Abstract
The intermolecular association of twelve combinations of six different Lewis acids and Lewis bases (i.e., R 3 A–BR′3 where A = B and Al; B = N and P; R = H, F, and C6F5; R′ = H, CH3, and C(CH3)3) was theoretically described by means of DFT calculations using the dispersion-corrected ωB97x-D and B97D functionals in conjunction with the 6-311++G(2d,2p) basis set including toluene as solvent through the PCM-SMD implicit solvent scheme. All the studied Lewis pairs appeared to be stable on the basis of computed BSSE-corrected interaction energies; however, the free energies of formation computed in solution (ΔG solv) indicate that three Lewis acid–base combinations can be considered frustrated Lewis pairs (FLPs). Besides, the four features that characterize FLPs are: (1) large distances between the acid and base centers, (2) negligible changes in the geometry of the acid, (3) weak interaction energies, and (4) non-covalent dispersion energy contributing to almost the entire interaction energy. In the present work, we introduce two ad hoc indexes intended to quantify separately the electronic and steric factors, which have a direct effect in the intermolecular association of Lewis acids and Lewis bases and can be used to distinguished FLPs from classical Lewis adducts. Based on the aforementioned ad hoc indexes, the existence of a new kind of complexes that are “intermediate” between classical complexes and FLPs is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In 1923, G. N. Lewis introduced his classical definition of acids and bases in order to rationalize the behavior of numerous chemical reactions [1]. According to Lewis’ definition, an acid is a molecule able to accept a pair of electrons, whereas a base is a molecule able to donate a pair of electrons. Thus, according to this, it can be stated that, at least in principle, a stable complex or adduct is always formed when acids and bases are combined as a consequence of the electron-donor/electron-acceptor interaction between the two species. The previous canon is considered one of the cornerstones in the chemistry of acids and bases, and it is also recognized as one of the most fundamental principles in organic as well as inorganic chemistry.
Even though most of the combinations of Lewis acids and bases result in a dative adduct, occasionally some combinations of bulky acids and bases appear to deviate from the simple Lewis rule [2–5]. Historically, this anomaly has been attributed to steric effects that preclude the encounter between the Lewis acid and base reactive centers. Therefore, the thermodynamic stability and other factors governing the formation of Lewis adducts have become an aspect of growing interest, specially since the non-conventional chemistry of the so-called frustrated Lewis pairs (FLP) was reported for first time in 2006 by D. W. Stephan [6, 7]. Stephan and collaborators showed, in an unprecedented experiment, that the combination of boranes and phosphines possessing bulky substituent groups is able to cleave the H–H bond under very mild conditions, representing the first example of a reversible H2 activation without the aid of a transition metal. This unusual reactivity is attributed to the use of a combination of a Lewis acid and a Lewis base in which the steric demand frustrates the formation of the classical dative adduct. As a result, the chemistry of FLPs has evolved in the last ten years as one of the most fructiferous strategies for metal-free activation of small molecules using both intermolecular and intramolecular combinations of Lewis acids and Lewis bases [8–11].
It must be emphasized that understanding the factors that affect the stability and reactivity of the association of Lewis acid and bases represents a great challenge for electronic structure calculations. Theoretical studies agree that the weak non-covalent interactions between Lewis acids and Lewis bases are the driving forces in the formation of FLPs [12–21]. This point has been addressed recently by Skara et al., who have investigated fourteen different Lewis pairs of various sizes using the Ziegler-Rauk energy decomposition in order to assess the relative contribution of: (1) the electrostatic and the orbital interactions, (2) the steric effects, and (3) the dispersion energy contribution to the total binding energy [20, 22]. In Skara’s work, the orbital interactions were computed by employing a natural orbital chemical valence (NOCV) analysis [23], whereas the non-covalent interactions were described by using the non-covalent interaction (NCI) method [24]. Interestingly, the results of the study revealed that the weak forces present between a Lewis acid and a Lewis base are the main energetic effects leading the mechanism of FLPs formation. More recently, state-of-the-art electronic structure methods have been also applied to investigate FLPs built up from Tris(pentafluorophenyl)borane (B(C6F5)3) and two phosphines (PR3; with R = 2,4,6-MeC6H2 and t-Bu) [21]. In agreement with the results of Skara et al., the latter study showed that the weak non-covalent interactions, in particular the dispersion interactions, are the driving factors for the formation of FLPs.
