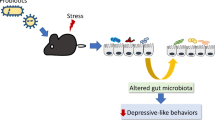
Abstract
Rational
Minocycline is a second-generation, semi-synthetic tetracycline, and has broad spectrum-antibacterial activity. Interestingly, many studies have demonstrated that minocycline is beneficial for depression, which may be due to its effects on neuroinflammation modulation. Recently, gut microbiota imbalance has been found in depression patient and animal models.
Objectives
Based on the fact of minocycline usually acting as an antibiotic and the relationship between depression, gut microbiota, and neuroinflammation, we designed this study to detect the effects of chronic minocycline treatment on antidepression, neuroinflammation, and gut microbiota modulation.
Results
Our results showed that minocycline treatment for 4 weeks, not acute treatment, exerted antidepressant effect in mice exposed to unpredictable chronic mild stress (CUMS). Further results suggested that chronic minocycline treatment inhibited neuroinflammation of hippocampus and altered species abundance and metabolites of gut microbiota. Meantime, we found that chronic minocycline treatment ameliorated intestinal barrier disruption and reduced the bacteriological indexes, such as diamine oxidase, C-reaction protein, and endotoxin in peripheral blood of CUMS mice.
Conclusions
To sum up, our findings confirm that chronic minocycline treatment exerts the antidepressant effect, inhibits neuroinflammation, and modulates gut microbiota. All of these imply that the antidepressant mechanism of chronic minocycline treatment is maybe due to the combined action of neuroinflammation and gut microbiota modulation, which need further prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a common mood disorder, accompanied by marked and persistent lack of energy, sadness, insomnia, and an inability to enjoy life. It is thought to be the result of a multi-factorial disease, including behavioral disturbance and immunological, metabolic, and neurotransmitter dysregulation (Berton and Nestler 2006; Dowlati et al. 2010). However, the specific mechanism involved is still not clear, so that the present antidepressant agents cannot meet the clinical need.
A body of evidence has shown that gut microbiota is important for the health of an individual, and may impact host health via the conversion of nondigestible carbohydrates to short-chain fatty acids (SCFAs) (Burokas et al. 2017), transformation of bile acids (Golubeva et al. 2017), action against pathogenic bacteria (Ostaff et al. 2013), and the modulation of host immune systems (Honda and Littman 2016). Recently, several literatures have found that gut microbiota imbalance is closely related to depression (Dash et al. 2015; Wong et al. 2016; Yu et al. 2017). Indeed, Lactobacillus strain has showed antidepressant effect in the animal model (Bravo et al. 2011), and a decrease in fecal Faecalibacterium has been observed in patients with major depressive disorder (Jiang et al. 2015). These findings about the gut microbiota and depression point out a new direction for new antidepressant drugs development.
Minocycline is a second-generation, semi-synthetic tetracycline that has been used against both gram-positive and gram-negative bacteria for over 30 years. But recently, more studies have focused on its non-antibiotic properties, and it has been considered to be beneficial for diseases with an inflammatory basis, including bullous dermatoses, neutrophilic diseases, pyoderma gangrenosum, sarcoidosis, aortic aneurysms, and autoimmune disorders (Garrido-Mesa et al. 2013). In the nervous system, minocycline has also been widely used to inhibit microglia (Kobayashi et al. 2013; Moller et al. 2016), and exerts its potential effects on the neuroinflammatory and/or neurodegenerative disorders, which have been confirmed in experimental models of Huntington disease (Chen et al. 2000), Parkinson’s disease (Du et al. 2001), amyotrophic lateral sclerosis (Zhu et al. 2002), and spinal cord injury (Tikka et al. 2001).
In several animal models, minocycline was found to decrease the expression of TNF-α and IL-1β in the prefrontal cortex and hippocampus and prevent the development of depression-like symptoms (Burke et al. 2014; Kreisel et al. 2014b). More importantly, minocycline has been potentially used to treat patients with depression (Murrough et al. 2018; Rosenblat and McIntyre 2018). Therefore, we detected the effects of chronic minocycline treatment on antidepression, neuroinflammation, and gut microbiota modulation in a mouse model of depression.
