Abstract
Geometric structures, stabilities, and electronic properties of Si λ n+1 and Si n Smλ (n = 1–9, λ = 0, −1) clusters have been investigated systematically using density functional method at four levels. Extensive searches for ground state structures were carried out by the comparison between simulated spectra and the measured photoelectron spectroscopy. The results show that Sm atom tends to occupy the low-coordinated position and edge-cap or face-cap on the silicon frames. The lowest energy structures of Si n Sm0/− favor planar structures for n = 1–3 and three-dimensional structures for n = 4–9. Based on the averaged binding energies and fragmentation energies, we predict that Si4Sm and Si2Sm− clusters have the higher relative stabilities. Furthermore, the patterns of HOMOs and derivatives of ρ for the most stable doped isomers are investigated to gain insight into the nature of bonding. The result shows that π-type or σ-type bonds are always formed among the Si atoms, and the interaction between the Sm and Si atoms is very weak. To achieve a deep insight into localization of charge and reliable charge-transfer information, the Mulliken population are analyzed and discussed. In addition, the electrostatic potential, which is well established as a guide to the interpretation and prediction of molecular behavior, is performed for the lowest energy structures of Si n Smλ (n = 1–9, λ = 0, −1) clusters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Silicon is the most important material for the semiconductor industry, with continuous miniaturization of the semiconductor devices. The properties of silicon-based clusters have attracted a great number of attentions. Particularly, the metal–silicon clusters have been extensively studied by both experimental [1–7] and theoretical [8–20] work, because the addition of “impurity” atoms can significantly alter their properties. For example, the notion of altering the electronic properties of silicon by doping group III (Ga, Al) and group V (P, As) elements into it has been in use for over half a century and has led to the revolutionary rise of modern computers.
Doping with transition metal atoms with unfilled d-shells is a promising approach to create clusters with new magnetic properties. Namely, unpaired d-electrons of TM (transition metal) atoms are envisioned as retaining their magnetic moments once encapsulated within a silicon cage. They might be able to serve as transitional materials between current silicon-based semiconductor technology and the nascent field of spintronics. To achieve this goal, several combined experimental spectrometric and theoretical studies have been carried out on doped silicon clusters Si n M with M = V, Mn, Ti, Cr, Mo, W, and Cu [1, 21–27]. In addition, our group has investigated the structures, stabilities, and electronic properties of Cu2Si n [28] and Ag2Si n clusters [29]. However, a considerable amount of evidence shows that silicon’s sp-orbitals interact strongly with the d-orbitals of the endohedral transition metal atom, thereby quenching the latter’s magnetic moment [6, 13, 14]. Because a nonzero magnetic moment arises from unpaired electrons and the stability of cluster relies heavily on its ability to attain a closed shell, it becomes apparent why these two essential requirements for a magnetic cluster-assembled material are mutually exclusive when the same electrons are responsible for both bonding and magnetism.
To bypass this constraint, Khanna and Jena first proposed the endohedral doping of silicon clusters with lanthanide (Ln) atoms. It is well known that these elements have the more localized f-electrons. These electrons are to a large extent which is not responsible for bonding. Lanthanide atoms with multiple unpaired f-electrons may retain a significant portion of their atomic magnetic moments even in the presence of a strongly interacting environment such as a silicon cage. Therefore, LnSi n clusters can be easily used to study magnetic properties [30] and interest in their potential applications spurred considerable activity over the past couple of years. Ohara et al. [31, 32] have previously reported experimental photoelectron spectra and water reactivities of TbSi − n (6 ≤ n ≤ 16). Kumar et al. [33] theoretically studied encapsulated fullerene-like neutral and anionic clusters, M@Si20 (M = La, Ac, Sm, Gd, Tm, Ce, Pa, Pu, Th, Np, Pm), and observed that Pa@Si20, Sm@Si20, Pu@Si20, Tm@Si20, and Gd@Si20 − retain rather significant magnetic moments in their most stable geometries. Bowen’s group have published the results of their experimental photoelectron spectroscopic studies of EuSi − n (3 ≤ n ≤ 7) [30] and LnSi − n (Ln = Yb, Eu, Sm, Gd, Ho, Pr; 3 ≤ n ≤ 13) [34] anionic clusters, thereby expanding the range of studied Ln-silicon systems to encompass half of the lanthanide series. However, the literature existed on lanthanide-containing silicon clusters is still limited.
