Abstract
Microwave (MW) digestion procedures with high sample throughput (simultaneous digestion of 36 or 80 samples) and procedural simplicity (disposable plastic tubes, or re-usable liners with screw-cap) were investigated for their efficiency in routine analyses of biological samples. Different digestion vessel materials were tested for metal leaching/adsorption and thermal resistance: quartz, glass, polyethylene (PE) and polystyrene (PS). For the instrumental quantification of Al, Bi, Cd, Co, Cr, Hg, Mn, Mo, Ni, Pb, Sb, and Tl at ultra-trace levels in urine, serum, and whole blood, sector field inductively coupled plasma mass spectrometry (SF-ICP–MS) was used. The different pretreatment conditions and vessels were evaluated in terms of contamination risk, effective power of detection, accuracy, and precision. Results of analyses of serum, urine and whole blood certified reference materials (CRMs) were fully satisfactory for almost all the analytes. In the case of Hg, Mo, and Tl in serum digested in plastic containers the results were just below the lower limit of uncertainty of the certified range. On the basis of the present data the following MW procedures can be suggested:
-
1.
for urine, digestion with nitric acid at atmospheric pressure in plastic vials;
-
2.
for serum, digestion with nitric acid at atmospheric pressure in glass vessels; and
-
3.
for whole blood, digestion under pressure in quartz tubes.
Because of the levels of the procedural blanks, Bi was not measurable at the concentrations expected in human fluids, and Al was accurately detectable in whole blood only.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is evidence that metals such as Al, Cd, Hg, Mn, and Pb play a role in the aetiology of neuro-degenerative diseases [1, 2, 3]. In particular, some association between Parkinson's (PD) and Alzheimer's (AD) diseases and environmental or occupational exposure to certain elements has been recognized [4, 5, 6]. In order to determine the influence of the environment on neuro-degeneration it is essential to measure the content of trace elements in the body fluids of healthy subjects and of patients affected by AD or PD. To address this problem an epidemiological study was launched in Italy to determine the levels of different body metals in these population groups in order to assess the role of exposure to specific metals as a potential co-factor of risk in the onset of these pathologies.
Bio-monitoring campaigns generally involve the testing of a large number of specimens and the development of routine analytical procedures with high throughput is thus essential. The unique combination of multi-element measurement capabilities and excellent detection power of inductively coupled plasma mass spectrometry (ICP–MS) makes this the technique of choice for epidemiological screening purposes and for ultra-trace analysis. Biological fluids nonetheless cause some matrix-induced variations in the ICP–MS signal, such as:
-
1.
signal suppression due to the high concentration of salts and, therefore, of easily ionizable metals (Na, K, etc.);
-
2.
signal enhancement on some analytical masses caused by the high content of organic carbon; and
-
3.
changes in sampling efficiency due to the gradual clogging of the torch injector and cones.
With regard to sample-preparation strategies, simple dilution of biological fluids could be preferred in order to minimize sample handling and save time, particularly when dealing with large-scale quantification. However, ICP–MS determinations may suffer from a significant decrease in sensitivity and signal stability when working with weakly homogeneous samples. During analysis of water-diluted body fluids, the deposition of organic components may cause clogging of the nebulizer, torch injector and cones, as well as requiring recurrent changing of plastic pipes and cleaning of ICP glassware [7, 8, 9, 10]. Riondato et al. found rapid clogging of the torch system when analysing human serum diluted 1+4 in water [10]. Krachler et al. had to dilute urine samples up to 50-fold with high-purity water to obtain accurate and reliable instrumental measurements for direct analysis by sector field mass spectrometry with an ultrasonic nebulizer [9]. On the other hand, when high dilution factors are applied there is the risk that the level of concentration will be too close to the detection limits (DLs). For these reasons, sample digestion still remains a suitable strategy for the accurate and routine analysis of biological fluids [11, 12]. A variety of decomposition procedures for biological matrices have been reported, including low-temperature ashing in a stream of oxygen, high-pressure ashing in quartz ampoules, ultraviolet photolysis, and microwave (MW)-assisted acid digestion in open or closed digestion vessels [13, 14, 15, 16, 17, 18, 19]. Ultraviolet irradiation is able to destroy the organic material contained in biological matrices such as urine and serum, although this method is expensive in terms of both reagents and time [9, 14]. In recent years, closed-vessel MW digestion has become the most popular method, because it implies efficient mineralization, reduced contamination risk, and relatively short preparation time. Many authors indicate MW digestion as the method of choice for the routine analysis of elements in biological matrices [16, 17, 18, 19]. Notwithstanding this, few MW digestion procedures have been investigated for large-scale measurements, mainly due to drawbacks closely connected with these systems, such as:
-
1.
the low number of vessels (max. 10) per reactor,
-
2.
the rather high volume of the liners (scarcely adequate during sample manipulation), and
-
3.
the time-consuming cleaning process after each cycle of digestion.