Materials and methods
Animals and treatments
Adult male C57BL/6 mice, aged 6–8 weeks, were used in this study. All experiments were approved by the Institutional Ethical Committee of the Fourth Military Medical University and performed following the guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996). The animals were housed randomly five per cage with ad libitum access to food and water. All mice were fed standard rodent chow. Mice were maintained at a temperature (24 ± 2 °C) and humidity (50–60%) controlled environment, with a 12 h light-dark cycle with lighting on at 7 a.m.
After being adapted to the environment for 1 week, the animals were randomly divided into different groups by body weight (n = 6–8). In Figs.1 and 2, mice were divided into five groups: (1) control group: mice were not stressed and were injected with saline when other groups receiving drug treatment; (2) unpredictable chronic mild stress (CUMS) group: mice exposed to CUMS for 6 weeks and were injected with saline as the control; (3) chronic imipramine (IMI): mice received intraperitoneal injection of imipramine (20 mg/kg, Cayman Chemical) for 4 weeks starting 2 weeks after CUMS; (4) chronic minocycline (MIN): minocycline (40 mg/kg, Cayman Chemical) was administered intraperitoneally daily for 4 weeks starting 2 weeks after CUMS; (5) acute minocycline (MIN): after experiencing the CUMS procedure, mice were administered 45 min before each behavioral test on the three consecutive days. In Figs. 3, 4, 5, and 6, mice were divided into three groups: control, CUMS, and chronic MIN based on the results. All the drugs were administered intraperitoneally in a volume of 10 mL/kg. The dose regimens have been previously found to be effective in counteracting chronic stress-induced behavioral changes (Gao et al. 2019; Han et al. 2014; Menard et al. 2017). Mice were killed immediately after the behavioral tests.
Chronic minocycline treatment reduced depressive-like behaviors induced by CUMS in mice. a The schedule of the study design. b Sample traces of locomotor activity in the open field test. After CUMS, the distance traveled (c) and the time spent in the center area (d) were measured in the OFT, and the immobility time were measured in the TST (e) and FST (f). n = 8 mice in each group. CUMS, unpredictable chronic mild stress; MIN, minocycline; IMI, imipramine; OFT, open field test; TST, tail suspension test; FST, forced swimming test. **P < 0.01 versus control group; #P < 0.05, ##P < 0.01 versus CUMS group
Chronic minocycline treatment decreased the levels of pro-inflammatory factors in the hippocampus. ELISA was used to detect the levels of IL-1β (a), IL-6 (b), and TNF-α (c) in hippocampus. n = 6 mice in each group. CUMS, unpredictable chronic mild stress; MIN, minocycline; IMI, imipramine. **P < 0.01 versus control group; #P < 0.05 < 0.01 versus model group
Chronic minocycline treatment reversed the imbalance of the gut microbiota induced by CUMS. a–d There was no significant difference in the Chao index (p = 0.633), Ace index (p = 0.635), Shannon index (p = 0.161), and Simpson index (p = 0.073) about species richness and diversity treated by CUMS or chronic minocycline. e, f LEfSe analysis showed the community composition and species abundance in the three groups. The circle graph radiated from inside to outside was a clustering tree, which represented the classification level of phylum (p), class (c), order (o), family (f), genus (g), or species (s) in turn. Yellow nodes represented species with no significant difference among groups, while red, green, and blue nodes represented microbial species that played an important role in each group. On the right side of the clustering tree, the microbial species represented by the nodes were summarized and annotated. g The bar chart showed the relative abundance of different species at the phylum level between different groups. h The bar chart showed the relative abundance of different species at the genus level between different groups. CUMS, unpredictable chronic mild stress; MIN, minocycline
Behavioral tests
After acclimatization for 7 days, mice were exposed to following stressors: restraint in plastic tube for 2 h, tilting cages to approximately 45° (without bedding), white noises, lights on during the dark phase, an empty cage with no nesting, dampen the bedding, and cage shaking. The duration and frequency of exposure were based on the procedure and were shown in previous study (Zhu et al. 2014). Mice suffering from restraint did not receive any other stress on that day. Above stressors were randomly arranged in a week, but the same stressor was discontinuously applied. The stress procedure lasted for 6 weeks followed by behavioral tests.