In this paper, we reported an extensive and systematical density functional theory (DFT) investigation on the small-sized neutral and anionic Si n Smλ (n = 1–9, λ = 0, −1) clusters. The pure silicon clusters were also studied by using identical methods and basis sets for comparison. The main objective of this research is to investigate the nature of interaction between silicon and samarium atoms. The various ground state minimum structures for Si n Smλ (n = 1–9, λ = 0, −1) are also obtained, which can provide significant help for such kind of cluster assemble materials.
2 Computational Methods
Geometrical structures’ optimizations and frequency analysis of Si n Smλ (n = 1–9, λ = 0, −1) clusters have been performed by the DFT method using the GAUSSIAN 03 program [35]. B3LYP density functional, which is Becke’s three-parameter functional [36] (B3) in conjunction with the correlation functional of Lee, Yang, and Parr (LYP) [37], was first employed for studying this system. Afterwards, all of the obtained isomers were re-optimized and their relative energies for the different isomers in a cluster were re-calculated using XC-M06 method. Furthermore, a selected set of the low-energy optimized structures was tested by B3PW91 [36, 38–40] and CCSD (t) [41–43] methods. This can guarantee the accuracy of our calculations. The basis sets labeled GENECP are the combination of 6-311 + G* [44] and MWB52 basis sets, which are employed for the Si and Sm atom, respectively. The ECP of MWB52 was developed by Dolg et al. [45, 46] for lanthanide metal atoms. A total of 46 + 4f 6 electrons are included in the lanthanide core, and the remaining 10 electrons are treated explicitly. Moreover, the adiabatic detachment energies (ADEs) were calculated using B3LYP, B3PW91, and CCSD (t) methods. The results comparing with experimental values [34] are presented in Table 1.
In this paper, the equilibrium geometries of pure silicon clusters were first studied based on the previous investigations. The pure silicon clusters in the range of 2–10 atoms have been well studied [47–51], and our obtained structures agree well with the previous results [47, 49]. To search for the lowest energy structure of Si n Smλ clusters, a large number of initial structures were obtained by placing the Sm atom on each possible site of the Si 0/− n host clusters as well as by substituting one Si atom using Sm in Si 0/− n+1 clusters. The previous studies [52, 53] on other lanthanide atoms doped silicon clusters are also employed as a guide. Due to the spin polarization, every initial structure is optimized at various possible spin multiplicities. In order to select most likely candidate structures, we have performed the simulated photoelectron spectroscopy (PES) spectra for obtained isomers and compared them with Bowen’s experimental spectra [34]. As is well known, well-resolved experimental PES spectra serve as electronic “fingerprints” of the underlying clusters.
3 Results and discussion
3.1 Geometrical structures
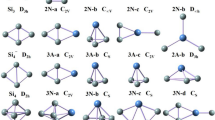
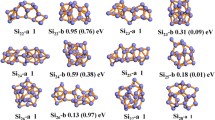
Based on the method that has been pointed out above, a large number of optimized isomers for Si n Smλ (n = 1–9, λ = 0, −1) clusters are obtained. We select the four most likely candidate isomers for each size and list them in Figs. 1, 2, and 3. According to their energies from low to high, the neutral isomers are designated by nN-a, nN-b, nN-c, and nN-d; the anions are designated by nA-a, nA-b, nA-c, and nA-d, where n represents the number of Si atoms in Si n Sm0/− clusters. In order to examine the effects of dopant Sm atom in silicon clusters, geometry optimizations of Si 0/− n (n = 2–10) clusters have been carried out using the identical method and basis set, and the lowest energy structures are also displayed for comparison. The corresponding relative energies, symmetries, electronic states, highest occupied molecular orbital (HOMO) energies, and LUMO energies for the selected pure and doped clusters are summarized in Table 2. Furthermore, the energetically low-lying isomers optimized at M06 XC level are shown in supplementary material (Fig. S1, S2, and S3) to verify our geometries.