In this work, a further investigation of twelve combination of different Lewis acids and Lewis bases (i.e., R 3 A–BR′ 3 where A = B and Al; B = N and P; R = H, F, and C6F5; R′ = H, CH3, and C(CH3)3) is presented in order to gain deeper insights into the stability of the intermolecular association between Lewis acids and Lewis bases. In particular, the energetic factors that control the overall thermodynamic stability of Lewis pairs in liquid toluene (i.e., a representative nonpolar solvent) is analyzed by means of DFT calculations performed by employing of the dispersion-corrected ωB97x-D and B97D functionals in conjunction with the 6-311++G(2d,2p) basis set. In contrast with previous theoretical studies that were focused on the analysis of FLPs, the combinations of Lewis acids and Lewis bases employed herein broaden the scenario from very strong classical Lewis adducts to FLPs possessing different degrees of electronic character (i.e., R groups with different electron-withdrawing and electron-donating character) as well as steric effects (i.e., R groups of various sizes). By employing the latter models, we expect to contribute to obtain a general perspective of the intermolecular association between Lewis acids and Lewis bases, with a description not biased toward the idea of “frustration” of the Lewis adducts. In more detail, the geometric and energetic changes occurring on the Lewis acids and Lewis bases are analyzed, and special emphasis in the contribution of the non-covalent dispersion interactions to the total energy is made. Moreover, two ad hoc indexes are introduced to account for the electronic and steric effects in the Lewis acids and the Lewis bases. Here, it is shown that these indexes can be easily calculated for the separated free gas-phase Lewis acids and Lewis bases, and they can be used to predict a priori whether a classical Lewis adduct or a FLPs is formed.
2 Computational details
All calculations of the present study were performed employing the Gaussian 09 suite of programs [25]. Equilibrium geometries were obtained using two dispersion-corrected exchange–correlation functionals, the long-range-corrected hybrid ωB97x-D functional [26–28] and the B97D functional [28] together with the large 6-311++G(2d,2p) basis set [29–31]. For a selected group dimers, we have also performed an analysis of the basis set size effect due to the inclusion of polarization and diffused functions. An ultra-fine grid was adopted for all the calculations since Lewis adducts have many soft vibrational modes. The Berni algorithm in redundant internal coordinates [32] was adopted for the geometry optimizations, and the thresholds for convergence were set to 0.00045 a.u. and 0.0003 a.u. for maximum force and root-mean-square (rms) force, respectively. The errors due to the basis set superposition (BSSE) were estimated by employing the standard counterpoise method as proposed by Boys and Bernardi [33]. Upon obtaining the equilibrium geometries of the models, a vibrational analysis was performed at the same level of calculation in order to confirm that the computed structures correspond to true minima in the potential energy surface. Subsequently, the resulting vibrational frequencies were employed to compute the zero-point energy and thermal corrections (i.e., ZPE and ET, respectively) in the ideal gas approximation at 298.15 K and 1 atm. Although previous studies have shown that both the ωB97x-D [20, 21] and B97D [12–19] functionals provide correct qualitative trends regarding the intermolecular association of Lewis acids and Lewis bases, it must be pointed out that free energy differences below 2 kcal/mol obtained with the present computational scheme are expected to be greatly affected by the non-negligible errors introduced in entropic contributions from the application of the rigid-rotor and the harmonic approximation for frequencies smaller than 100 cm−1. In order to take into account the effect of the solvent, the polarizable continuum model with the radii and non-electrostatic terms for SMD Cramer and Truhlar solvation model was adopted (PCM-SMD). A dielectric constant value of 2.3741, corresponding to liquid toluene, was considered to perform single point calculations on the equilibrium geometry of the systems as obtained at the gas phase. A concentration correction of 1.89 kcal mol−1 in the calculation of solvation free energies was used to account for the change in conditions when going from 1 atm to 1 M concentration (i.e., when going from gas phase to a solution regime) [34–36]. For all dimers, the charge transfer extension between the Lewis acid and the Lewis base was assessed by means of the Quantum Theory of Atoms in Molecule (QTAIM) by using the EXTREME and PROAIM programs of the Bader’s group [37–39].
For the steric effect analysis, a measure of the substituent volumes is obtained through the Weizsacker kinetic energy functional,
The Weizsacker energy was used because it has proven to be an indication of the changes in the volume of a given system [40–42], and it can be easily calculated by employing the wavefunction file (i.e., WFN output), obtained with the program Gaussian, through our in-home implementation of the Becke integration methodology based on the atomic fuzzy Voronoi polyhedral [43]. For each atomic basin, the radial integration has been performed using 40 points in the Chebyshev’s quadrature, whereas for the angular part, the Lebedev’s quadrature method with 194 points has been employed.
3 Results and discussion
For the sake of clarity, the present Section has been divided into three parts: (a) the analysis of the factors that contribute to the thermodynamic stability of the intermolecular association of Lewis acids and Lewis bases, (b) the geometric and energetic changes upon the formation of the Lewis adducts, and (c) an evaluation on the interplay between electronic and steric factors in the intermolecular association of Lewis acids and Lewis basis.
