Abstract
Rationale
Cocaine can increase inflammatory neuroimmune markers, including chemokines and cytokines characteristic of innate inflammatory responding. Prior work indicates that the Toll-like receptor 4 (TLR4) initiates this response, and administration of TLR4 antagonists provides mixed evidence that TLR4 contributes to cocaine reward and reinforcement.
Objective
These studies utilize (+)-naltrexone, the TLR4 antagonist, and mu-opioid inactive enantiomer to examine the role of TLR4 on cocaine self-administration and cocaine seeking in rats.
Methods
(+)-Naltrexone was continuously administered via an osmotic mini-pump during the acquisition or maintenance of cocaine self-administration. The motivation to acquire cocaine was assessed using a progressive ratio schedule following either continuous and acute (+)-naltrexone administration. The effects of (+)-naltrexone on cocaine seeking were assessed using both a cue craving model and a drug-primed reinstatement model. The highly selective TLR4 antagonist, lipopolysaccharide from Rhodobacter sphaeroides (LPS-Rs), was administered into the nucleus accumbens to determine the effectiveness of TLR4 blockade on cocaine-primed reinstatement.
Results
(+)-Naltrexone administration did not alter the acquisition or maintenance of cocaine self-administration. Similarly, (+)-naltrexone was ineffective at altering the progressive ratio responding. Continuous administration of (+)-naltrexone during forced abstinence did not impact cued cocaine seeking. Acute systemic administration of (+)-naltrexone dose-dependently decreased cocaine-primed reinstatement of previously extinguished cocaine seeking, and administration of LPS-Rs into the nucleus accumbens shell also reduced cocaine-primed reinstatement of cocaine seeking.
Discussion
These results complement previous studies suggesting that the TLR4 plays a role in cocaine-primed reinstatement of cocaine seeking, but may have a more limited role in cocaine reinforcement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Occasional cocaine use can progress to uncontrolled abuse and dependence in some individuals, resulting in health, interpersonal, and occupational challenges that interfere with healthy daily functioning. In addition to neuronal mechanisms, neuro-immune signaling may contribute to cocaine use disorder (CUD) (Hill et al. 2010; Valdizán et al. 2012; Theberge et al. 2013; Korpi et al. 2017) (Hutchinson et al. 2008; Hutchinson and Watkins 2014; Lacagnina et al. 2017; Bachtell et al. 2017; Hofford et al. 2019). The evidence for altered immune function in CUD has been indicated in clinical populations of cocaine users. For instance, individuals diagnosed with CUD have increased peripheral levels of the proinflammatory cytokine, interleukin-6, and decreased levels of the anti-inflammatory cytokine, interleukin-10 (Moreira et al. 2016). Treatment-seeking cocaine-dependent individuals also exhibited increased acute levels of the pro-inflammatory cytokine, tumor necrosis factor-alpha, in response to stress-related or drug-associated cue imagery (Fox et al. 2012). CUD symptom severity was also associated with greater alterations in peripheral proinflammatory markers, indicating a correlative relationship between behavioral symptoms of CUD and immune activation (Araos et al. 2015).
The significance of these inflammatory changes and their impact on brain function are not fully understood, but immune-related signaling molecules are known to impact neurotrophic and neuromodulatory functions (Pedraz et al. 2015; Araos et al. 2015; Moreira et al. 2016; Levandowski et al. 2016; García-Marchena et al. 2019; Soder et al. 2020; Stamatovich et al. 2021). Anti-inflammatory drugs, such as minocycline and ibudilast, alter drug-related behaviors and relevant neural activity in pre-clinical addiction models, and clinical studies have found some promising, but limited, efficacy with these drugs (Liu et al. 2006; Chen et al. 2009; Beardsley et al. 2010; Fujita et al. 2012; Attarzadeh-Yazdi et al. 2014; Bell et al. 2015; Arezoomandan and Haghparast 2016; Worley et al. 2016; Cooper et al. 2016, 2017; Metz et al. 2017; Birath et al. 2017; Arezoomandan et al. 2018; Li et al. 2020).
The Toll-like receptor 4 (TLR4) system, an innate immune receptor complex expressed primarily by microglia in the brain, is involved in psychostimulant- and opioid-induced immune activation (Jacobsen et al. 2014; Bachtell et al. 2017; Wu and Li 2020). TLR4 signaling initiates an acute inflammatory cytokine response by recognizing molecular patterns associated with pathogens, such as lipopolysaccharide (LPS) on the cell walls of gram-negative bacteria (Kawai and Akira 2007). TLR4 signaling is also activated by molecular markers of cellular damage (e.g., high mobility group box 1 (HMGB1)) and some exogenous molecules exogenous or xenobiotics (Northcutt et al. 2015). Single injections of cocaine or multiple days of cocaine self-administration initiate transcriptional expression of inflammation-related genes in regions of the mesolimbic dopamine (DA) system that is well-characterized for its involvement in drug reinforcement and relapse (Northcutt et al. 2015; Brown et al. 2018; Burkovetskaya et al. 2020). Multiple lines of evidence suggest that TLR4 is involved in cocaine-induced inflammatory signaling in the brain (Northcutt et al. 2015; Wang et al. 2019). Importantly, pharmacological manipulation of TLR4, both systemically and within the ventral tegmental area (VTA), alters nucleus accumbens (NAc) DA release in response to psychostimulants and opioids (Northcutt et al. 2015; Tanda et al. 2016; Wang et al. 2019).
The involvement of TLR4 in cocaine reinforcement and seeking models has been explored with TLR4-specific agonists and antagonists. Notably, (+)-naloxone and (+)-naltrexone are stereo-specific, blood-brain barrier permeable (+)-enantiomers of opioid receptor antagonists that are antagonists for TLR2 and TLR4 (Hutchinson et al. 2008; Northcutt et al. 2015; Zhang et al. 2018; Wang et al. 2019). Unlike the (-)-enantiomers, the (+)-enantiomers are devoid of opioid receptor activity making them interesting compounds for exploring TLR2/4-induced effects. These drugs block TLR4 via competitive binding at the myeloid differentiation factor 2 (MD2) LPS pocket of the TLR4 complex (Zhang et al. 2018). Additionally, LPS from the bacterial species Rhodobacter sphaeroides (LPS-Rs) has been well-characterized as a TLR4 antagonist, although it is not blood-brain barrier permeable and must be administered intracranially when targeting specific brain regions. In accordance with the hypothesis that cocaine induces neuroimmune signaling through TLR4, TLR4 antagonists, such as (+)-naltrexone, (+)-naloxone, and LPS-Rs, block pro-inflammatory neuro-immune transcriptional expression to cocaine in isolated microglia preparations and in vivo within the brain parenchyma (Northcutt et al. 2015). In addition to blocking pro-inflammatory cytokine expression, (+)-naltrexone and (+)-naloxone reduce both operant responding for cocaine and expression of conditioned preference for psychostimulants in some experimental conditions (Northcutt et al. 2015; Tanda et al. 2016; Wang et al. 2019). In addition to (+)-naltrexone and (+)-naloxone, intracranial administration of LPS-Rs into the VTA reduced the reinstatement of operant responding for cocaine but not sucrose (Brown et al. 2018). Together, these studies point to a role of the TLR4 system in cocaine reinforcement and drug-seeking; however, some questions remain regarding the efficacy of these drugs in models of motivated drug-taking and drug-seeking (Tanda et al. 2016; Yue et al. 2019).