3.1.1 Bare silicon clusters Si 0/− n (n = 2–10)
As shown in Figs. 1, 2, and 3, our ground state structures of Si n and Si − n (n = 2–10) clusters are in good agreement with the isomers reported in previous studies [47, 54, 55]. For the smallest clusters Si2 and Si2 −, the ground states are calculated to be 3Σg and 2Σg, respectively. Their bond lengths are calculated to be (2.253, 2.187), (2.281, 2.197), and (2.271, 2.190) at CCSD (t), B3LYP, and B3PW91 levels. These results agree well with the experimental studies. The Si2 has an experimental bond length 2.246 Å [54, 55], and the experimental bond length of Si2 − is 2.127 as given by Nimlos et al. [54]. The ground state structures of both neutral and anionic Si 0/− n clusters change from planar to three-dimensional (3D) structure at n = 5. The studied neutral and anionic silicon clusters tend to have the similar structures except for n = 6, and almost all of them have high symmetry.
3.1.2 Si n Smλ (n = 1–9, λ = 0, −1) clusters
The B3LYP density functional was first employed for studying this system. Henceforth, the values of bond length and relative energies are given at B3LYP level, unless mentioned otherwise. At B3LYP level, our obtained ground state of SiSm is shown to be 1Σ, with 3.387 Å bond length. Upon an extra electron attachment, the ground state of anion SiSm− will change to be 2Σ, and the bond length is shortened to 3.355 Å. Unfortunately, there are no experimental and theoretical results for the smallest SiSm0/− monomer available. Our theoretical results need to be further verified by experiments. All possible initial structures of Si2Sm0/− clusters, i.e., linear structures (D ∞h , C ∞v ), and triangle structures (acute angle or obtuse angle) are optimized with the different spin multiplicities. The triangle structures (2 N-a and 2A-a) with acute angle are found to be the most stable isomers for neutral and anionic clusters. Both of them can be obtained by replacing one Si atom by Sm in the corresponding ground state Si 0/−3 clusters. Furthermore, the lowest energy isomer of 3 N-a and 3A-a can also be derived from ground state Si 0/−4 clusters by this method.
Moving on to the next bigger cluster, we found that the lowest energy structure for Si4Sm0/− (Fig. 1) begin to show 3D appearance with C s symmetry. They can be seen as Si4 clusters capped by a Sm atom. When the number of silicon atoms is up to 5, no low-lying planar structure is found in our calculations. For Si6Sm, as shown in Fig. 2, it can be seen that the lowest energy has C s symmetry with 1A′ state, while the ground state structures of Si6Sm− has C 2v symmetry and 2A′ electronic state. Both of the lowest energy structures of neutral and anionic Si7Sm have C s symmetry, and they can be obtained by adding one Si atom on the Si 0/−7 clusters. For Si8Sm0/− clusters, when the top Si atom of the ground state structure for Si 0/−9 is substituted by a Sm atom, their ground state structures can be obtained. As shown in Fig. 3, we showed four low-energy isomers for Si9Sm0/−, respectively. All of them have the Sm atom face-capped on silicon clusters. According to the above discussions, we discuss the general features observed in these clusters. Starting at n = 4, the lowest energy structures of neutral and anionic doped clusters begin to show appearance of 3D geometries. The ground state structures of neutral and anionic Si n Sm0/− clusters have the similar structures except for n = 5, 6, and 9. The Sm atom tends to occupy the low-coordinated position and edge-cap or face-cap on the silicon clusters.
3.2 Comparison between the simulated PES spectra and the measured spectra
A PES experiment on Si n Sm− (n = 3–13) was carried out using a magnetic-bottle time-of-flight photoelectron spectrometer equipped with a laser vaporization cluster source by Bowen’s group [34]. As we all know, well-resolved PES spectra serve as electronic “fingerprints” of the underlying clusters and can be used for comparisons with theoretical simulations. In this case, we have performed the simulated spectra by adding the relative energies of the orbitals (ΔE n = E (HOMO-n) − E HOMO) to the VDE, and then, they are fitted with a unit-area Gaussian function of 0.2 eV full width at half maximum. The VDEs of each cluster anion correspond to the first peak maximum of each spectrum in Fig. 4. Due to the nonadiabatic interactions and anharmonic resonances are not included during our calculations, it is not possible to quantitatively compare calculated intensities with experimental ones, but the positions and the general shape of the peaks overall agree with experimental spectra. As can be seen in Fig. 4, the numbers of distinct peaks of simulated photoelectron spectra in the low-binding-energy range of ≤4.5 eV and their relative positions overall agree with the experimental spectra. Those increase the confidence in the reliability of the ground state structure isomers obtained.