3.1 The thermodynamic stability of the intermolecular association of Lewis acids and Lewis bases
The different Lewis acids and Lewis bases that form the twelve pairs (1–12) considered in the present work are reported in Table 1 together with their corresponding BSSE-uncorrected and BSSE-corrected interaction energy (ΔE and ΔE(BSSE), respectively) obtained at the ωB97x-D/6-311++G(2d,2p) (first line) and B97D/6-311++G(2d,2p) (second line) level of calculation. Dimers 1–4 correspond to the cases where the substituent groups in the acids (i.e., boranes and alanes) and bases (i.e., amines and phosphines) are H; dimers 5–8 are the cases where the substituents in the acids are F and the substituents in the bases are CH3; and finally, dimers 9–12 are systems where the substituents on the acid are C6F5 and the substituents on the base are C(CH3)3. Taking into consideration the latter descriptions, it can be stated that pairs 5–8 represent cases of increasing acidity/basicity character and modest or negligible changes concerning the steric effects when compared to 1–4, whereas the pairs 9–12 represent systems of increasing acidity/basicity character as well as increasing steric effects with respect to 1–4. From the ΔE(BSSE) data reported in the column 5 of Table 1, it can be observed that for the two functionals employed, all the considered combinations resulted in stable complexes (i.e., ΔE(BSSE) < 0). However, the degree of stability in each case is different, allowing the various complexes to be classified into four categories as follows: very strong if the interaction energy is lower than −30 kcal/mol (a category containing the two AlF3 complexes 7–8), strong if the interaction energy is between −30 and −20 kcal/mol (5 complexes), weak if the interaction energy is between −20 and −10 kcal/mol (4 complexes), and very weak if the interaction energy is above −10 kcal/mol (1 complex). In addition, it is observed that for the complexes 1–8 and 12, the ΔE calculated using the ωB97x-D functional is between 2 and 4 kcal/mol stronger than the B97D values, in contrast, for the three weaker bonds, 9–11, the B97D functional predict tighter bonds; in spite of these differences, both functionals show the same stability trend. Upon comparison of columns 4 and 5, it is observed that the BSSE values (i.e., the ΔE(BSSE) − ΔE difference) depend on the size of the substituent groups of the Lewis pairs as follows: The larger the components of a pair, the larger its BSSE. The resulting BSSE values for both functionals are within the 0.36–0.25 kcal/mol, 1.36–2.03 kcal/mol, and 2.37–3.39 kcal/mol ranges for the groups of dimers 1–4, 5–8, and 9–12, respectively. In column 6 of Table 1, the values of the gas-phase enthalpy computed at 298.15 K and 1 atm, ΔH gas, are reported. The experimental value of ΔH gas for complex 1 is −31.1 ± 1.0 kcal/mol [44], and this value is 4 kcal/mol larger than our best prediction. However, our values of ΔH gas are in very well agreement with the values calculated for MP2 and six DFT functionals with similar basis set [45]. When subtracting former values from the ΔE(BSSE) ones, the contribution of the zero-point energy and the thermal corrections to the enthalpy are obtained. In contrast to the BSSE values, a particular trend is not found regarding either the zero-point energy or the thermal corrections to the enthalpy. The sum of these corrections spans the 0.97–4.19 kcal/mol range for ωB97x-D functional and 1.21–3.64 kcal/mol for the B97D functional, being the two greatest amounts the quantities associated with 1 and 12 in both functionals (i.e., H3B–NH3, (C6F5)3Al–P(C(CH3)3)3, respectively). Values reported in column 7 are the gas-phase free energy of formation (ΔG gas) computed at 298.15 K and 1 atm. The difference between these data and the gas-phase enthalpy corresponds to the gas-phase entropic components (−TΔS gas), which is a positive quantity for all cases and span the 8.83–16.87 kcal/mol range for both functionals. Previous works have remarked that the gas-phase entropy is the most destabilizing component in the intermolecular association of Lewis pairs [19–21], and it could lead to the formation of FLPs. In agreement with the latter statement, the largest value obtained in the present study for both functionals corresponds to the (C6F5)3Al–P(C(CH3)3)3 pair (12), which possesses bulky R substituents. However, it must be indicated that relatively large −TΔS gas values are also found in other pairs including some of those possessing less bulky R groups. The last thermodynamic quantities reported in Table 1 correspond to the PCM-SMD free energy values (ΔG solv). By analyzing the computed ΔG solv values for both functionals, it can be pointed out that the formation of dimers 9, 10, and 11 is not favorable from the thermodynamic point of view, and they can be considered as FLPs. The last Lewis pair (12) is particularly interesting because, albeit it has the theoretic conditions to give rise to a FLP, its formation is slightly favorable according to its ΔG solv value of −7.42 kcal/mol for ωB97x-D and −4.46 kcal/mol for B97D. A plausible explanation for this is that 12 has some characteristics of a classic Lewis adduct as well as a FLP; thus, it represents an “intermediate” system.
It is remarkable for each level of calculation that the zero-point energy, the gas-phase thermal corrections to the enthalpy, and the entropic correction to the gas-phase free energy are approximately similar in each of the complexes (i.e., deviations not larger than 3 kcal/mol) in spite of the fact that these systems are different regarding the electron-withdrawing/electron-donating character of the R substituent groups or the nature of the centers of the Lewis acid and Lewis base (with the notable exception of 12). We notice that the average increase in energy from ΔE(BSSE) to ΔG solv is 13.74 kcal/mol for the ωB97x-D level (11.76 kcal/mol for B97D) and 11.08 kcal/mol for the ωB97x-D level (15.04 kcal/mol for B97D) from ΔE(BSSE) to ΔG solv for the bulky substituents (9–12). These values are slightly lower than values previously reported; for instance, Skara et al. have obtained values for fourteen FLPs with an increment in energy between 16.4 and 21.5 kcal/mol (an average of 18.29 kcal/mol) from ΔE(BSSE) to ΔG solv using ωB97x-D/6-311++G(d,p), and Bannwarth, Hansen, and Grimme have reported a value of 13.3 kcal/mol for the pair B(C6F5)3–P(t-Bu)3 from ΔE(BSSE) to ΔG gas and 12.4 kcal/mol from ΔE(BSSE) to ΔG solv by employ the COSMO-RS solvation model [21].