After CUMS procedure, mice were tested for open field test (OFT) on day 43, forced swimming test (FST) on day 44, and tail suspension test (TST) on day 45. Thirty minutes before the tests, each mouse was brought into the testing room. All tests were conducted between 9:00 a.m. to 13:00 p.m., and the schedule of the whole studies was shown in Fig. 1a. Scorers were blind to the treatments.
Open field test (OFT)
Mice were tested in a square arena (30 cm × 30 cm × 30 cm) with clear Plexiglass walls and a white floor (JL Behv-LAM-Shanghai jiliang software, China). After the mice were placed in the center of the arena, their exploration track was recorded for 15 min by using a video camera fixed above. The total distance traveled and time spent in central area were analyzed by using the motion tracking system (Med Associates, St. Albans, VT).
Tail suspension test (TST)
Mice were suspended at a height of 50 cm with adhesive tape placed approximately 2 cm from the tip of its tail. During a 5-min session, mice initially struggled to face upward and climb to a solid surface. When the animal stops struggling and hangs immobile, it is considered to have “given up.” Longer periods of immobility are characteristic of a depressive-like state. The duration of immobility was manually recorded. Immobility was defined when the animals hung passively without limb movement.
Forced swimming test (FST)
Mice were placed in a big plastic basin (100 cm × 50 cm × 30 cm, filled with 23–25 °C water) and forced to swim for 5 min. The duration of immobility behavior (floating in water without active movements of forepaws) was measured manually. After the test, mice were covered by a dry towel and then placed in a warm cage until they were dry.
Analysis of pro-inflammatory cytokines in hippocampus
Hippocampus tissues with equal weight (100 mg) from each group were homogenized with 1 mL PBS containing 1% protease inhibitor. The homogenates were centrifuged at 12000 rpm at 4 °C for 5 min. The supernatant fractions were separated and used for inflammatory cytokines detection. IL-1β, IL-6, and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, USA) according to the manufacturer’s protocols.
Analysis of plasma bacteriological indexes
Blood samples were obtained by eyeball extraction. Plasma diamine oxidase (DAO) activity was measured by a commercial kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instruction. Plasma levels of D-lactate and C-reaction protein (CRP) were determined by ELISA kit (R&D Systems, USA) in accordance with the manufacturer’s protocol. Plasma endotoxin level was tested using a chromogenic limulus amebocyte lysate kit (Shanghai Med & Chem Institute, China) according to the manufacturer’s protocols.
Histological examination
The distal ileum fixed in paraformaldehyde were embedded in paraffin, sliced, and stained with hematoxylin and eosin (H&E). Pathological evaluation was performed using light microscopy (Olympus, Japan) by two investigators who were blind to the experimental treatments.
Immunofluorescence staining
After the behavioral tests, mice were anesthetized with diethyl ether and perfused with 4% polyformaldehyde. The separated brains were dehydrated by sucrose gradient, and then frozen to cut 30 μm-thick coronal sections containing hippocampus. After PBS rinses, sections were incubated in 0.1% Triton X-100 for 10 min at 4 °C and then incubated with 3% BSA for 1 h. Sections were then stained with rabbit anti-Iba1 (1:100; Abcam, ab178847) overnight at 4 °C, followed by incubation with secondary antibodies conjugated with FITC (1:200, Boster Bio-Technology, Wuhan, China). The nucleus was stained with DAPI dye. Images were collected using an Olympus Confocal laser scanning FV1000 microscope (Olympus, Tokyo, Japan).