The purpose of this study was, thus, to tentatively develop pretreatment procedures combining high sample throughput, speed and ease of operation, and low contamination risk, i.e., the use of multiple batch reactors (up to 80 vessels) filled with disposable vessels in which the whole manipulation (sample collection, digestion, and determination) could be carried out. Different sample MW digestion procedures were, therefore, compared with the aim of examining their potential applicability in the routine analysis of large quantities of biological fluids. Plastic, glass, and quartz digestion vessels were tested for metal leaching or adsorption and for thermal resistance. Sector field (SF) ICP–MS was used for the quantification of Al, Bi, Cd, Co, Cr, Hg, Mn, Mo, Ni, Pb, and Tl in digested samples of urine, serum and whole blood. The accuracy and precision of the methods were checked against appropriate certified reference materials (CRMs).
Experimental
Instrumentation
The SF-ICP–MS system used was a Thermo Finnigan Element model (Bremen, Germany) equipped with a standard sample-introduction device (Meinhardt-type glass nebulizer; water-cooled Scott spray chamber; torch with guard electrode device; platinum interface cones). The following ICP operating conditions were used: RF power, 1200 W; gas flow rates, 14.0 L min−1 (plasma), 0.90 L min−1 (auxiliary), and 0.85 L min−1 (nebulizer); sample uptake rate, 0.4–0.6 mL min−1; mass resolution, 300 m/Δm (low resolution, LR, for Bi, Cd, Hg, Mo, Pb, Tl) and 3000 m/Δm (medium resolution, MR, for Al, Cr, Co, Mn, Ni); no. of scans in LR and MR mode, 25; total time of analysis, 2 min. Daily instrument optimization was also performed by monitoring the BaO+:Ba+ ratio, in order to obtain values lower than 0.001.
Samples were MW-digested in a Milestone Ethos 900-Mega II oven (FKV Milestone, Milan, Italy). Two different MW multiple batch reactors (MultiPREP Rotors, FKV Milestone) fitted with fibre-optic temperature control systems were tested:
-
1.
the MultiPREP 80 rotor (with 80 reaction vessels, for digestion at atmospheric pressure) in combination with three kinds of reaction tube: 15 mL conical tubes in polyethylene (PE) or polystyrene (PS) (Falcon, Franklin Lakes, NJ, USA) and 20 mL glass liners (FKV Milestone); and
-
2.
the MultiPREP 36 rotor (with 36 reaction vessels, for digestion under pressure) equipped with liners made of glass and quartz (FKV Milestone).
Reagents and samples
Ultrapure grade HNO3 and HCl (Carlo Erba, Milan, Italy) and Milli-Q de-ionized water (PBI International, Milan, Italy) were used. Prior to MW-treatment PE and PS conical tubes were carefully decontaminated with a 10% mixture of HCl and HNO3 overnight, followed by repeated rinsing with de-ionized water. In addition, glass and quartz vessels were processed through repeated acid-assisted MW cleaning cycles. All disposable lab-ware, such as pipette tips, was rinsed with HNO3 and de-ionized water. Calibrations in the standard addition mode and the use of the internal standard (IS) correction provided adequate means for dealing with matrix-induced variations and instrumental drifts. Calibrants and IS solutions were prepared by diluting 1 mg mL−1 single-element standard solutions (Spex, Edison, NJ, USA). Calibration ranges were:
-
1.
in urine, 2.0–8.0 ng mL−1 for Cd, Co, Cr, Hg, Mn, Ni, and Tl; 5.0–20.0 ng mL−1 for Pb, and 20.0–80.0 ng mL−1 for Al;
-
2.
in serum, 0.125–0.5 ng mL−1 for Bi, Cd, Co, Cr, Hg, Mn, Ni, and Tl; 5.0.0–20.0 ng mL−1 for Al, and 0.5–2.0 ng mL−1 for Mo;
-
3.
in blood, 0.25–1.0 ng mL−1 for Cd, Co, Cr, Hg, and Ni; 2.5–10.0 ng mL−1for Mn, and 10.0–40.0 ng mL−1 for Pb.