We end this section with a comment regarding the basis set employed. B97D optimization and frequencies calculations performed on the complexes 1–4 with different basis sets: 6-31G(d,p), 6-31++G(d,p), 6-311G(2d,2p), and 6-311++G(2d,2p), present differences of less than 1.42 kcal/mol for ΔE and 1.89 kcal/mol for ΔG gas, being the largest deviation found in the case of the alanes (i.e., 3 and 4). It is important to notice that these differences are specially observed when diffused functions are included to both the 6-311G(d,p) and the 6-311G(2d,2p) basis sets, and it is also observed that diffusion functions tend to enhance the bond. Interestingly, a similar trend is obtained for the complex 12; however, in this particular case the inclusion of diffuse functions produces a large change. In going from the 6-31G(p,d) to the 6-31++G(d,p) the ΔE change from −26.77 kcal/mol to −28.10 kcal/mol, and from 6-311G(2d,2p) to 6-311++G(2d,2p), a decrease from −26.91 kcal/mol to −28.92 kcal/mol is observed. The latter observation applies also for computed ΔG gas values. Clearly, the inclusion of diffuse functions in the basis set, as employed in this work, seems to be mandatory for the study of these complexes, specially when bulky substituents are employed.
3.2 Geometric and energetic changes upon the combination of the Lewis acids and Lewis bases
Conceptually, the formation of a Lewis pair can be ideally divided into two steps: (1) the distortion of the Lewis acid and the Lewis base to the geometry that these species adopt when compose the complex and (2) the subsequent establishment of a binding interaction between the acid and the base reactive centers. Thus, it results reasonable to analyze the interaction energies obtained for systems 1–12 as a sum of the above-mentioned contributions by considering the following expression:
where E AB(X) is the energy of X with the geometry in the dimer AB and E 0(X) is the optimized energy for X. ΔE(A) and ΔE(B) are always destabilizing terms, whereas the last term, ΔE(AB), corresponds to the stabilizing effects due to polarization, exchange, and charge transfer between the Lewis acid and the Lewis base at the fixed complex geometry. The terms of the energy decomposition in Eq. (1) are presented in Table 2, for the two functional employed in this work, together with some relevant geometrical features of systems 1–12. Table 2 also includes the change in the dispersion energy correction, ΔE DISP, obtained at the ωB97x-D and B97D levels for the different Lewis acid–base pairs upon comparison with their free components. Results reported in Table 2 are commented in a more detailed manner in the following paragraphs. As a complement of the geometrical values presented in Table 2, in Fig. 1, we show the structures of the optimized complexes as obtained at the ωB97x-D/6-311++G(2d,2p) level with some relevant geometrical parameters. Needless to say, similar structures are obtained with the B97D functional.
In general terms, the bond distance between the Lewis acid and the Lewis base can be ascertained from the covalent distances between the electron-donor and electron-acceptor atoms; therefore, the trend, B–N < B–P ≈ Al–N < Al–P, is expected for the studied molecules. From the data reported in Table 2, it is observed that the latter trend applies only in the case of the Lewis pairs 1–8, whereas much larger values (almost twice as larger) were observed for the bulkier systems (9–11), excluding pair 12, where some favorable interaction is evident from the calculated shorter distance.
From a structural consideration, it can be stated that the trigonal planar structure of free Lewis acids must be deformed to acquire a pseudo-tetrahedral configuration in order to interact with a Lewis base. In view of the latter rule, the angle R–A–B can be used as an indicator of the degree of change in the geometry of a Lewis acid when being part of a Lewis adduct. In principle, large deviations from 90° (i.e., characteristic of an undeformed acid) are associated with significant geometrical distortions and great distortion energies. In classic Lewis adducts, this deformation tends to be large, being more significant for boranes than for alanes. On the other hand, for the case of FLPs, almost negligible changes in the R–A–B angle are determined.
As explained previously, the BSSE, the thermal correction to the enthalpy, the entropic factor, and the solvation energy produce an increase in the interaction energy in the order of 15–20 kcal/mol for bulky substituents like complexes 9–12 (Table 1) and in the order of 10–12 kcal/mol for the small substituents (1–8). This increment produces a positive free energy of complexation for weak and very weak interactions, being this characteristic commonplace in FLPs.