Photoelectron spectra of Si n Sm− (n = 3–9) measured at 266 nm (The spectra are taken from Ref. [36]. Copyright 2009 American Chemical Society). Simulated photoelectron spectra for the lowest energy structures of Si n Sm− (n = 3–9) clusters at the B3LYP level
3.3 Relative stabilities
In order to investigate the relative stabilities of the ground state Si n Smλ (n = 1–9, λ = 0, −1) clusters, we have calculated the averaged binding energies E b (n) and fragmentation energies ΔE(n). Considering the influence of impurity atom on the small pure clusters, all of the above calculations are compared with the pure Si λ n+1 (n = 1–9, λ = 0, −1) clusters. For Si n Smλ (λ = 0, −1) clusters, E b (n) and ΔE(n) are defined as the following formula:
where E(Si), E(Siλ), E(Si n Smλ), and E(Si n−1Smλ) denote the total energies of Si, Smλ, Si n Smλ, and Si n−1Smλ clusters, respectively.
For Si λ n+1 (λ = 0, −1) clusters, E b (n) and ΔE(n) are defined as follows:
where E(Si), E(Siλ), E(Si λ n ), and E(Si λ n+1 ) denote the total energies of Si, Siλ, Si λ n , and Si λ n+1 clusters, respectively.
The E b (n) and ΔE(n) values of the lowest energy Si λ n+1 and Si n Smλ (n = 1–9, λ = 0, −1) clusters against the corresponding number of the Si atoms are plotted in Fig. 5. The features of size evolution are best viewed, and the peaks of curves correspond to those clusters with enhanced local stabilities. For pure Si n+1 and Si − n+1 clusters, the averaged binding energies increase gradually with the cluster size increasing at n ≤ 5; then, the curves become to slow down. The curve of Si − n+1 is higher than that of the corresponding sized Si n+1 clusters, reflecting that the stability is enhanced when the cluster attach an extra electron. The dissociation energies of Si2, Si2 −, SiSm, and SiSm− dimers are 3.065, 3.829, 0.641, and 0.395 eV, respectively. These reflect that the Si–Si interaction is much stronger than that of Si–Sm. For both E b (n) and ΔE(n) curves, the visible peak occurs at Si4 and Si10 −, hinting that they are more stable than their neighboring clusters.
For Si n Smλ (n = 1–9, λ = 0, −1) clusters, the averaged binding energies are lower than those of the corresponding sized pure silicon clusters, reflecting that the stability of Si 0/− n clusters cannot be enhanced when Sm atom is doped into them. The two curves increase gradually with the cluster size increasing for the entire size. Fragmentation energy involves the energy that a Si atom is separated from Si n Sm0/− clusters. Two conspicuous maxima are found at Si4Sm and Si2Sm−. Accordingly, this indicates that Si4Sm and Si2Sm− clusters are the most stable clusters in their respective species.
3.4 Orbital and bonding properties analysis
The highest occupied–lowest unoccupied molecular orbital (HOMO–LUMO) energy gap, which represents the ability of molecule to participate into chemical reaction in some degree, has been examined. In a sense, it can provide an important criterion to reflect the chemical stability of clusters. A large value of the HOMO–LUMO energy gap is related to an enhanced chemical stability. In contrast, a small one corresponds to a high chemical activity. For the most stable Si λ n+1 and Si n Smλ (n = 1–9, λ = 0, −1) clusters, HOMO and LUMO energies are listed in Table 1. In addition, the HOMO–LUMO energy gaps against the cluster size are also plotted in Fig. 6. As shown in Fig. 6, there are no apparent odd–even alternation behaviors in the four curves, but they show very irregular behaviors. The HOMO–LUMO energy gaps of pure Si n+1 clusters show the same tendency with our previous results [28]. For Si n Sm and Si n Sm− clusters, the remarkable peaks occur at Si9Sm, SiSm−, and Si4Sm− clusters, which imply that these clusters have the larger HOMO–LUMO gaps than others and possess dramatically enhanced chemical stability. For Si8Sm cluster, the gap is very small compared to the neighbor’s, indicating that the chemical activity of Si8Sm is stronger than that of its neighboring clusters.