Two ISs were used in LR mode, namely 115In (1 ng mL−1) in the case of 100Mo, 114Cd, 123Sb, and 195Pt (1 ng mL−1) for 202Hg, 205Tl, 209Bi, and 208Pb. In the MR mode 45Sc (1 ng mL−1) as IS was adequate for all the analytes under study, i.e., 27Al, 59Co, 52Cr, 55Mn and 60Ni.
All manipulations were performed in a Class 100 clean room (Tamco, Rome, Italy). Certified reference materials were Clinchek Control-lyophilized human urine, human serum, and human whole blood (Recipe, Munich, Germany). The lowest concentration level for each CRM was used. The CRMs were employed both for the accuracy study and for precision tests.
Control of contamination
The well-known excellent capability of SF-ICP–MS to quantify trace and ultra-trace elements in biological fluids could be negatively affected by paying scarce attention to contamination sources. For these reasons, strict control of the entire analytical procedure, including the pretreatment step, was adopted (see also the previous sections). Specimen handling was restricted to a minimum. The samples were analysed in the same container in which digestion was carried out. Moreover, a small amount of reagents was used, while only a minimal dilution factor was required, thanks to the low concentration of organic constituents in the MW-digested matrices. Furthermore, the contribution to the element contamination (mainly Al, Bi, Sn, etc.) from the pump tubing and nebulizer was effectively limited by 10 min washing with 0.005% EDTA solution followed by a rinse with 5% HNO3 for about 10 min, before starting analytical sequence. Release of material from the sampler and skimmer cones was reduced by the formation of a salt layer on the interface by aspiration of diluted urine samples for 10 min prior to daily instrument tuning.
Sample preparation
Microwave digestion procedures were developed as a function of the complexity of the matrix, by optimizing the type of reagent, the reagent/sample volume ratio, the temperature, the MW power ramp and the time. Two or more blank digests were prepared for each series of samples in order to measure the level of contamination. Urine samples were MW-digested at atmospheric pressure (MultiPREP 80 rotor) in PE or PS conical tubes and glass vessels on the basis of the following scheme: 1 mL of the sample; 0.5 mL of HNO3; temperature ramp, 10 min up to 60 °C and 30 min at 60 °C. Samples were diluted 1+4 by adding de-ionized water directly into the reaction vessels. For serum samples the following digestion in MultiPREP 80 rotor was performed: vessels, PE or PS tubes and glass liners; sample volume, 1 mL; reagent, 1 mL HNO3; temperature program, 10 min up to 80 °C and 30 min at 80 °C. The final 1+4 dilution with de-ionized water was achieved in the same digestion container. Whole blood was digested under pressure conditions (up to 15 bar) in glass and quartz closed vessels (MultiPREP 36 rotor). The following scheme was adopted: 1 mL of sample, 2 mL of HNO3, temperature up to 130 °C in 15 min and at 130 °C for 20 min. The digested solutions were diluted in the reaction containers up to 10 mL with de-ionized water. For all digested samples the deionized water used for final dilutions contains the ISs.
Results and discussion
Spectral interference
The severity of problems associated with spectral interference in biological matrices has long been recognized. To reduce the weight of spectral interference on the masses under study, the following general principles were adopted:
-
1.
evaluation of the effective non-significance of some types of interference due to the low concentration of the interfering metal in the matrix considered (e.g., 191Ir18O on 209Bi; 192Os16O on 208Pb; 189Os16O on 205Tl);
-
2.
acceptance of a compromise between deterioration of detection power and higher instrumental resolution; and
-
3.
acceptance of the impossibility of separating some interference from the analytical mass, even in high-resolution mode (e.g., 98Mo16O and 114Sn on 114Cd).
Table 1 reports the operational conditions chosen for the analysis, i.e., the isotopes for quantification and the level of resolution selected. By using MR mode the effective control of interference enables the determination of elements such as Al, Co, Cr, Mn, and Ni, which would otherwise be severely affected, while preserving adequate instrumental sensitivity. The most abundant isotopes for each element were selected, with only two exceptions, Mo and Ni. The m/z 98 of the former cannot be taken into account because it is heavily affected by 40Ar58Fe, due to the high Fe content in blood samples. For the latter, the isobaric overlapping of the 58Fe signal prevents the use of 58Ni. Mathematical corrections are unavoidable for accurate 114Cd determination in biological samples, as instrumental separation of the interference of 114Sn and 98Mo16O is not possible, even in high-resolution mode.