The most intriguing observation concerning FLPs is the fact that the contribution of the non-covalent dispersion interactions (ΔE DISP) is larger than ΔE for the two DFT functional used in this work; however, as clearly observed in Table 2, B97D dispersion energy corrections are consistently much larger than the ωB97x-D values for all complexes. The large values of ΔE DISP in the case of B97D functional is indicative of an overestimation of the dispersion interaction, as was previously reported [47]. Previous works have revealed that although several kinds of weak interactions such as dispersion, π–π stacking, C–H···π interactions, weak hydrogen bonding, and halogen bonding are present in FLPs [20], the dispersion forces are dominant since they counteract the destabilizing factors caused by the steric factors of the bulky substituents [12–21]. In fact, this effect forces to reconsider the conception of steric hindrance as purely repulsive [46]. Bulky substituents like terbutyl and pentafluorophenyl produce a huge stabilization via dispersion forces via C–H···π interactions and C–H…F–C short contacts between 1.8 and 2.0 Å. Thus, the steric demand results from a balance between the repulsive Pauli interactions and the attractive dispersive forces. From data in Table 2, it is observed that the complex between Al(C6F5)3 and P(t-Bu)3 (12) is unique in the sense that the dispersion energy accounts for a large part of the binding energy (60 % in the case ωB97x-D and 110 % in the case of B97D); however, this pair cannot be considered a FLP because it represents a thermodynamic stable system as determined on the basis of its free energy computed in solvent (Table 1). From the data in Table 2, it is also observed that the three pairs identified as FLPs (9–11), on the basis of their positive ΔG solv values (Table 1), are characterized by the following four properties: (1) very large distances between the acid and base center (i.e., A–B > 4 Å), (2) almost negligible changes in the geometry of the Lewis acid (i.e., R–A–B bond angle close to 90° and ΔE(A) ≈ 0), (3) weak BSSE-corrected interactions energies (see Table 1) and ΔE(AB) (i.e., ΔE(BSSE) and ΔE(AB) > −20 kcal/mol), and (4) very large values of the change in the dispersion energy, ΔE DISP, which is the primary stabilizing factor.
3.3 The interplay between electronic and steric effects in the intermolecular association of FLPs
In this section, a rationalization of the changes in interaction energy associated with the stabilization of Lewis adducts is addressed. With this purpose, Table 3 shows a compilation of electronic and steric descriptors of the bond between the twelve complexes of the present study. As previously indicated, systems 1–8 have been identified as classic Lewis complexes, whose stability can be rationalized, almost entirely, on the basis of electronic factors. The most popular and simplest electronic descriptor for Lewis pairs is the energy difference between the LUMO of the Lewis acid and the HOMO of the Lewis base, \(\eta = \varepsilon_{\text{LUMO}} \left( {\text{Acid}} \right) - \varepsilon_{\text{HOMO}} \left( {\text{Base}} \right)\). However, other global reactivity descriptors within the framework of the hard–soft acid–base principle have been proposed [48] as quantities that relate the charge transfer with the binding energy in a simple way. The fourth column of Table 3 show the calculated values of η (in eV) for the twelve Lewis dimers obtained with the two functionals employed in the present study. This descriptor is approximated from the Kohm–Sham orbital energies at each level of theory. Clearly, η using ωB97x-D functional is always much larger than the results obtained with the B97D functional; however, the two sets of data present an acceptable correlation (r 2 = 0.964). For compounds 1–4, the interaction energy (ΔE) reported in Table 1 decreases in the following order 1 > 3 > 2 > 4 in agreement with the values of η that are found to be 9.51 eV (3.36 eV) for B–N, 9.59 eV (3.84 eV) for Al–N, 9.69 eV (3.87 eV) for B–P, and 9.82 eV (4.36 eV) for Al–P. The correlation between ΔE and η for complexes 1–4 is r 2 = 0.959 for the ωB97x-D functional and r 2 = 0.856 for B97D. Additionally, the fifth column in Table 3 present the calculated QTAIM charge transfer from the Lewis base to the Lewis acid (Δq). As in the case of η, Δq present a strong correlation with the value of ΔE for the first four complexes: r 2 = 0.993 for the ωB97x-D functional and r 2 = 0.970 for B97D. The same behavior applies for the case of 5–8 for which the computed η are 8.10 eV (2.37 eV) for Al–N, 8.33 eV (2.77 eV) for Al–P, 9.39 eV (4.20 eV) for B–N, and 9.52 e V (4.60 eV) for B–P. As in the case of the first four complexes, for complexes 5–8 both η (r 2 = 0.856 for ωB97x-D and r 2 = 0.888 for B97D) and Δq (r 2 = 0.987 for ωB97x-D and r 2 = 0.970 for B97D) present acceptable correlations with ΔE. Interestingly, the order of the frontier orbital gap is reverse in the case of systems possessing bulkier substituents (i.e., 9–12). The strongest complex 12 has the largest η value, 6.60 eV (1.50 eV), whereas the weakest system 9 has the smallest, 5.71 eV (0.30 eV). Also, charge transfer in the FLP examples, 9–11, is almost negligible. Clearly, for the last four complexes, steric factors play a relevant role in determining the interaction.