In order to gain insight into the nature of the bonding, we have analyzed the HOMO for the most stable Si n Sm0/− clusters. The patterns of HOMOs are displayed in Fig. 7. These MOs provide insight into the observed special features and the nature of bonding in Sm-doped and pure silicon clusters. In pure Si2 dimer, we found that the π-type bond is formed between Si and Si atoms. However, when Sm substitutes one Si atom, the π-type bond disappears but with mixed Si p characters. Upon an electron attachment, the Sm s character emerges in SiSm− cluster. As for 2N-a structure, the two silicon atoms form σ2p -type bond. When an electron attach to 2N-a, the σ2p -type bond is changed to be π-type bond but with mixed Sm-s character. Across all the HOMOs of the most stable Si n Sm0/− clusters, the π-type or σ-type bonds are always formed among the Si atoms. In some clusters, the Sm atoms form σ bonding through an overlap between vacant in-plane Si p orbitals and valence Sm s orbitals. In the other clusters, the Sm atoms only show s character or have no orbital density localized. In addition, the interaction between the Sm and Si atoms is very weak. This may be related to the electronic configuration of Sm atom that is characterized by a closed shell and two 6s valence electrons (5s 2 p 66s 2), while the electronic configuration of Si atom is (1s 22s 2 p 63s 2 p 2).
To reveal the nature of chemical bonding for Si–Sm bond, we used the AIM (atoms in molecules) model to analyze the derivatives of electron density in the ground state Si n Sm0/− (n = 1–9) clusters. The electron density [ρ(r BCP)], Laplacian [∇2 ρ(r BCP)], bond ellipticity (ε), and curvature λ 3 at bond critical points (BCP), which contain a wealth of chemical information, are summarized in Table 3. The BCP are between Sm atom and the nearest Si atoms. The bond ellipticity is a quantity defined as ε = (λ 1/λ 2) − 1, where λ i are eigenvalues of the Hessian matrix of electron density at a BCP. The Laplacian of electron density, which is the trace of the Hessian matrix of ρ, can be used as criteria to classify the interaction between atoms. When the electron density ρ(r BCP) itself is large, and the Laplacian at the BCP ∇2 ρ(r BCP) is less than zero and large in absolute value, the electron density is concentrated in the internuclear region, and the bond will be referred to as a shared interaction or covalent bond. This type of bond is always strong. In contrast, a positive Laplacian at the BCP suggests a closed-shell system. At the BCP of the closed-shell interaction, the electron density is depleted. That is to say these interactions are relatively weak. As for Si–Sm bond BCP in Table 3, we can see that the ∇2 ρ(r BCP) is positive, and its absolute value is small. Furthermore, the electron density is also relatively small. In this case, the interactions between Si and Sm atoms are dominated by the contraction of electronic charge away from the interatomic surface toward the nuclei and relatively weak.
3.5 Electronic properties
The Mulliken population (MP) can provide the localization of charge and reliable charge-transfer information. Here, the MP for the lowest energy species of Si n Smλ (n = 1–9, λ = 0, −1) have been calculated and summarized in Table S1 and S2, respectively. As shown in Table S1, it can be clearly seen that the samarium atoms in neutral Si n Sm clusters possess positive charges ranging from 0.139e to 1.233e, while most of the Si atoms possess negative charges. This indicates that the electron transfer from Sm atom to the Si n frames, namely samarium atom, acts as electron donor in all the neutral clusters. This may be due to the electronegativity of Sm (1.17) that is much smaller than Si (1.98); therefore, the silicon atoms has a stronger ability to attract electrons. Even in the anionic Si n Sm− clusters, most of the Sm atoms are still with positive charges. Only in SiSm−, Si4Sm−, and Si7Sm− clusters, the Sm atoms possess −0.122e, −0.108e, and −0.106e charges, respectively.