Performance of digestion procedures
The MW digestion procedures tested in this study involve the use of multi-batch reactors that permit the simultaneous mineralization of numerous samples, i.e., 36 or 80, in one cycle. The digestion containers used were also easy to close without specific tools (screw-cap vials). These properties make it possible to achieve high sample throughput and simplicity of operation, essential requirements when a large number of samples is to be processed, maintaining at the same time a sufficient mineralization efficiency. This last characteristic was confirmed by the value of the total organic carbon (TOC) remaining after the digestion step, i.e., 0.35, 2.15, and 5.50 g L−1 for urine, serum, and blood analytical solutions. Moreover, the temperature probe located inside the reaction vial and the fact that the same vessel is used for digestion and quantification are also important in keeping digestion conditions and pre-analytical contamination under control. All these characteristics help to ensure the overall reliability and accuracy of the analysis.
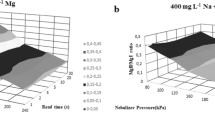
Figure 1 shows the stability pattern of signals acquired in LR and MR modes during a 5-h period of SF-ICP–MS analysis of serum diluted 1+4 with water or MW-digested and then diluted 1+4. When serum was diluted in water the stability of the analytical signal was clearly scant for both elements shown in the figure, Mo in LR mode and Cr in MR. By improving the homogeneity of the analytical solutions, mineralization reduced the risk of clogging the devices used for introducing samples and substantially enhanced the long-term stability of the instrument. Similar profiles were obtained for the other analytes.
Table 2 reports the SF-ICP–MS detection limits calculated as three times the standard deviation of analytical replicates of a 5% HNO3 solution, as well as the blank concentrations obtained after different MW digestion procedures. It should be noted that the blank concentration for each container is the mean of 20 reagent blanks that were MW-processed on different days and hence also reflects the variability between preparation batches. The instrumental DLs reported here are in good agreement for almost all the elements under consideration with those obtained by Rodushkin et al. for analogous HNO3 solutions analysed by SF-ICP–MS, while for Cd, Cr, Mn, Ni and Tl they were slightly better [17]. The DLs found here for Cr and Ni are also similar to those obtained by Krachler et al. in SF-ICP–MS analysis of a simple 5% HNO3 solution [9].
Despite the good quality of the instrumental DLs achieved, the real limiting factor for successful quantification is the level of the procedural blanks. All the pretreatments described (which differed according to vessels and/or digestion conditions) gave similar blank contributions for almost all the analytes, except for Al and Pb. In these two cases the lowest blank value was obtained by means of mineralization under pressure in quartz liners, whilst glass vials released a significant amount of Al and Pb, especially when this material was stressed under pressure.
On the other hand, taking into account the ranges of concentration of elements expected in the urine, serum, and blood of unexposed subjects (see columns 8–10 in Table 2), the digestion blanks found by the authors are suitable for quantification of most of the elements under consideration, with the exception of Bi. In effect, the high blank concentration of Bi and the very low natural content of this element in biological fluids hampered its quantification. Moreover, Al determination can be carried out only in whole blood by means of mineralization under pressure in quartz vials, at least for non-exposed subjects. Special attention should also be paid during the analysis of Cr, Hg, and Ni in urine, and Pb in serum, as their blank levels are close to the lower limits of ranges of the concentrations expected for unexposed subjects [20, 21, 22]. However, the blank levels obtained were fully adequate for the analysis of the CRMs (level I) used in this study and presumably satisfactory for quantifying elements in the biological fluids of occupationally or environmentally exposed persons in whom levels of concentration may reasonably be expected to be higher than normal.
Table 3 summarizes the concentration of the analytes in the human urine control material as determined by SF-ICP–MS after mineralization in three different reaction tubes. For all elements and vessels considered there was fully satisfactory agreement between the certified values and those measured. The only exception was for Al processed in glass liners, which resulted in not negligible contamination due to leaching from the tube, although the value found was within the reference range. The precision over 20 replicates of the same digested CRM solution was always better than 8%. Plastic materials may be preferred in routine analyses because they are disposable, inexpensive, and easy to decontaminate. In particular, polystyrene vials were more suitable because of their better thermal resistance.
Table 4 reports the concentrations of the analytes in the serum CRM after mineralization in normal conditions carried out in different reaction vessels. Digestion in plastic containers produced results very close to—and in the cases of Hg, Mo and Tl, below—the lower limit of the reference range. On the other hand, it is known that some elements (e.g., Hg) could be adsorbed on the plastic wall of the containers. The precision was also fairly high (between 4 and 30%), suggesting scant homogeneity of the digested solutions, i.e., incomplete destruction of the serum matrix. This was due to the necessary compromise adopted between the thermal resistance of the plastics and the need to reach high temperatures (up to 80 °C) in order to obtain homogeneous and well-mineralized solutions. However, serum mineralization in glass liners led to recoveries ranging between 93% for Ni and 122% for Hg, and to precision ranging between 2% for Cd and Mo and 9% for Bi. These figures demonstrate that glass vessels are suitable for accurate and reliable analysis of the elements listed in Table 4. The data for accuracy and precision in Table 5 relate to the elements analysed in whole blood CRMs after mineralization under pressure in glass and quartz liners. The data for recovery and reliability obtained for both container materials tested were satisfactory. However, the procedure of pre-conditioning and decontaminating the glass liners resulted to be a step more critical than in the case of the quartz vials.