Even if the concept of steric effects is commonly invoked to explain phenomena occurring at the molecular level, finding a unique definition of steric descriptors has been referred to as one of the most elusive problems in chemistry [49, 50]. One reason for this is the fact that steric effects are not linked to any direct physical observable, and they are, therefore, subject to interpretation. In the case of a Lewis pair, the steric effects come from two main sources: (1) on one hand, it is evident that steric effects increase with the volume of the substituents attached to the acid and base center and (2) on the other hand (although this is not completely evident), the steric effects depend on the size of the reactivity center. Therefore, centers belonging to the second row of the periodic table (Al or P) present lower steric effects than first period centers for a given R substituent group. It must be indicated that the second assumption is related to the simple observation that the energy necessary to deform a Lewis acid or a Lewis base is lower for second period atoms. Based on these assumptions, an ad hoc steric index can be introduced by dividing the “volume” of the substituent by the “radii” of the Lewis acid or Lewis base center. It must be noted that the previous principle is independent of the precise definition of “volume” or “radii.”
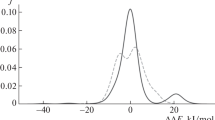
Changes in Weizsacker kinetic energy, T W [ρ] (Eq. 1), are related to the changes in the volume of the system; therefore, a simpler estimation of the steric contribution of the substituents in the Lewis acid and the Lewis base can easily be associated with the change in the Weizsacker energy when they are compared with a reference substituent (i.e., R = R′ = H). In view of this, the steric volume for a substituent R in the Lewis acid is defined as \(T_{\text{W}} \left( {{\text{AR}}_{3} } \right) - T_{\text{W}} \left( {{\text{AH}}_{3} } \right)\), where A = B or Al. In a similar fashion, the steric volume for a substituent in the Lewis base is defined as \(T_{\text{W}} \left( {{\text{BR}}_{3} } \right) - T_{\text{W}} \left( {{\text{BH}}_{3} } \right)\), where B = N or P. In order to consider the effect of the different centers, the contributions of the Lewis acid and the Lewis base are divided by the Brag–Slater radii of the reactive center (in atomic units) [50]. On this manner, the steric indexes \({\text{SI}}^{\text{ACID}} \left( {{\text{AR}}_{3} } \right) = \frac{{T_{\text{W}} \left( {{\text{AR}}_{3} } \right) - T_{\text{W}} \left( {{\text{AH}}_{3} } \right)}}{{{\text{R}}\left( {\text{A}} \right)}}\) and \({\text{SI}}^{\text{BASE}} \left( {{\text{BR}}_{3}^{\prime } } \right) = \frac{{T_{\text{W}} \left( {{\text{BR}}_{3}^{\prime } } \right) - T_{\text{W}} \left( {{\text{BH}}_{3} } \right)}}{{{\text{R}}\left( {\text{B}} \right)}}\) are introduced for Lewis acids and Lewis bases, respectively. For a Lewis pair, the steric index is defined as the mean of the steric indexes of the individual Lewis acid and Lewis base, \(\Delta {\text{SI}} = \frac{{{\text{SI}}^{\text{ACID}} \left( {{\text{AR}}_{3} } \right) + {\text{SI}}^{\text{BASE}} \left( {{\text{BR}}_{3}^{{\prime }} } \right)}}{2}\). This ad hoc steric effect definition is more illustrative for the present case than the changes in the Weizsacker energy associated with the dimer formation that tends to be negative due to a diminishing effect in the molecular volume of the complex when compared to the free Lewis acid and Lewis base [36–39]. The values of SIACID, SIBASE, and ΔSI are reported in Table 3 (sixth to eight column) in Hartree/Bohr. Figure 1 shows a plot of the electronic index, defined as η −1 in units of eV−1, versus the steric index ΔSI in units of Hartree/Bohr for the twelve complexes studied in the work using the ωB97x-D functional (a similar plot is obtained using the B97D functional). Three zones can be clearly identified in this plot: (1) the lower-left corner characterized by the presence of all the eight classical adducts for which the electronic index is lower than 1 eV−1 and the steric index never reaches 500 Hartree/Bohr, (2) the top-right corner characterized by the presence of FLPs for which the electronic factors exceed 1 eV−1 and the steric index is above 1000 Hartree/Bohr, and (3) a zone between i and ii, where the system, identified as “intermediate” (12), resides having an electronic factor between 0.5 and 1.5 eV−1 and a steric index between 600 and 1000 Hartree/Bohr. Before concluding, some comments on the limitations of our approach must be conveyed. The numerical values of the electronic index described above (η −1) can change slightly when different methodologies and basis sets are adopted; however, previous works [51] show that the relative trends might be maintained regardless the computational method employed. In contrast, the steric index introduced is approximately independent of the level of calculation because it is based in the electron density and a standard definition of atomic radii. Therefore, it is expected that similar conclusions could be obtained when adopting other levels of theory.
4 Conclusions
We thoroughly investigate the interactions between twelve Lewis pairs that span from classical adducts to FLPs, with the purpose of gaining deeper insights into the factors associated with their thermodynamic stability. On the basis of their binding energies, all complexes are stable at different degrees; however, due to thermal corrections to the enthalpy, entropic factors, and solvation effects, a positive ΔG solv value was computed for three complexes, which were identified as FLPs. When compared to classical complexes, the three identified FLPs show the following unique characteristics: (1) The bond distance between the acid and base center is larger than 4.0 Å, a distance in which negligible interactions between the centers is expected, (2) the geometry of the Lewis acid is almost undeformed, (3) the interaction energy is negative but larger than −15 kcal/mol (weak interaction), and (4) the contribution from non-covalent dispersion term represents the largest contribution to the total interaction energy.