To further understand TLR4 behavioral pharmacology in the context of CUD, we assessed the impact of TLR4 antagonists in cocaine self-administration and cocaine-seeking models. We first evaluated the continuous administration of (+)-naltrexone during cocaine self-administration to evaluate the impact of TLR4 antagonism on (1) acquisition and maintenance of cocaine self-administration, (2) cocaine reinforcement using a progressive ratio (PR) schedule of reinforcement, and 3) cocaine seeking during forced abstinence and following extinction training.
Materials and methods
Animals
A total of 188 male Sprague-Dawley rats (Envigo, Indianapolis, IN), weighing between 330 and 350 g upon arrival, were singly housed in a temperature (72 °F) and humidity (40%) controlled animal vivarium on a standard 12-h light/ 12 h dark cycle. Rats were provided standard rat chow (Envigo, Indianapolis, IN) and water ad libitum throughout the entirety of the study unless otherwise noted. All experiments were performed during the light cycle. All procedures were conducted per institutional guidelines outlined by the Institutional Animal Care and Use Committee at the University of Colorado Boulder, an Association for Assessment and Accreditation of Laboratory Animal Care International accredited institution.
Drugs
Cocaine HCl (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile saline (0.9% NaCl) at a working concentration of 5 mg/mL and filtered daily through a 200-nm filter before self-administration. (+)-Naltrexone was synthesized and provided by Dr. Kenner Rice of the Drug Design and Synthesis Section, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. (+)-Naltrexone was synthesized as (+)-naltrexone.HCl.4H2O with added waters of hydration to improve solubility. All doses are reported as (+)-naltrexone free base. Selection of doses and administration methods is based on prior behavioral work assessing similar outcome variables and work evaluating the pharmacokinetics of (+)-naltrexone (Theberge et al. 2013; Northcutt et al. 2015; Brown et al. 2018). Northcutt et al. (2015) reported that administration of 10 mg/kg (+)-naltrexone subcutaneously was absorbed very rapidly (tmax = 0.25 h), and the half-life of (+)-naltrexone was 1.19 ± 0.063 h. Administration of 10-mg/kg (−)-naltrexone (SC) showed a similar pharmacokinetic profile with (+)-naltrexone suggesting that absorption and distribution characteristics are similar between the two enantiomers. Thus, subcutaneous mini-pump dosing was based on prior work using osmotic mini-pump delivery of either (−)-naltrexone or (+)-naltrexone (Hill et al. 2010; Valdizán et al. 2012; Theberge et al. 2013; Korpi et al. 2017). Surgically implanted subcutaneous osmotic mini-pumps (ALZET, Cupertino, CA) delivered 15 mg/kg/day (+)-naltrexone for up to 14 days as reported previously (Theberge et al. 2013). Acute administration of (+)-naltrexone was administered as two half-doses (up to 60 mg/kg in total) 30 min apart to improve pharmacokinetic absorption and distribution throughout the testing session. The lipophilic nature of (+)-naltrexone makes it ideal for distribution following a systemic administration, but not a localized brain infusion. Therefore, the classical TLR4 antagonist, Lipopolysaccharide from the species Rhodobacter sphaeroides (LPS-RS), was selected given its well-characterized specificity for the TLR4 complex and comparative lack of diffusion. LPS-Rs (Sigma-Aldrich, St. Louis, MO) was administered intracranially at a dose of 5 μg/side based on prior work (Northcutt et al. 2015; Brown et al. 2018; Wang et al. 2019). The non-steroidal anti-inflammatory drug Ketofen (3 mg/kg) and the antibiotic Baytril (3 mg/kg) were dissolved in sterile saline (0.9% vol./wt.) and administered post-operatively immediately following surgery.
Surgical catheter implantation and cocaine self-administration procedures
Silastic tubing catheters were intravenously implanted into the right jugular veins of rats to allow for intravenous (iv) self-administration of cocaine HCl (0.5 mg/kg/inf., Sigma-Aldrich, St. Louis, MO). Following 5–7 days of recovery from surgery, rats began self-administration procedures in sound-attenuating operant chambers (Med-Associates, Georgia, VT) equipped with two retractable levers, a fan, a house light, and discrete cue lights located directly above the retractable levers. Commencement of self-administration sessions was occasioned by the illumination of the house light and the presentation of two levers located on one side of the chamber. Intravenous infusions of 100-μL cocaine HCl (0.5 mg/kg/infusion) were contingent upon lever presses on the drug-paired lever. Presses on the inactive lever had no programmed consequences. Infusions of cocaine occurred simultaneously with 5-s illumination of a discrete cue light located directly above the active, drug-paired lever. A 20-s time-out period followed cocaine infusion and was occasioned by extinguishing of the house light. Cocaine self-administration procedures were performed using a fixed-ratio 1 (FR1), fixed-ratio 5 (FR5), and progressive ratio (PR) schedule of reinforcement. During extinction procedures, drug-paired lever presses no longer resulted in iv cocaine infusions, drug-paired cue light illumination, or extinguishing of the house light.
Intracranial pharmacological microinjections
Rats were stereotaxically implanted with bilateral 26 ga. guide cannulae (PlasticsOne, Roanoke, VA) extending 8 mm below the plastic pedestal. Guide cannulae were stereotaxically inserted into the NAc Shell (A/P: +1.8, M/L: +0.8, D/V: −5.6) or NAc Core (A/P: +1.7, M/L: +1.5, D/V: −5.7) using coordinates adapted from Paxinos and Watson (2013). Placements were anatomically verified post-mortem via visual inspection of 1-μl cresyl violet microinjections via the surgically implanted guide cannula. Stereotaxic surgeries were performed during the same surgery as the catheter implantation. Dental cement fixed to 4 skull screws was used to secure the guide cannula to the skull. Microinjections of LPS-Rs (5 μg/side, 1-μl vol.) were delivered via a bilateral microinjector that was inserted into the guide cannulae and extended 1 mm beyond the tip of the cannulae. Microinjections of LPS-Rs were delivered at a rate of 200 nL/min. Following completion of the injection, the microinjector remained in the cannula for an additional 2 min to allow for diffusion of the drug. During control microinjections, rodents received 1-μL injections of sterile H2O at a rate of 200 nL/min.
