icat was linearly dependent on the concentration of AA over the range 0.006–3.0 mmol L–1 for the (Fc-PAH/PVS)2 film-covered electrode. The dynamic detection range is much better than that of Fc-PAH monolayer-covered Au electrodes, in which the oxidation current was proportional to the concentration of ascorbic acid in the range of 0.05–1.0 mmol L–1 [20]. In other words, the sensitivity of the Fc-PAH monolayer-covered electrode can be improved by modification with the multilayer film. In addition, the time required to reach 95% of the steady-state current was less than 10 s after addition of AA, suggesting a rapid current response of the PEM-modified electrode [20]. In the presence of a high concentration of AA, however, interference arose from the direct oxidation of AA on the Au electrode surface, because part of the AA diffuses into the PEM film to reach the electrode surface (Fig. 4, voltammogram e).
Permselective inner blocking layers improve the high concentration range of AA
Effect of the PEM blocking layers
It has been reported that a (PAH/PVS)2PAH film-coated Pt electrode almost completely suppressed the diffusion of AA and uric acid to the electrode surface. The (PAH/PVS)2PAH film-coated electrode was successfully used to determine H2O2 in the presence of these interferences [4]. Here it may be interesting to check the permeation of AA to the (PAH/PVS)2 film-covered Au electrode. Figure 6 shows CV associated with the oxidation of AA at bare and (PAH/PVS)2 film-covered Au electrodes. Irreversible oxidation of AA occurs at around 0.5 V on the bare electrode; the electron-transfer kinetics is rather sluggish owing to the fouling of the electrode surface by the oxidation products of AA [23]. The (PAH/PVS)2 film-coated electrode, on the other hand, gave no peak in the potential range 0–0.5 V (Fig. 6b). This shows that the (PAH/PVS)2 film successfully blocked access of AA to the electrode surface. A size-exclusion effect may be responsible for this blocking. It has been reported that one bilayer of (PAH/PVS) film can suppress the permeation of uric acid [4]. Therefore, the (PAH/PVS)2 film-coated electrode can also prevent interference from uric acid.
Electron-transfer mechanism for (PAH/PVS) n (Fc-PAH/PVS)5 film-covered Au electrode
Electron transfer is a short-distance interaction decaying exponentially with the separation between the donor and acceptor [24]. We previously observed that a Fc-PAH layer can transfer electrons through a (PAH/PVS)3 spacer inserted between the Fc PEM and the electrode [9]. This means that an electron transfer mechanism might be varied by adjusting the thickness of the blocking layer inserted. Figure 7 shows the dependence of anodic current (ipa) on the scan rate (υ) or the square root of scan rate \((\surd \upsilon )\) for different (PAH/PVS) blocking layers inserted between the electrode and (Fc-PAH/PVS)5 film. Evidently, the (Fc-PAH/PVS)5 film-coated electrode gave reversible redox peaks originating from the Fc+/Fc couple in the film, with the peak current increasing linearly with scan rate (Fig. 7a, line a), suggesting diffusion-free electron transfer between the Fc-PAH and the electrode. Upon insertion of two blocking layers between (Fc-PAH/PVS)5 and the electrode, the peak current is greatly suppressed. For the Au/MPS/(PAH/PVS)2/(Fc-PAH/PVS)5 film-covered electrode ipa was linearly dependent on \(\surd \upsilon ,\) and not on υ (Fig. 7b), indicating that the electron transfer is diffusion-controlled. These results revealed that the (PAH/PVS)2 film cannot block the heterogeneous electron transfer.
a Relationship between anodic current (ipa) and scan rate (υ). b Relationship of anodic current (ipa) and the square root of scan rate \((\surd \upsilon)\) for different blocking layers inserted into the (Fc-PAH/PVS)5 PEM film and Au electrode. CV was performed in 10 mmol L–1 Tris–HCl buffer containing 0.1 mol L–1 NaCl (pH 7.0). (PAH/PVS)2(Fc-PAH/PVS)5 film-modified Au electrode (filled squares), (Fc-PAH/PVS)5 film-modified Au electrode (filled circles)
Improvement of the upper detection limit of the (Fc-PAH/PVS) film-coated electrodes
As discussed above, the (PAH/PVS)2 film-covered electrode hampered direct access of AA to the electrode surface. For this purpose, the Au electrode surface was covered with a (PAH/PVS)2 film before deposition of the (Fc-PAH/PVS)5 layer to enhance the upper detection limit. CV of (PAH/PVS)2(Fc-PAH/PVS)5 film-modified electrodes was carried out in the presence of ascorbic acid to evaluate the electrocatalytic activity of PEM films. Figure 8 shows CV of a (PAH/PVS)2(Fc-PAH/PVS)5 film-modified Au electrode in the presence of ascorbic acid. The icat increased with increasing concentration of AA up to 20 mmol L–1 without any deterioration in the shape of CV, confirming that the direct oxidation of AA does not occur in the 0–0.5 V region within this concentration range. Thus, the dynamic range of the electrode is highly improved by the inner blocking (PAH/PVS)2 layer. The calibration curve for AA is shown in Fig. 9. The lower and upper detection limits of the (PAH/PVS)2(Fc-PAH/PVS)5 film-modified Au electrode were found to be 0.1 and 25 mmol L–1, respectively. The dynamic range for AA is 0.1–25 mmol L–1. It should be noted here that the improvement of the upper detection limit of the (PAH/PVS)2(Fc-PAH/PVS)5 film-modified Au electrode relied on the use of a (PAH/PVS)2 PEM film as an inner layer, instead of using conventional polymer films. The PEM film was found to hamper diffusion of ascorbic acid to the Au electrode surface without blocking electron transfer from Fc-PAH to the Au surface.