In order to rationalized these results, we introduce a classical electronic index based on the difference in energy between the LUMO of the acids and the HOMO of the base, and an ad hoc steric index that takes into account two considerations: (1) the larger the volume of the substituents attached to the acid or the base center, the more important the steric effect and (2) the larger the center radii, the less significant the steric effect. In our definition, we take as a reference for the steric index the Lewis acids and Lewis bases with H as substituents where the volume of the substituents was estimated using the Weizsacker kinetic energy functional. On this way, we could discriminate classical adducts and FLPs using a plot of the electronic versus the steric indexes. Moreover, a third kind of intermolecular complexes was identified. We described this system as an “intermediate” complex, whose free energy in solution is slightly negative (i.e., characteristic of a classical complexes), but its non-covalent dispersion energy contributes to most of the total interaction energy (i.e., characteristic of a FLP).
In the present work, our ad hoc indexes were employed for discriminating FLPs from classical adducts. However, it must be pointed out that these indexes can also be employed for the classification of the different reactivity character (in terms of reversible uptake of H2, irreversible uptake of H2, or no reactivity with H2) of the members of a particular FLP family. This particular idea is a matter of future investigations in this field.
As a final remark, it can be indicated that this work employs a restricted sampling of twelve combinations of acids and bases, in which only three of them can be considered as FLPs; therefore, the generalizations presented could be considered at a first instance quite speculative. Certainly, a more extended scrutiny of Lewis acid–base systems is mandatory to confirm the potential of the present approach to classify intermolecular complexes between Lewis pairs. However, we anticipate few changes in the overall distribution of the plot shown in Fig. 2 when considering a more extended group of cases since the studied complexes span a broad spectrum in terms of centers and substituents.
Plots of the electronic index defined as the inverse of the difference between the LUMO of the acid and the HOMO of the base versus the steric index defined as the volume of the substituents relative to H divided by the radii of the reactivity center (see the text for details and units) at the ωB97x-D/6-311++G(2d,2p) level
References
Lewis GN (1923) Valence and the structure of atoms and molecules. Chemical Catalogue Company, New York
Brown HC, Schlesinger HI, Cardon SZ (1942) Studies in stereochemistry. I. Steric strains as a factor in the relative stability of some coördination compounds of boron. J Am Chem Soc 64:325–329
Brown HC, Kanner B (1966) Preparation and reactions of 2,6-Di-t-butylpyridine and related hindered bases. A case of steric hindrance toward the proton. J Am Chem Soc 88:986–992
Wittig G, Benz E (1959) Über das Verhalten von Dehydrobenzol gegenüber nucleophilen und elektrophilen Reagenzien. Chem Ber 92:1999–2013
Tochtermann W (1966) Structures and reactions of organic ate-complexes. Angew Chem Int Ed Engl 5:351–371
Welch GC, San Juan RR, Masuda JD, Stephan DW (2006) Reversible, metal-free hydrogen activation. Science 314:1124–1126
Welch GC, Stephan DW (2007) Facile heterolytic cleavage of dihydrogen by phosphines and boranes. J Am Chem Soc 129:1880–1881
Stephan DW, Erker G (2010) Frustrated Lewis pairs: metal-free hydrogen activation and more. Angew Chem Int Ed 49:46–76
Stephan DW (2015) Frustrated Lewis pairs: from concept to catalysis. Acc Chem Res 48:306–316
Stephan DW, Erker G (2014) Frustrated Lewis pair chemistry of carbon, nitrogen and sulfur oxides. Chem Sci 5:2625–2641
Stephan DW, Erker G (2015) Frustrated Lewis pair chemistry: development and perspectives. Angew Chem Int Ed 54:6400–6441
Rokob TA, Hamza A, Stirling A, Soos T, Papai I (2008) Turning frustration into bond activation: a theoretical mechanistic study on heterolytic hydrogen splitting by frustrated Lewis pairs. Angew Chem Int Ed 47:2435–2438
Rokob TA, Hamza A, Papai I (2009) Rationalizing the reactivity of frustrated Lewis pairs: thermodynamics of H2 activation and the role of acid–base properties. J Am Chem Soc 131:10701–11710
Hanza A, Stirling A, Rokob TA, Papai I (2009) Mechanism of hydrogen activation by frustrated Lewis pairs: a molecular orbital approach. Int J Quantum Chem 109:2416–2425
Momming CM, Fromel S, Kehr G, Frohlich R, Grimme S, Erker G (2009) Reactions of an intramolecular frustrated Lewis pair with unsaturated substrates: evidence for a concerted olefin addition reaction. J Am Chem Soc 131:12280–12289
Grimme S, Kruse H, Goerigk L, Erker G (2010) The mechanism of dihydrogen activation by frustrated Lewis pairs revisited. Ang Chem Int Ed 49:1402–1405
Schimmer B, Grimme S (2010) Electric field induced activation of H2-can DFT do the job? Chem Commun 46:7942–7944