Experiment 1: Effects of TLR4 antagonism on the acquisition of cocaine self-administration and subsequent cocaine seeking
To test the effects of TLR4 antagonism on the acquisition of cocaine self-administration, rats were implanted with subcutaneous osmotic mini-pumps (ALZET, model 2ML2, Cupertino, CA) to allow continuous delivery of 15 mg/kg/day (+)-naltrexone or saline for 14 days during cocaine self-administration. One day after mini-pump implantation, sated rats were allowed to lever press for iv cocaine infusions on an FR1 reinforcement schedule in 2-h sessions. Mini-pumps were removed before the 14th self-administration session and rats resumed cocaine self-administration for a total of 17 self-administration sessions. To evaluate the latent effects of continuous (+)-naltrexone during cocaine self-administration on subsequent cocaine seeking, cocaine-paired lever responding was extinguished in seven daily 2-h extinction sessions. Reinstatement of cocaine seeking was conducted in three separate test sessions using a within-subjects design. The first reinstatement test evaluated cue-induced cocaine seeking in a 3-h session. The session included an initial 2-h period under extinction conditions that was immediately followed by a 1-h reinstatement test period. During the reinstatement test period, 5 non-contingent presentations of the cocaine-associated cue (5-s) were delivered every 2-min over the first 10-min of the session, and responses at the previously cocaine-paired lever also resulted in a 5-s illumination of the cocaine-associated cue. The second and third reinstatement tests were counterbalanced to evaluate cocaine (or saline)-primed reinstatement of cocaine seeking. The drug-primed reinstatement session occurred under extinction conditions. An injection of cocaine (15 mg/kg, ip) or sterile saline (0.9% vol./wt., ip) was administered immediately before the 2-h reinstatement session.
Experiment 2: Effects of TLR4 antagonism on cocaine’s reinforcing properties
The effects of TLR4 antagonism were evaluated on the motivation to self-administer cocaine using PR-maintained cocaine self-administration. Rats acquired cocaine self-administration on an FR1 schedule for five daily 2-h sessions similar to Exp. 1 before being transitioned to an FR5 schedule of reinforcement for an additional five 2-h self-administration sessions. Immediately following one self-administration session using a PR schedule of reinforcement, rats were implanted with subcutaneous osmotic mini-pumps (ALZET, model 2ML1, Cupertino, CA) to allow continuous delivery of 15 mg/kg/day (+)-naltrexone or saline for 7 days. After mini-pump implantation, rats resumed testing using a PR schedule of reinforcement for 4 additional sessions. The progression for response/injection ratios was determined according to [5e(injection number × 0.2)]−5 (e.g., 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50). In a separate cohort of rats, PR responding was evaluated following an acute pretreatment of (+)-naltrexone (0, 30, 60 mg/kg, ip) administered before the PR test session. The (+)-naltrexone pretreatment was administered as two half-dose injections (0, 15, 30 mg/kg, ip, respectively) administered 30 min apart to improve pharmacokinetic absorption and distribution. Rats were tested following vehicle and either 30 mg/kg or 60 mg/kg (+)-naltrexone with the order of the pretreatments counterbalanced to avoid ordering effects.
Experiment 3: Acute (+)-naltrexone before sucrose self-administration did not alter responding for sucrose
Prior work demonstrates that high doses of (+)-naltrexone inhibit the self-administration of food pellets (Tanda et al. 2016). Here, we assess whether (+)-naltrexone administered as 2 half-dose injections impacts the self-administration of a sucrose reward. Rats (n = 6) were placed on a limited diet with access to 15 g of standard rat chow per day and were trained to self-administer banana-flavored sucrose pellets (45 mg/pellet) on an FR1 schedule of reinforcement for 4 daily 2-h sessions. Following FR1 training, rats were advanced to an FR5 schedule of reinforcement for 4 daily additional 2-h sessions. Rats then underwent testing in four 2-h sessions where they received 2 injections of (+)-naltrexone (0, 7.5, 15, 30 mg/kg, ip) at 30-min intervals for a total of 0, 15, 30, or 60 mg/kg (+)-naltrexone. Immediately following the 2nd injection, rats were placed in operant chambers to self-administer sucrose pellets on an FR5 schedule during 2-h sessions. The order of the pretreatments counterbalanced to avoid ordering effects.
Experiment 4: Effect of TLR4 antagonism on cocaine seeking during abstinence
The effect of TLR4 antagonism was evaluated on the incubation of cue-seeking following cocaine self-administration. Rats acquired cocaine self-administration on an FR1 schedule for ten daily 2-h sessions similar to Exp. 1. During the 14-d forced abstinence period, (+)-naltrexone was chronically delivered via subcutaneous mini-pumps (ALZET, model 2ML2, Cupertino, CA) to allow a continuous dose of 15 mg/kg/day (+)-naltrexone or vehicle. Rats experienced a 30-min cue-seeking test session on withdrawal day (WD) 1 to assess initial cue-seeking and match group assignments (vehicle vs. (+)-naltrexone). Immediately after the test session, rats were implanted with the subcutaneous mini-pump. Rats were tested for the incubation of cue seeking in a 3-h session conducted on WD 14. During the cue-seeking tests, responding on the drug-paired lever resulted in the cue light illumination, but no cocaine delivery.
Experiment 5: Effect of TLR4 antagonism on cocaine-primed reinstatement of cocaine seeking following extinction training
The effect of TLR antagonism was tested on cocaine-primed reinstatement of extinguished cocaine seeking. Rats acquired cocaine self-administration on an FR1 schedule for ten 2-h daily sessions similar to Exp. 1 followed by seven daily 2-h extinction sessions. A pretreatment of (+)-naltrexone (0, 15, 30 mg/kg, ip) was administered as two half-dose injections (0, 7.5, 15 mg/kg, ip, respectively) administered 30 min apart before the 2-h drug-primed reinstatement of cocaine seeking session.
Experiment 6: Effect of nucleus accumbens TLR4 antagonism on cocaine-primed reinstatement of cocaine seeking following extinction training
The effect of TLR antagonism within the core and shell subregions of the nucleus accumbens was tested on cocaine-primed reinstatement of extinguished cocaine seeking. Following cocaine self-administration and extinction as in prior experiments, drug-primed reinstatement of cocaine seeking session was evaluated following a pretreatment of LPS-Rs (5 μg/side) administered into the NAc Shell or NAc Core. All rats were tested under control conditions (Vehicle/Saline).
Statistics
Statistical analyses were performed using the GraphPad Prism (v9.4 for Mac, San Diego, CA). A 2-factor mixed-design analysis of variance (ANOVA) with sessions and treatment as the factors was used to evaluate the impact of (+)-naltrexone administration on cocaine self-administration studies using the dependent measures of cocaine infusions and lever responding. Responding during extinction training was analyzed in a 3-factor mixed-design ANOVAs with sessions and lever as within-subjects factors and treatment as a between-subject factor. Drug-paired and inactive lever responding during the seeking tests were analyzed separately in ANOVAs with the session and treatment as the factors. Self-administration and extinction data include more than 3 repeated measures and may lack sphericity across the repeated sampling. The Geisser-Greenhouse correction was used to adjust the degrees of freedom in determining the critical F-value. Significant main and interactive effects were followed by simple main effects tests and post hoc tests (Tukey’s multiple comparison test). Statistical significance was determined as p < 0.05.