Here it may be interesting to compare the dynamic range of the (PAH/PVS)2(Fc-PAH/PVS)5 film-modified Au electrode with those of other Fc-modified electrodes. The dynamic range of carbon-paste electrodes containing β-cyclodextrin-Fc inclusion complex was reported to be 0.0005–1.0 mmol L–1 [19]. An Fc-containing dimyristoyl phosphatidylcholine film-modified glassy carbon electrode had an upper detection of 5 mmol L–1 [21]. Therefore, it is evident that the upper detection limit of the (PAH/PVS)2(Fc-PAH/PVS)5 film-modified Au electrode is much higher than those of the Fc-modified carbon paste and glassy carbon electrodes.
Conclusions
The Fc-PAH/PVS film-modified electrode was successfully used to electrochemically catalyze the oxidation of AA. The electrocatalytic current depended on the concentration of AA in the range 6 μmol L–1–3 mmol L–1 for a (Fc-PAH/PVS)2 film-covered electrode. On the other hand, the upper detection limit of the Fc-PEM film-coated electrodes was extended up to 25 mmol L–1 ascorbic acid by using a (PAH/PVS)2 blocking layer.
References
Decher G (1997) Science 277:1232–1237
Yang X, Johnson S, Shi J, Holesinger T, Swanson B (1997) Sens Actuators B 45:87–92
Li D, Jiang Y, Li Y, Yang X, Lu L, Wang X (2000) Mater Sci Eng C 11:117–119
Hoshi T, Saiki H, Kuwazawa S, Tsuchiya C, Chen Q, Anzai J (2001) Anal Chem 73:5310–5315
Yu A, Caruso F (2003) Anal Chem 75:3031–3037
Hodak J, Etchenique R, Calvo J, Singhal K, Bartlett N (1997) Langmuir 13:2708–2716
Hou S, Yang K, Fang H, Chen H (1998) Talanta 47:561–567
Calvo J, Etchenique R, Pietrasanta L, Wolosiuk A, Danilowicz C (2001) Anal Chem 73:1161–1168
Liu A, Kashiwagi Y, Anzai J (2003) Electroanal 15:1139–1142
Liu A, Anzai J (2003) Langmuir 19:4043–4046
Davies B, Austin J, Partridge A (eds) (1991) Vitamin C: its chemistry and biochemistry. The Royal Society of Chemistry, Cambridge
Jain A, Chaurasia A, Verma K (1995) Talanta 42:779–787
Jin L, Zhao G, Fang Y (1994) Chem J Chin Univ 15:189–194
Retna Raj C, Ohsaka T (2001) Chem Lett 607–608
Komatsu M, Fujishima A (2003) Bull Chem Soc Jpn 76:927–933
Gao Z, Huang H (1998) J Chem Soc Chem Commun 2107–2108
Raoof B, Ojani R, Kiani A (2001) J Electroanal Chem 515:45–51
Pournaghi-Azar H, Ojani R (1995) Talanta 42:1839–1848
Zhang G, Wang X, Shi X, Sun T (2000) Talanta 51:1019–1025
Zhang S, Fu Y, Sun C (2003) Electroanal 15:739–746
Wang J, Wu Z, Tang J, Wang E (2001) Electroanal 13:1093–1097
Sauerbrey GZ (1959) Z Phys 15:206–214
Raj CR, Ohsaka T (2001) J Electroanal Chem 496:44–49
Closs G, Miller J (1988) Science 240:440–443
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, A., Anzai, Ji. Ferrocene-containing polyelectrolyte multilayer film-covered electrodes: electrocatalytic determination of ascorbic acid and use of inner blocking layers to improve the upper detection limit of the electrodes. Anal Bioanal Chem 380, 98–103 (2004). https://doi.org/10.1007/s00216-004-2728-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-004-2728-5