Rokob TA, Bako I, Stirling A, Hamza A, Papai I (2013) Reactivity models of hydrogen activation by frustrated Lewis pairs: synergistic electron transfers or polarization by electric field? J Am Chem Soc 135:4425–4437
Zeonjuk LL, Vankova N, Mavrandonakis A, Heine T, Roschenthaler GV, Eicher J (2013) On the mechanism of hydrogen activation by frustrated Lewis pairs. Chem Eur J 19:17413–17424
Skara G, Pinter B, Top J, Geerlings P, De Proft F, De Vleeschouwer F (2015) Conceptual quantum chemical analysis of bonding and noncovalent interactions in the formation of frustrated Lewis pairs. Chem Eur J 21:1–11
Bannwarth C, Hansen A, Grimme S (2015) The association of two “frustrated” Lewis pairs by state-of-the-art quantum chemical methods. Isr J Chem 55:235–242
Ziegler T, Rauk A (1977) On the calculation of bonding energies by the Hartree Fock Slater method. I. The transition state method. Theor Chim Acta 46:1–10
Mitoraj MP, Michalak A, Ziegler T (2009) A combined charge and energy decomposition scheme for bond analysis. J Chem Theor Comp 5:962–975
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin, KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision C.01. Gaussian, Inc., Wallingford, CT
Chai J-D, Head-Gordon M (2008) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128:084106
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comp Chem 27:1787–1799
Raghavachari K, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. 20. Basis set for correlated wave-functions. J Chem Phys 72:650–654
McLean AD, Chandler GS (1980) Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z = 11–18. J Chem Phys 72:5639–5648
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods. 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80:3265–3269
Schelegel HB (1987) Optimization of equilibrium geometries and transition structures. Adv Chem Phys 67:249–286
Boys SF, Bernardi F (1970) Calculation of small molecular interactions by differences of separate total energies—some procedures with reduced errors. Mol Phys 19:553–566
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Scalmani G, Frisch MJ (2010) Continuous surface charge polarizable continuum models of solvation. I. General formalism. J Chem Phys 132:114110
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Biegler-Koning FW, Bader RFW, Tang TH (1982) Calculation of the average properties of atoms in molecules. II. J Comp Chem 3:317–328
Available from http://www.chemistry.mcmaster.ca/aimpac
Liu S (2007) Steric effects: a quantitative description from density functional theory. J Chem Phys 126:244103
Rong C, Lu T, Liu S (2014) Dissecting molecular descriptors into atomic contributions in density functional reactivity theory. J Chem Phys 140:024109
Fang D, Piquelman JP, Liu S, Cisneros GA (2014) DFT-steric-based energy decomposition analysis of intermolecular interactions. Theor Chem Acc 133:1484
Rincon L, Almeida R (2012) Is the Hammett’s constant free of steric effects? J Phys Chem A 116:7323–7530
Haaland A (1989) Covalent versus dative bonds to main group metals, a useful distinction. Angew Chem Int Ed Eng 28:992–1007
Gilbert TM (2004) Tests of the MP2 model and various DFT models in predicting the structures and B–N bond dissociation energies of amine-boranes (X3C)mH3−mB–N(CH3)nH3−n (X = H, F; m = 0–3; n = 0,3): Poor performance of the B3LYP approach for dative B–N bonds. J Phys Chem A 108:2550–2554
Wagner JP, Schreiner PR (2015) London dispersion in molecular chemistry-reconsidering steric effects. Angew Chem Int Ed Eng 54:12274–12296
Kocman M, Jurecka P, Dubecky M, Otyepka M, Cho Y, Kim KS (2015) Choosing a density functional for modeling adsorptive hydrogen storage: reference quantum mechanical calculations and a comparison of dispersion-corrected density functionals. Phys Chem Chem Phys 17:6423–6432
Changrakumar KRS, Pal S (2002) A systematic study on the reactivity of Lewis acid–base complexes through the local hard-soft acid–base principle. J Phys Chem A 106:11775–11781
Pinter B, Fievez T, Bickelhaupt FM, Geerlings P, De Proft F (2012) On the origin of the steric effect. Phys Chem Chem Phys 14:9846–9854
Slater JC (1965) Quantum theory of molecules and solids. McGraw-Hill, New York
Chermette H (1999) Chemical reactivity indexes in density functional theory. J Comp Chem 20:129–154
Acknowledgments
This work has been performed by employing the resources of the USFQ’s High Performance Computing system (HPC-USFQ). The authors would like to thank USFQ’s chancellor and collaboration grants program for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “CHITEL 2015 - Torino - Italy.”
Rights and permissions
About this article
Cite this article
Becerra, M., Real-Enriquez, M., Espinosa-Gavilanes, C. et al. On the thermodynamic stability of the intermolecular association between Lewis acids and Lewis bases: a DFT study. Theor Chem Acc 135, 77 (2016). https://doi.org/10.1007/s00214-016-1829-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1829-5