Results
Experiment 1: Effects of TLR4 antagonism on the acquisition of cocaine self-administration and subsequent extinction and cocaine seeking
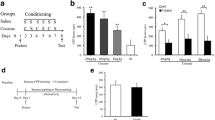
This experiment assessed the direct effects of subcutaneous delivery of (+)-naltrexone on the acquisition and maintenance of cocaine self-administration and the latent effects of continuous (+)-naltrexone delivery during cocaine self-administration on cocaine seeking during extinction training and cue-induced and drug-primed reinstatement testing (Fig. 1a). No differences were observed in the acquisition of cocaine self-administration between the (+)-naltrexone (n = 10) and vehicle-treated (n = 7) groups (Fig. 1b, treatment effect: F1,15 <1, NS, session: F6.12,91.73 = 5.60, p <0.001, treatment × session interaction: F16,240 <1, NS). No effect of previous treatment with (+)-naltrexone on extinction responding was found, although rats in both groups pressed the active lever more frequently than the inactive lever during the initial extinction sessions (Fig. 1c, treatment: F 1,15 = 1.08, p = .32, lever: F.25, 3.7 = 36.35, p < .01, treatment × session × lever interaction: F6,90 = 2.15, p = .06). Rats in both groups displayed cue-induced cocaine seeking as indicated by greater active lever responding during the reinstatement compared with the extinction sessions (session: F1,15 = 15.41, p < .001, treatment × session: F1,15 < 1, NS). No differences were found between the rats treated with (+)-naltrexone and vehicle during self-administration on cue-induced reinstatement test (Fig. 1d, treatment: F1,15 < 1, NS). Rats in both groups also showed robust drug-primed reinstatement of cocaine seeking with greater active lever responding during reinstatement compared with extinction (session: F1,15 = 17.77, p < .001, treatment × session: F1,15 < 1, p = .35), but no group differences were found during the drug-primed reinstatement session (Fig. 1e, treatment: F1,15 < 1, NS).
Effects of continuous TLR4 antagonism via osmotic minipump on the acquisition of cocaine self-administration and subsequent extinction and cocaine seeking. a Timeline illustrating the experimental design. b Subcutaneous mini-pump delivery of (+)-Naltrexone for ~14 days (shaded region) did not alter the acquisition or maintenance of cocaine self-administration as indicated by the mean ± SEM number of cocaine infusions. c (+)-Naltrexone administration during the self-administration phase had no impact on subsequent extinction of previously drug-paired or inactive lever responding (mean ± SEM). Prior (+)-naltrexone administration during the self-administration phase had no impact on subsequent cocaine-seeking induced by cocaine-associated cues (d) or a cocaine priming injection (e). Data are the mean ± SEM number of drug-paired lever responses. Vehicle n = 7; (+)-naltrexone n = 10
Experiment 2: Effects of TLR4 antagonism on cocaine’s reinforcing properties
This experiment assessed PR operant responding for cocaine in rats treated with continuous (+)-naltrexone (15 mg/kg/day) via osmotic mini-pumps (Fig. 2a). After acquiring the FR1 response, rats were advanced to an FR5 schedule of reinforcement (Fig. 2b). One day after minipump implantation, rats self-administered cocaine on a PR schedule for 4 consecutive days. (+)-Naltrexone delivered through an osmotic mini-pump had no effect on cocaine infusions during PR responding (Fig. 2c, treatment: F1,13 <1, NS, session: F2.21, 28.72 = 2.92, p = .07), treatment × session: F4,52 <1, NS) or cumulative lever responses (Fig. 2d, treatment: F1,13 <1, NS, session: F1.69, 21.96 = 1.902, p = .18), treatment × session: F4,52 <1, NS). These findings indicate that continuous (+)-naltrexone does not alter the motivation to acquire cocaine in a PR testing procedure.
Effects of continuous TLR4 antagonism on the motivation to acquire cocaine using a progressive ratio schedule of reinforcement. a Timeline illustrating the experimental design. b Cocaine intake (mean ± SEM number of cocaine infusions) on an FR5 schedule was equivalent before mini-pump implantation for continuous delivery of (+)-naltrexone during progressive ratio testing. Continuous delivery of (+)-naltrexone (n = 7; shaded region) did not alter the number of cocaine infusions (c) or the number of cumulative active lever responses (d) during progressive ratio testing compared with the vehicle group (n = 8)
The effects of acute injections of (+)-naltrexone administered immediately before the PR session were also used to evaluate 2 additional (+)-naltrexone doses in a separate cohort of rats (Fig. 3a). After acquiring the FR1 response and being advanced to an FR5 schedule of reinforcement (Fig. 3b), rats then self-administered cocaine on a PR schedule. During the PR sessions, rats received counterbalanced acute injections of either vehicle or (+)-naltrexone (30 mg/kg or 60 mg/kg, ip). No effect of treatment dose was observed on PR infusions (Fig. 3c, treatment: F2,23 = 2.80, p = .081) or cumulative lever presses (Fig. 3d, treatment: F2,23 = 1.77, p = .19) suggesting that (+)-naltrexone did not alter the motivation to acquire cocaine using a PR testing procedure.
Effects of acute TLR4 antagonism on the motivation to acquire cocaine using a progressive ratio schedule of reinforcement. a Timeline illustrating the experimental design. b Cocaine intake (mean ± SEM number of cocaine infusions) on an FR5 schedule was stable before evaluating the effects of vehicle (n = 14), 30 mg/kg (+)-naltrexone (n = 8), and 60 mg/kg (+)-naltrexone (n = 6) on progressive ratio performance using a modified within-subject design. Acute administration of (+)-naltrexone failed to alter the number of cocaine infusions (c) or the cumulative active lever responses (d) during progressive ratio testing compared with the vehicle group
Experiment 3: Acute (+)-naltrexone before sucrose self-administration did not alter responding for sucrose
Prior work has demonstrated that (+)-naltrexone reduces operant responding for a food reward (Tanda et al. 2016). Here, we tested the effects of (+)-naltrexone on operant responding for sucrose using an administration procedure designed to maximize the bioavailability of (+)-naltrexone (Fig. 4a). Food-restricted rats were trained to self-administer sucrose pellets on an FR1 schedule of reinforcement for 4 daily 2-h sessions followed by an additional 4 sessions on an FR5 schedule of reinforcement. Rats (n = 6) then underwent testing over 4 days during which they received pretreatment of (+)-naltrexone (0, 15, 30, or 60 mg/kg, ip). Surprisingly, rats continued to respond robustly at the active lever for sucrose pellets regardless of the (+)-naltrexone pretreatment (Fig. 4, treatment: F3, 30 <1, NS, lever (F1,10 = 78.10, p <.0001), treatment × lever (F3,30 <1; NS). These findings indicate that (+)-naltrexone does not have a major impact on motivation to respond for natural rewards.