The electrostatic potential (ESP) created by the nuclei and electrons of a molecule in the surrounding space is well established as a guide to the interpretation and prediction of molecular behavior. An important feature of the ESP is that it is a real physical property that can be determined experimentally by diffraction methods, as well as computationally. In this work, we used self-consistent-field (SCF) molecular orbital methods to compute ESP. The ESP \( V(\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {r} ) \) evaluated at point specified by the position vector \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {r} \) is given by:
where Z A is the nuclear charge of atom A, \( \overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {R}_{A} \) is the position of the nucleus of this atom, and \( \rho (\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {r^{\prime}}) \) is the electron density as a function of the position vector \(\overset{\lower0.5em\hbox{$\smash{\scriptscriptstyle\rightharpoonup}$}} {r^{\prime}}\). The equation being in atomic units with the electronic charge is taken as unity. The isosurface of ESP of the lowest energy Si n Smλ (n = 10–9, λ = 0, −1) species are displayed in Fig. S4. As we can see from Fig. S4, the transparent envelopes (contiguous envelope enclosing the molecular framework) represent the isosurface of ESP. The big and small balls represent Si and Sm atoms, respectively. As shown in indicator, red transparent envelopes represent negative potential, while the blue transparent envelopes represent positive potential. In all the neutral clusters, the color of the transparent envelopes changes gradually from red to blue. The transparent envelopes close to the Sm atom always show blue color appearance, indicating that the surrounding of Sm atom is positive potential. On the other hand, the transparent envelopes close to Si atoms always show the negative potential. These are in accord with the above charge analysis. The samarium atoms in the neutral Si n Sm clusters possess positive charges, while most of the Si atoms are with negative charges. In addition, the transparent envelopes close to the region between Si n frame and Sm atom show white ring pattern, which correspond to the weak Si–Sm interaction. As for the anionic clusters, the whole of transparent envelopes show red color appearance, indicating that the surrounding of entire cluster is negative potential. This may be due to the attachment of the extra electron.
4 Conclusions
The geometrical structures, stabilities, and electronic properties of small pure silicon clusters Si λ n+1 and Sm-doped silicon clusters Si n Smλ (n = 1–9, λ = 0, −1) have been investigated using DFT at four levels. All the results are summarized as follows.
-
1.
Extensive searches for the ground state structures were carried out with the aid of comparisons between the simulated spectra and experimental PES data. The results showed that small-sized neutral and anionic silicon clusters tend to have high symmetry. For Si n Sm0/− clusters, the lowest energy structures favor planar structures for n = 1–3 and 3D structures for n = 4–9. The Sm atom tends to occupy the low-coordinated position and edge-cap or face-cap on the silicon clusters.
-
2.
By calculating the averaged binding energies and fragmentation energies, the Si4Sm and Si2Sm− clusters are tested to have the higher relative stabilities. The Si9Sm, SiSm−, and Si4Sm− clusters have the larger HOMO–LUMO gaps indicating their enhanced chemical stability. In order to gain insight into the nature of the bonding, the patterns of HOMOs and the derivatives of ρ for the most stable doped isomers are investigated. The result show that π-type or σ-type bonds are always formed among the Si atoms, and the interaction between the Sm and Si atoms is very weak.
-
3.
The MP results indicate that the electron transfer from Sm atom to the Si n frames in all the neutral clusters, namely samarium atom, acts as electron donor. Correspondingly, the transparent envelopes close to the Sm atom always show blue color appearance, indicating that the ESP close to Sm atom is positive, while for the anionic clusters, the whole of transparent envelopes show red color appearance