The present results suggest the following procedures for the routine determination in biological fluids of the trace elements considered:
-
1.
for urine (1 mL), MW digestion with 0.5 mL of HNO3 at atmospheric pressure in plastic tubes (preferably PS) and 1+4 final dilution (Al and Bi cannot be accurately quantified using this method);
-
2.
for serum (1 mL), MW digestion under normal conditions with 1 mL of HNO3 in glass vessels and 1+4 final dilution (again with the exception of Al and Bi, which cannot be efficiently analysed); and
-
3.
for blood (1 mL), MW digestion under pressure with 2 mL of HNO3 in quartz vessels and 1:10 water dilution (only Bi could not be detected).
Conclusions
The main advantages of the MW digestion-based strategies proposed here for the treatment of biological fluids are the short sample mineralization time and the high rates of sample throughput, i.e., 40 min for simultaneous digestion of from 36 to 80 samples, and the ease of execution of the entire procedure.
Thanks to these characteristics, the MW procedures tested are really useful for the monitoring of Cd, Co, Cr, Hg, Mo, Mn, Ni, Sb, and Tl in the urine, serum and blood of non-exposed or exposed subjects, as well as for the screening of Al, albeit only in whole blood matrix. The value of the procedural blanks was still the limiting factor in accurately quantifying Bi and, in part, also Al in the human fluids under study.
References
Pamphlett R, McQuilty R, Zarkos K (2001) Neurotoxicology 22:401–410
Verity MA (1999) Neurotoxicology 20:489–498
Bressler J, Kim K, Chakraborti T, Goldstein G (1999) Neurochem Res 24:595–600
Montgomery EB (1995) Toxicology 97:3–9
Doll R (1993) Age Ageing 22:138–153
Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ (1999) Neurotoxicology 20:239–248
Mingorance MD, Perez-Vazquez ML, Lachica M (1993) J Anal At Spectrom 8:853–858
Vandecasteele C, Vanhoe H, Dams R (1993) J Anal At Spectrom 8:781–786
Krachler M, Alimonti A, Petrucci F, Forastiere F, Caroli S (1998) J Anal At Spectrom 13:701–705
Riondato J, Vanhaecke F, Moens L, Dams R (1997) J Anal At Spectrom 12:933–937
Alimonti A, Petrucci F, Santucci B, Cristaudo A, Caroli S (1995) Anal Chim Acta 306:35–41
Vaughan MA, Baines AD, Templeton DM (1991) Clin Chem 37:210–215
Knapp G, Raptis SE, Kaiser G, Tolg G, Schramel P, Schreiber B (1981) Fresenius Z Anal Chem 308:97–103
Schramel P, Wendler I (1998) Fresenius J Anal Chem 361:487–491
Begerow J, Turfeld M, Dunemann L (2000) J Anal At Spectrom 15:347–352
Rodushkin I, Ödman F, Branth S (1999) Fresenius J Anal Chem 364:338–346
Rodushkin I, Ödman F, Olofsson R, Axelsson MD (2000) J Anal At Spectrom 15:937–944
Teresa M, Vasconcelos SD, Tavares HMF (1997) Sci Total Environ 205:189–199
Kunze J, Koelling S, Reich M, Wimmer MA (2000) Fresenius J Anal Chem 366:165–166
Caroli S, Alimonti A, Coni E, Petrucci F, Senofonte O, Violante N (1994) CRC 24:363–398
Iyengar GV (1998) Radiat Phys Chem 51:545–560
Rodushkin I, Element 2 Finnigan Mat Application Flash Report no. E11, 12/01 Part no.1990950
Acknowledgements
This work is a part of the Neurotox Project funded by the Italian Ministry of Health (Project no. 1AB/F, 2002–2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bocca, B., Alimonti, A., Forte, G. et al. High-throughput microwave-digestion procedures to monitor neurotoxic elements in body fluids by means of inductively coupled plasma mass spectrometry. Anal Bioanal Chem 377, 65–70 (2003). https://doi.org/10.1007/s00216-003-2029-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2029-4