Effects of TLR4 antagonism on the self-administration of sucrose pellets. a Timeline illustrating the experimental design. b Sucrose responding during the FR5 sessions was stable and robust before evaluating the effects of vehicle or naltrexone (15, 30, and 60 mg/kg, ip) using a within-subject design (n = 6) on FR5 responding for sucrose pellets. c Acute administration of (+)-naltrexone failed to alter the number of lever responses (left) or the total sucrose consumed (right) compared with the vehicle treatment. Data are presented as the mean ± SEM number of active and inactive lever responses
Experiment 4: Effect of TLR4 antagonism on cocaine seeking during abstinence
This experiment assessed the effects of chronic subcutaneous osmotic mini-pump delivery of (+)-naltrexone during abstinence on cue-reinforced cocaine seeking (Fig. 5a). Rats self-administered cocaine during 15 consecutive 2-h daily sessions (Fig. 5b) and were tested during a 30-m cue-reinforced drug-seeking session on WD1. Rats were then implanted with subcutaneous osmotic mini-pumps delivering (+)-naltrexone (15 mg/kg/day) or vehicle and remained in the home cage. On WD14, rats were tested on a 3-h cue-reinforced drug-seeking session. A main effect of abstinence day was found indicating that cue-reinforced lever pressing during the first 30 min increased from WD1 to WD14 (session: F1,13 = 14.00, p < .01). However, no effect of (+)-naltrexone treatment was observed (Fig. 5c, treatment: F1,13 <1 , NS), treatment × session: F1,13 <1, NS). No effect of treatment was found on total lever presses during 1-h bins (Fig. 5d, treatment: F1,13 = 1.43, p = .25, time: F1.87, 24.28 = 24.13, p <.0001, treatment × Time: F2,26 <1, NS) or over the entirety of the 3-h session (Fig. 5e, treatment: t13 = 1.20, p = .25). These findings suggest the (+)-naltrexone administered during abstinence is not sufficient to reduce subsequent cocaine seeking similar to findings observed with another psychostimulant drug, methamphetamine (Theberge et al. 2013).
Effects of TLR4 antagonism during the abstinence phase on incubated cue-induced cocaine seeking. a Timeline illustrating the experimental design. b Cocaine intake (mean ± SEM number of cocaine infusions) was stable before the implantation of the osmotic mini-pump. c Cue seeking during the 30-m test on day 1 and the first 30-m of the 3-h test on day 14 indicate that cocaine-seeking (mean ± SEM number of drug-paired lever responses) increased to a similar magnitude in both the vehicle and (+)-naltrexone treated rats (*p < 0.05 compared with day 1). d Evaluation of hourly cocaine cue seeking (mean ± SEM number of drug-paired lever responses) on abstinence day 14 also indicated a similar distribution of seeking between the treatment groups. e Total responding (mean ± SEM number of drug-paired lever responses) during the 3-h cue-seeking test was not significantly different between the vehicle and (+)-naltrexone treated groups. Vehicle n = 7; (+)-naltrexone n = 8
Experiment 5: Effect of TLR4 antagonism on cocaine-primed reinstatement of cocaine seeking following extinction training
Inhibition of TLR4 receptors in the ventral tegmental area was previously shown to be sufficient to inhibit drug-primed reinstatement of cocaine seeking (Brown et al. 2018). Here, we assessed the effects of multiple (+)-naltrexone doses on drug-primed reinstatement of cocaine seeking (Fig. 6a). Rats were trained to self-administer cocaine for 15 consecutive 2-h daily sessions (Fig. 6b), and lever responding was extinguished in seven 2-h extinction sessions (Fig. 6c). Rats were then administered vehicle or (+)-naltrexone (15 or 30 mg/kg, ip) before a drug-primed reinstatement session or vehicle control reinstatement session. Pretreatment of 30-mg/kg (+)-naltrexone significantly reduced active lever presses during the drug-primed reinstatement session (Fig. 6d, cocaine-prime: F1,51 = 37.81, p < .0001, treatment: F2,51 = 3.66, p = .033, interaction: F2,51 = 3.71, p = .031). Post hoc Tukey’s comparison revealed that the 30-mg/kg (+)-naltrexone group was significantly reduced compared with the vehicle-treated group during drug-primed reinstatement session (t51 = 6.67, p < .001), although rats treated with 30 mg/kg (+)-naltrexone still displayed significant cocaine seeking compared with the vehicle group.
Effects of TLR4 antagonism on drug-primed reinstatement of cocaine seeking. a Timeline illustrating the experimental design. b Cocaine intake (mean ± SEM number of cocaine infusions) was stable before extinction training and reinstatement testing. c Rats were extinguished in seven daily 2-h extinction sessions. Data indicate the mean ± SEM number of drug-paired and inactive lever responses. d Rats were administered a priming injection of either cocaine (15 mg/kg, ip) or vehicle to elicit cocaine seeking. Before the priming injection, rats were administered either vehicle or (+)-naltrexone (15 or 30 mg/kg, ip). All pretreatment groups displayed significant drug-paired lever responding following the cocaine priming injection compared with a vehicle priming injection (*p < 0.05 compared with respective vehicle prime). Administration of 30 mg/kg (+)-naltrexone decreased cocaine-primed seeking compared with vehicle pretreatment (#p < 0.05). Veh/Veh n = 7; 15 mg/kg (+)-naltrexone/Veh n = 5; 30 mg/kg (+)-naltrexone/Veh n = 8; Veh/Cocaine n = 13; 15 mg/kg (+)-naltrexone/cocaine n = 6; 30 mg/kg (+)-naltrexone/cocaine n = 18
Experiment 6: Effect of nucleus accumbens TLR4 antagonism on cocaine-primed reinstatement of cocaine seeking following extinction training
Prior work demonstrates that the ventral tegmental area is a brain region where TLR4 is implicated in drug-primed cocaine seeking (Brown et al. 2018). We sought to extend those findings by assessing the effect of the TLR4 antagonist LPS-Rs delivered into the NAc Shell (n = 16) or NAc Core (n = 8) on drug-primed reinstatement (Fig. 7a). Rats self-administered cocaine in daily 2-h sessions (Fig. 7b), and lever responding was extinguished in 7 daily 2-h extinction sessions (Fig. 7c). Delivery of LPS-Rs (5 μg/side) into the NAc Shell reduced drug-primed reinstatement of cocaine seeking compared to the vehicle-treated group (NAc Shell and NAc Core pooled to form single vehicle group, n = 16) and LPS-Rs NAc Core group (Fig. 7d, treatment: F3,60 = 6.28, p < .001, lever: F1,60 = 38.53, p <.0001, treatment × lever: F3,60 = 8.71, p < .0001). The post hoc comparison indicates that the vehicle-treated group (t120 = 8.37, p <.0001) and LPS-Rs NAc Core treated group (t120 = 6.40, p < .0001) were both significantly different than the vehicle saline-prime control group (n = 24). These findings suggest that TLR4 in the NAc Shell, but not the NAc Core, plays a role in cocaine seeking induced by a cocaine priming injection.