References
Beck SM (1989) J Chem Phys 90:6306
Jaeger JB, Jaeger TD, Duncan MA (2006) J Phys Chem A 110:9310
Janssens E, Gruene P, Meijer G, Woste L, Lievens P, Fielicke A (2007) Phys Rev Lett 99:063401
Koyasu K, Atobe J, Akutsu M, Mitsui M, Nakajima A (2007) J Phys Chem A 111:42
Ohara M, Koyasu K, Nakajima A, Kaya K (2003) Chem Phys Lett 371:490
Zheng W, Nilles JM, Radisic D, Bowen KH (2005) J Chem Phys 122:071101
Hiura H, Miyazaki T, Kanayama T (2001) Phys Rev Lett 86:1733
Kumar V, Kawazoe Y (2001) Phys Rev Lett 87:045503
Miyazaki T, Hiura H, Kanayama T (2002) Phys Rev B 66:121403
Hagelberg F, Xiao C, Lester WA (2003) Phys Rev B 67:035426
Han JG, Shi YY (2001) Chem Phys 266:33
Jackson K, Nellermoe B (1996) Chem Phys Lett 254:249
Khanna SN, Rao BK, Jena P (2002) Phys Rev Lett 89:016803
Khanna SN, Rao BK, Jena P, Nayak SK (2003) Chem Phys Lett 373:433
Kumar V (2004) Comput Mater Sci 30:260
Kumar V (2006) Comput Mater Sci 35:375
Kumar V (2006) Comput Mater Sci 36:1
Kumar V, Majumder C, Kawazoe Y (2002) Chem Phys Lett 363:319
Reveles UJ, Khanna SN (2006) Phys Rev B 74:035435
Sen P, Mitas L (2003) Phys Rev B 68:155404
Ngan VT, Gruene P, Claes P, Janssens E, Fielicke A, Nguyen MT, Lievens P (2010) J Am Chem Soc 132:15589
Ngan VT, Janssens E, Claes P, Lyon JT, Fielicke A, Nguyen MT, Lievens P (2012) Chem Eur J 18:15788
Ngan VT, Pierloot K, Nguyen MT (2013) Phys Chem Chem Phys 15:5493
Claes P, Ngan VT, Haertelt M, Lyon JT, Fielicke A, Nguyen MT, Lievens P, Janssens E (2013) J Chem Phys 138:194301
Beck SM (1987) J Chem Phys 87:4233
Lau JT, Hirsch K, Klar P, Langenberg A, Lofink F, Richter R, Rittmann J, Vogel M, Zamudio-Bayer V, Möller T, Issendorff BV (2009) Phys Rev A 79:053201
Kong XY, Xu HG, Zheng W (2012) J Chem Phys 137:064307
Shao P, Kuang XY, Ding LP, Zhong MM, Wang ZH (2012) Phys B 407:4379
Zhao YR, Kuang XY, Wang SJ, Li YF, Lu P (2011) Z Naturforsch 66:353
Grubisic A, Wang HP, Ko YJ, Bowen KH (2008) J Chem Phys 129:054302
Ohara M, Miyajima K, Pramann A, Nakajima A, Kaya K (2002) J Phys Chem A 106:3702
Ohara M, Miyajima K, Pramann A, Nakajima A, Kaya K (2007) J Phys Chem A 111:10884
Kumar V, Singh AK, Kawazoe Y (2006) Phys Rev B 74:125411
Grubisic A, Ko YJ, Wang H, Bowen KH (2009) J Am Chem Soc 131:10783
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision E.01. Gaussian, Wallingford
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Perdew JP (1991) In: Ziesche P, Eschrig H (eds) Electronic structure of solids. Akademie Verlag, Berlin, pp 11–20
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671
Perdew JP, Wang Y (1992) Phys Rev B 45:13244
Purvis GD, Bartlett RJ (1982) J Chem Phys 76:1910
Scuseria GE, Janssen CL, Schaefer HF III (1988) J Chem Phys 89:7382
Scuseria GE, Schaefer HF III (1989) J Chem Phys 90:3700
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650
Dolg M, Stoll H, Savin A, Preuss H (1989) Theor Chim Acta 75:173
Dolg M, Stoll H, Preuss H (1989) J Chem Phys 90:1730
Yang JC, Xu WG, Xiao WS (2005) J Mol Struct Theochem 719:89
Pouchan C, Bégué D, Zhang DY (2004) J Chem Phys 121:4628
Jackson K, Pederson MR, Porezag D, Hajnal Z, Frauenheim T (1997) Phys Rev B 55:2549
Binggeli N, Chelikowsky JR (1997) Phys Rev Lett 75:493
Kostko O, Leone SR, Duncan MA, Ahmed M (2010) J Phys Chem A 114:3176
Zhao GF, Sun JM, Gu YZ, Wang YX (2009) J Chem Phys 131:114312
Liu TG, Zhao GF, Wang YX (2011) Phys Lett A 375:1120
Nimlos MR, Harding BL, Ellison GB (1987) J Chem Phys 87:5116
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure, constants of diatomic molecules, vol IV. Van Nostrand Reinhold, New York
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, CG., Pan, LJ., Shao, P. et al. Structures, stabilities, and electronic properties of the neutral and anionic Si n Smλ (n = 1–9, λ = 0, −1) clusters: comparison with pure silicon clusters. Theor Chem Acc 134, 34 (2015). https://doi.org/10.1007/s00214-015-1623-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1623-9