Western blot analysis
Frozen segments of the tissue were homogenized in ice-cold RIPA lysis buffer containing 1× protease inhibitor cocktail. Equivalent amounts of protein were resolved using 9% SDS-PAGE gel and transferred to a nitrocellulose membrane. After incubation with the secondary antibodies, the proteins were observed using enhanced chemiluminescence (ECL, GE Healthcare Pharmacia, Uppsala, Sweden). The following primary antibodies were used: ZO-1 (1:500; Proteintech, 21773-1-AP), Claudin-1 (1:500; Abcam, ab180158), Occludin (1:500; Proteintech, 13409-1-AP), Iba1 (1:1000; Abcam, ab178847), IL-1β (1:500; Abcam, ab200478), TNF-α (1:1000; Abcam, ab9739), and β-actin (1:10000; Sigma, A5316). The secondary antibody was horseradish peroxidase-conjugated goat antibody to rabbit or mouse immunoglobulin. The densitometric analysis of Western blots was conducted using a ChemiDoc XRS (Bio-Rad, Hercules, CA) and quantified using Quantity One version 4.1.0 (Bio-Rad).
Analysis of fecal microbiota community diversity
The collected feces of mice were stored at−80 °C until DNA extraction. The DNA was extracted from 250 mg samples using the QIAamp DNA Stool Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. The V4-V5 region of bacterial 16S-rRNA genes was amplified using the universal primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 926R (5′-CCGTCAATTCMTTTGAGTTT-3′) (Baker et al. 2003; Liu et al. 2008; Wang and Qian 2009). The primers also contained the Illumina 5′ overhang adapter sequences for two-step amplicon library building, following manufacturer’s instructions for the overhang sequences. The Illumina/Nextera XT Index kit (Illumina Inc., San Diego. CA, USA) with dual 8-base barcodes were used for multiplexing. Prior to library pooling, the barcoded PCR products were purified using aDNA gel extraction kit (Axygen, China) and quantified using the FTC-3000 TM real-time PCR. The libraries were sequenced by 2 × 300 bp paired-end sequencing on the MiSeq platform using MiSeq v3 Reagent Kit (Illumina) at Tiny Gene Bio-Tech Co., Ltd. (Shanghai). The 16S sequences were analyzed using a combination of software mothur (version 1.33.3), UPARSE (use arch version v8.1.1756, http://drive5.com/uparse/), and R (version 3.2.3). For the α-diversity analysis, Shannon, Simpson, Chao, and Ace indexes were calculated using mothur and plotted by R. For the β-diversity metrics, the weighted and unweighted UniFrac distance matrices were calculated using mothur and were visualized with Principal Coordinate Analysis (PCoA) and tree by R. The Bray-Curtis metrics were calculated by R and were visualized also by R.
Metabonomic analysis based on gas chromatography-mass spectrometry detector (GC-MSD)
The cecum and proximal colons were collected, and were homogenated into supernatant by centrifugation. The procedure of sample preparation for GC-MSD analysis was described in previous study (Choe et al. 2012). Agilent 7890A was used for gas chromatography. The chromatographic column was Agilent DB5-MS (30 m × 250 μm × 0.25 μm). The mass spectrometer used Agilent 5975C MSD. In shunting injection part, 1 μL injection, shunting ratio 10:1 was set. Injection port temperature is 250 °C; MSD interface temperature is 230 °C. The initial temperature of the heating program is 60 °C and lasts for 1 min. Then it was heated to 300 °C at 10 °C/min and lasts for 10 min. Ion source temperature is 250 °C; quadrupole pole temperature is 150 °C; solvent delay time is 5.9 min; the detection range of the instrument is 50–600 m/z.
Agilent Chrom Station software (Agilent Technologies, USA) was used to convert the original data into a common (NetCDF) format. In R software platform, erah package was used for peak identification, retention time alignment, automatic integration, and other preprocessings. Then, the number of sample features was screened to obtain a matrix containing sample names and relative quantification. GMD database was used for data spectral comparison, and the results with high retention similarity and the best matching retention index were selected to obtain a qualitative visualization matrix including retention time, retention index, metabolite name, and matching factor.