Effects of TLR4 antagonism in the nucleus accumbens on drug-primed reinstatement of cocaine seeking. a Timeline illustrating the experimental design. b Cocaine intake (mean ± SEM number of cocaine infusions) was stable before extinction training and reinstatement testing. c Rats were extinguished in seven daily 2-h extinction sessions. Data indicate the mean ± SEM number of drug-paired and inactive lever responses. d Rats were administered a priming injection of either cocaine (15 mg/kg, ip) or vehicle to elicit cocaine seeking. Before the priming injection, rats were administered either LPS-Rs (5 μg/side) or vehicle in the NAc Shell or NAc Core. A cocaine priming injection induced significant drug-paired lever responding in rats receiving an intra-cranial vehicle injection or intra-NAc Core administration of LPS- Rs (*p < 0.05 compared with vehicle prime). Administration of LPS-Rs into the NAc Shell, but not the NAc Core, resulted in diminished cocaine-primed seeking (#p < 0.05 compared with vehicle pretreatment). e Histological plates illustrating the placements of the intra-NAc Shell and intra-NAc Core microinfusions. Rats with misplaced infusions (shell n = 2; core n = 2) were excluded from all analyses. Veh (core and shell)/Veh n = 24; Veh (core and shell)/cocaine n = 16; core LPS-Rs/cocaine n = 8; shell LPS-Rs/cocaine n = 16
Discussion
This study sought to further investigate the role of TLR4 in cocaine self-administration and cocaine-seeking using a pharmacological antagonist approach. Acute administration of the TLR4 antagonist (+)-naltrexone reduced drug-primed reinstatement of cocaine seeking. Bilateral intracranial administration of LPS-Rs into the NAc Shell also reduced drug-primed reinstatement suggesting the NAc Shell as a site where cocaine may interact with TLR4 to induce cocaine seeking. These results are consistent with the previous reports from our laboratory suggesting a role for the TLR4 system in cocaine-elicited drug seeking (Northcutt et al. 2015; Brown et al. 2018). The impact of TLR4 inhibition appears specific to drug-primed reinstatement since (+)-naltrexone administration during forced abstinence did not alter cue-reinforced cocaine seeking. This finding is similar to that observed on methamphetamine seeking when (+)-naltrexone was administered during abstinence (Theberge et al. 2013).
The TLR4 system has previously been investigated in rodent self-administration models. Results have generally indicated a role for TLR4 in drug reinforcement and seeking behaviors, but findings have been drug-specific and, in some studies, complicated by the generalization of TLR4 antagonist effects to non-drug reinforcers (e.g., sucrose reward). In psychostimulant drug reinforcement and reward-related models, (+) naloxone and (+)-naltrexone blocked methamphetamine and cocaine conditioned place preference and reduced cocaine operant self-administration (Northcutt et al. 2015; Wang et al. 2019). Similarly, a genetic approach using the C3H/HeJ mouse strain that possesses a spontaneous point mutation that impedes TLR4/NF-κB signaling, illustrates that C3H/HeJ TLR4-mutant mice self-administered less cocaine on both fixed- and progressive-ratio schedules of reinforcement (Poltorak et al. 1998; Northcutt et al. 2015). Both pharmacological and genetic approaches did not detect an effect of TLR4 manipulation during sucrose self-administration, suggesting specificity to cocaine-related behaviors (Northcutt et al. 2015). Interpretation of reduction in cocaine self-administration of C3H/HeJ mice warrants caution, however, as these mice have a homozygous 20% inversion of chromosome 6, limiting conclusions about the specificity of observed effects to TLR4. A more recent study found that TLR4 (−/−) mice exhibit reduced cocaine locomotor sensitization and conditioned place preference thresholds with no differences in novel object exploration, open-field locomotor activity, or sucrose preference between TLR4 knockout and wild-type mice (Kashima and Grueter 2017). Only one prior study has examined the effect of TLR4 blockade on the reinstatement of cocaine-seeking, a model of relapse-like behavior. In this study, intra-cranial administration of the TLR4 antagonist LPS-Rs into the VTA reduced drug-primed reinstatement of cocaine seeking, but the identical treatment did not alter reinstatement of sucrose seeking elicited by non-contingent delivery of sucrose pellets (Brown et al. 2018). In contrast to TLR4 blockade, activation of TLR4 appears to exert opposite behavioral effects. For instance, Tortorelli et al. (2015) found that activation of TLR4 with LPS resulted in cocaine locomotor sensitization to a sub-threshold dose of cocaine. Additionally, TLR4 activation with LPS in the VTA was sufficient to reinstate cocaine-seeking (Brown et al. 2018). Translational evidence linking early life stress and TLR4 neuro-immune signaling to the subsequent propensity for cocaine abuse indicates a dynamic interplay among neuro-immune function, development, and cocaine abuse (Lo Iacono et al. 2018). Together, these studies suggest a bi-directional relationship between TLR4 blockade, which generally reduces cocaine behaviors, and TLR4 activation, which appears to heighten cocaine sensitivity and initiate drug-seeking behaviors.
Our results support a role for TLR4 in the reinstatement of cocaine seeking, but do not support a role of TLR4 in cocaine reinforcement as reported previously (Northcutt et al. 2015; Tanda et al. 2016). Unlike the prior studies, the current studies implanted osmotic minipump to chronically deliver (+)-naltrexone. A similar discrepancy was observed with different types of TLR4 antagonist administration on opioid self-administration. Acute delivery of (+)-naloxone or (+)-naltrexone inhibited self-administration of remifentanil while chronic delivery of (+)-naltrexone via an osmotic minipump failed to alter heroin self-administration (Hutchinson et al. 2012; Theberge et al. 2013; Tanda et al. 2016; Yue et al. 2019). Chronic drug delivery via an osmotic minipump has several advantages over repeated injections including reduced animal stress, consistent drug levels, increased drug efficacy, and generally fewer side effects. However, constant drug delivery over about a 2-week period may produce adaptations within the TLR4 system and its downstream targets that render (+)-naltrexone less efficacious. Alternatively, other physiological adaptations such as metabolic tolerance may reduce the overall bioavailability of (+)-naltrexone when administered via an osmotic minipump. Many differences in pharmacokinetics and pharmacodynamics between these drug delivery routes likely play a role in these discrepant findings, limiting interpretation of the precise mechanisms that account for our observed differences in cocaine reinforcement. Future studies may benefit from assessing distinct molecular and synaptic consequences of TLR4 antagonists administered through different routes and time periods.
Concerns regarding the specificity of the effects of TLR4 pharmacological antagonists emerged following replication attempts in opioid and cocaine reinforcement models. Tanda et al. (2016) found that (+)-naltrexone reduced cocaine self-administration. However, reductions in sucrose self-administration were also observed, suggesting non-specific effects (Tanda et al. 2016). Conflicting findings regarding the effects of these pharmacological compounds on non-drug reward behaviors, including null results in this report, may point to discrepancies across various models and dosing regimens used by different laboratories. One notable difference between the present dosing regimen and those of Tanda et al. (2016) and Yue et al. (2019) is the timeframe during which (+)-naltrexone was delivered. In the current studies, (+)-naltrexone was administered via two doses 30 min apart or osmotic minipump to achieve a stable steady-state concentrations within the brain. This dosing regimen was selected because of previous findings that determined a short half-life (~1 h) for (+)-naltrexone (Northcutt et al. 2015). This strategy was also used in previous reports from Northcutt et al. (2015) and Zhang et al. (2018) with similar doses and efficacy. Other strategies to manipulate TLR4, including transgenic rodent and pharmacological methods, have found results specific to drug reward compared to natural reward, memory, and locomotor behaviors, all of which argue against the interpretation that TLR4 effects are generalized across behavioral outcomes.
The role of neuro-immune function in drug abuse has received greater attention recently, and investigations are not limited to TLR4. Widespread transcriptional and translational changes of neuroimmune-related genes have been found following cocaine self-administration in rodents and non-human primates (Vallender et al. 2017; Burkovetskaya et al. 2020). The breadth of cocaine-induced neuroimmune changes indicates the need to further investigate their involvement in drug abuse-related behaviors. Given widespread changes in neuroimmune gene expression following cocaine experience, it is not surprising that, to date, several pleiotropic immune signaling molecules have been found that alter psychostimulant behaviors and mesolimbic DA function (Calipari et al. 2018; Hofford et al. 2019; Gao et al. 2020). Similarly, other receptors, including subtypes of the TLR family and the endoplasmic reticulum stress pathway, appear to be involved in cocaine-induced inflammation (Guo et al. 2015; Liao et al. 2016). Finally, neuroimmune interactions are not limited to psychostimulants, as indicated by studies in alcohol, opioid, and nicotine drug models (Hutchinson et al. 2012; Theberge et al. 2013; Northcutt et al. 2015; Crews et al. 2017; Linker et al. 2020). Together, this work highlights the potential importance of neuro-immune interactions in cocaine abuse and the complexity of using immune-related treatment approaches.
References
Araos P, Pedraz M, Serrano A et al (2015) Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity. Addict Biol 20:756–772. https://doi.org/10.1111/adb.12156
Arezoomandan R, Haghparast A (2016) Administration of the glial cell modulator, minocycline, in the nucleus accumbens attenuated the maintenance and reinstatement of morphine-seeking behavior. Can J Physiol Pharmacol 94:257–264. https://doi.org/10.1139/cjpp-2015-0209
Arezoomandan R, Riahi E, Haghparast A (2018) Minocycline increases firing rates of accumbal neurons and modifies the effects of morphine on neuronal activity. Addict Biol 23:1055–1066. https://doi.org/10.1111/adb.12557
Attarzadeh-Yazdi G, Arezoomandan R, Haghparast A (2014) Minocycline, an antibiotic with inhibitory effect on microglial activation, attenuates the maintenance and reinstatement of methamphetamine-seeking behavior in rat. Prog Neuropsychopharmacol Biol Psychiatry 53:142–148. https://doi.org/10.1016/j.pnpbp.2014.04.008
Bachtell RK, Jones JD, Heinzerling KG et al (2017) Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend 180:156–170. https://doi.org/10.1016/j.drugalcdep.2017.08.003
Beardsley PM, Shelton KL, Hendrick E, Johnson KW (2010) The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur J Pharmacol 637:102–108. https://doi.org/10.1016/j.ejphar.2010.04.010
Bell RL, Lopez MF, Cui C et al (2015) Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol 20:38–42. https://doi.org/10.1111/adb.12106
Birath JB, Briones M, Amaya S et al (2017) Ibudilast may improve attention during early abstinence from methamphetamine. Drug Alcohol Depend 178:386–390. https://doi.org/10.1016/j.drugalcdep.2017.05.016
Brown KT, Levis SC, O’Neill CE et al (2018) Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav Immun 67:130–138. https://doi.org/10.1016/j.bbi.2017.08.012
Burkovetskaya ME, Small R, Guo L et al (2020) Cocaine self-administration differentially activates microglia in the mouse brain. Neurosci Lett 728:134951. https://doi.org/10.1016/j.neulet.2020.134951
Calipari ES, Godino A, Peck EG et al (2018) Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat Commun 9:9. https://doi.org/10.1038/s41467-017-01881-x
Chen H, Uz T, Manev H (2009) Minocycline affects cocaine sensitization in mice. Neurosci Lett 452:258–261. https://doi.org/10.1016/j.neulet.2009.01.078
Cooper ZD, Johnson KW, Pavlicova M et al (2016) The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict Biol 21:895–903. https://doi.org/10.1111/adb.12261
Cooper ZD, Johnson KW, Vosburg SK et al (2017) Effects of ibudilast on oxycodone-induced analgesia and subjective effects in opioid-dependent volunteers. Drug Alcohol Depend 178:340–347. https://doi.org/10.1016/j.drugalcdep.2017.04.029
Crews FT, Walter TJ, Coleman LG, Vetreno RP (2017) Toll-like receptor signaling and stages of addiction. Psychopharmacology (Berl) 234:1483–1498. https://doi.org/10.1007/s00213-017-4560-6
Fox HC, D’Sa C, Kimmerling A et al (2012) Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol 27:156–166. https://doi.org/10.1002/hup.1251
Fujita Y, Kunitachi S, Iyo M, Hashimoto K (2012) The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol Biochem Behav 101:303–306. https://doi.org/10.1016/j.pbb.2012.01.005
Gao S-Q, Zhang H, He J-G et al (2020) Neuronal HMGB1 in nucleus accumbens regulates cocaine reward memory. Addict Biol 25:e12739. https://doi.org/10.1111/adb.12739
García-Marchena N, Barrera M, Mestre-Pintó JI et al (2019) Inflammatory mediators and dual depression: potential biomarkers in plasma of primary and substance-induced major depression in cocaine and alcohol use disorders. PLoS One 14:e0213791. https://doi.org/10.1371/journal.pone.0213791
Guo M-L, Liao K, Periyasamy P et al (2015) Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 11:995–1009. https://doi.org/10.1080/15548627.2015.1052205
Hill KG, Sable HJK, Ferraro Iii FM, Kiefer SW (2010) Chronic naltrexone treatment and ethanol responsivity in outbred rats. Alcohol Clin Exp Res 34:272–279. https://doi.org/10.1111/j.1530-0277.2009.01090.x
Hofford RS, Russo SJ, Kiraly DD (2019) Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur J Neurosci 50:2562–2573. https://doi.org/10.1111/ejn.14143
Hutchinson MR, Northcutt AL, Hiranita T et al (2012) Opioid activation of Toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32:11187–11200. https://doi.org/10.1523/JNEUROSCI.0684-12.2012
Hutchinson MR, Watkins LR (2014) Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 76(Pt B):218–227. https://doi.org/10.1016/j.neuropharm.2013.05.039
Hutchinson MR, Zhang Y, Brown K et al (2008) Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of Toll-like receptor 4 (TLR4). Eur J Neurosci 28:20–29. https://doi.org/10.1111/j.1460-9568.2008.06321.x
Jacobsen JHW, Watkins LR, Hutchinson MR (2014) Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. Int Rev Neurobiol 118:129–163. https://doi.org/10.1016/B978-0-12-801284-0.00006-3
Kashima DT, Grueter BA (2017) Toll-like receptor 4 deficiency alters nucleus accumbens synaptic physiology and drug reward behavior. Proc Natl Acad Sci USA 114:8865–8870. https://doi.org/10.1073/pnas.1705974114
Kawai T, Akira S (2007) Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13:460–469. https://doi.org/10.1016/j.molmed.2007.09.002
Korpi ER, Linden A-M, Hytönen HR et al (2017) Continuous delivery of naltrexone and nalmefene leads to tolerance in reducing alcohol drinking and to supersensitivity of brain opioid receptors. Addict Biol 22:1022–1035. https://doi.org/10.1111/adb.12393
Lacagnina MJ, Rivera PD, Bilbo SD (2017) Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology 42:156–177. https://doi.org/10.1038/npp.2016.121
Levandowski ML, Viola TW, Prado CH et al (2016) Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug Alcohol Depend 167:140–148. https://doi.org/10.1016/j.drugalcdep.2016.08.010
Li MJ, Briones MS, Heinzerling KG et al (2020) Ibudilast attenuates peripheral inflammatory effects of methamphetamine in patients with methamphetamine use disorder. Drug Alcohol Depend 206:107776. https://doi.org/10.1016/j.drugalcdep.2019.107776
Liao K, Guo M, Niu F et al (2016) Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation 13:33. https://doi.org/10.1186/s12974-016-0501-2
Linker KE, Gad M, Tawadrous P et al (2020) Microglial activation increases cocaine self-administration following adolescent nicotine exposure. Nat Commun 11:306. https://doi.org/10.1038/s41467-019-14173-3
Liu HQ, Zhang WY, Luo XT et al (2006) Paeoniflorin attenuates neuroinflammation and dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease by activation of adenosine A1 receptor. Br J Pharmacol 148:314–325. https://doi.org/10.1038/sj.bjp.0706732
Lo Iacono L, Catale C, Martini A et al (2018) From traumatic childhood to cocaine abuse: the critical function of the immune system. Biol Psychiatry 84:905–916. https://doi.org/10.1016/j.biopsych.2018.05.022
Metz VE, Jones JD, Manubay J et al (2017) Effects of ibudilast on the subjective, reinforcing, and analgesic effects of oxycodone in recently detoxified adults with opioid dependence. Neuropsychopharmacology. https://doi.org/10.1038/npp.2017.70
Moreira FP, Medeiros JRC, Lhullier AC et al (2016) Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend 158:181–185. https://doi.org/10.1016/j.drugalcdep.2015.11.024
Northcutt AL, Hutchinson MR, Wang X et al (2015) DAT isn’t all that: cocaine reward and reinforcement requires toll like receptor 4 signaling. Mol Psychiatry. https://doi.org/10.1038/mp.2014.177
Paxinos G, Watson, C (2013) The Rat Brain in Stereotaxic Coordinates. Netherlands: Elsevier Science.
Pedraz M, Martín-Velasco AI, García-Marchena N et al (2015) Plasma concentrations of BDNF and IGF-1 in abstinent cocaine users with high prevalence of substance use disorders: relationship to psychiatric comorbidity. PLoS One 10:e0118610. https://doi.org/10.1371/journal.pone.0118610
Poltorak A, He X, Smirnova I et al (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088. https://doi.org/10.1126/science.282.5396.2085
Soder HE, Berumen AM, Gomez KE et al (2020) Elevated neutrophil to lymphocyte ratio in older adults with cocaine use disorder as a marker of chronic inflammation. Clin Psychopharmacol Neurosci 18:32–40. https://doi.org/10.9758/cpn.2020.18.1.32
Stamatovich S, Lopez-Gamundi P, Suchting R et al (2021) Plasma pro- and anti-inflammatory cytokines may relate to cocaine use, cognitive functioning, and depressive symptoms in cocaine use disorder. Am J Drug Alcohol Abuse 47. https://doi.org/10.1080/00952990.2020.1828439
Tanda G, Mereu M, Hiranita T et al (2016) Lack of specific involvement of (+)-naloxone and (+)-naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology 41:2772–2781. https://doi.org/10.1038/npp.2016.91
Theberge FR, Li X, Kambhampati S et al (2013) Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biological psychiatry 73:729–737. https://doi.org/10.1016/j.biopsych.2012.12.019
Tortorelli LS, Engelke DS, Lunardi P et al (2015) Cocaine counteracts LPS-induced hypolocomotion and triggers locomotor sensitization expression. Behav Brain Res 287:226–229. https://doi.org/10.1016/j.bbr.2015.03.054
Valdizán EM, Díaz A, Pilar-Cuéllar F et al (2012) Chronic treatment with the opioid antagonist naltrexone favours the coupling of spinal cord μ-opioid receptors to Gαz protein subunits. Neuropharmacology 62:757–764. https://doi.org/10.1016/j.neuropharm.2011.08.029
Vallender EJ, Goswami DB, Shinday NM et al (2017) Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug Alcohol Depend 175:9–23. https://doi.org/10.1016/j.drugalcdep.2017.01.030
Wang X, Northcutt AL, Cochran TA et al (2019) Methamphetamine activates Toll-like receptor 4 to induce central immune signaling within the ventral tegmental area and contributes to extracellular dopamine increase in the nucleus accumbens shell. ACS Chem Neurosci 10:3622–3634. https://doi.org/10.1021/acschemneuro.9b00225
Worley MJ, Heinzerling KG, Roche DJO, Shoptaw S (2016) Ibudilast attenuates subjective effects of methamphetamine in a placebo-controlled inpatient study. Drug Alcohol Depend 162:245–250. https://doi.org/10.1016/j.drugalcdep.2016.02.036
Wu R, Li J-X (2020) Toll-like receptor 4 signaling and drug addiction. Front Pharmacol 11:603445. https://doi.org/10.3389/fphar.2020.603445
Yue K, Tanda G, Katz JL, Zanettini C (2019) A further assessment of a role for Toll-like receptor 4 in the reinforcing and reinstating effects of opioids. Behav Pharmacol. https://doi.org/10.1097/FBP.0000000000000474
Zhang X, Cui F, Chen H et al (2018) Dissecting the innate immune recognition of opioid inactive isomer (+)-naltrexone derived Toll-like receptor 4 (TLR4) antagonists. J Chem Inf Model 58:816–825. https://doi.org/10.1021/acs.jcim.7b00717
Funding
This work was supported by institutional funds, US Public Health Service grant DA 033358, and the intramural research programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism, National Institute on Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brown, K.T., Levis, S.C., O’Neill, C.E. et al. Toll-like receptor 4 antagonists reduce cocaine-primed reinstatement of drug seeking. Psychopharmacology 240, 1587–1600 (2023). https://doi.org/10.1007/s00213-023-06392-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06392-w