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM) for at least three independent experiments. Data analysis was conducted using Prism 7.0 (GraphPad, San Diego, CA, USA). The data were analyzed by one-way analysis of variance (ANOVA) first. When there was a significant interaction, further analysis was carried out using Tukey’s HSD post hoc tests for multiple comparisons. Principal component analysis (PCA) and partial least square-discriminant analysis (PLS-DA) was used to analyze the gut microbiota metabolites. Kruskal-Wallis nonparametric test and LDA effect size analysis (LEfSE) was used to analyze gut microbiota composition. In all cases, p < 0.05 was considered statistically significant.
Results
Chronic minocycline treatment exerts antidepressant effect in CUMS mice
Firstly, mice were exposed to a 6-week CUMS procedure, and the depression-like behaviors were detected using the OFT, TST, and FST on days 43, 44, and 45, respectively. One-way ANOVA showed significant effects of treatments on locomotor (F4,35 = 10.99, p < 0.01) and exploratory activity (F4,35 = 11.85, p < 0.01) in OFT, and immobility time in FST (F4,35 = 6.738, p < 0.01) and TST (F4,35 = 6.291, p < 0.01). Compared to the control, CUMS mice exhibited decreased locomotor (Fig. 1b, c; q = 8.596, p < 0.01) and exploratory activity (Fig. 1d; q = 8.468, p < 0.01) in OFT, and increased immobility time in FST (Fig. 1e; q = 6.751, p < 0.01) and TST (Fig. 1f; q = 6.416, p < 0.01). Then we determined whether minocycline had the antidepressant effect. Meantime, imipramine, a classical tricyclic antidepressant, was used as the positive control. In the OFT (Fig. 1b–d), both the total distance traveled (q = 5.078, p < 0.01) and the time spent in central area (q = 4.86, p = 0.0125) by mice treated with chronic minocycline were significantly more than those of CUMS mice. Furthermore, the chronic minocycline treatment significantly reduced the immobility time both in the TST (Fig. 1e, q = 4.837, p = 0.0307) and FST (Fig. 1f, q = 4.27, p = 0.0356) compared with CUMS mice. Imipramine had the similar effects with chronic minocycline treatment on the total distance traveled (q = 4.908, p = 0.011) and the time spent in central area (q = 5.461, p < 0.01) in the OFT (Fig. 1b–d), and the immobility time in the TST (Fig. 1e, q = 4.837, p = 0.013) and FST (Fig. 1f, q = 4.34, p = 0.031), but no significant differences was found in the group receiving acute minocycline treatment (p > 0.05, Fig. 1b–f). These data suggested chronic minocycline treatment, not acute minocycline, and had the same antidepressant effect as imipramine in CUMS mice.
Chronic minocycline treatment inhibits neuroinflammation of hippocampus in CUMS mice
Due to the anti-inflammatory activity of minocycline and the role of neuroinflammation in depression, we measured the pro-inflammatory factors in the hippocampus. One-way ANOVA showed significant effects of treatments on the levels of IL-1β (F4,25 = 7.403, P < 0.01), IL-6 (F4,25 = 5.761, P < 0.01) and TNF-α (F4,22 = 4.896, p < 0.01). Further post hoc tests showed that CUMS significantly enhanced the levels of IL-1β (Fig. 2a, q = 7.178, p < 0.01), IL-6 (Fig. 2b, q = 6.384, p < 0.01), and TNF-α (Fig. 2c, q = 5.739, p < 0.01) in the hippocampus compared to the control group, and all the increases of the three cytokines were significantly inhibited by chronic minocycline treatment (q = 5.306, p < 0.01 in Fig. 2a; q = 4.785, p = 0.018 in Fig. 2b; q = 4.947, p = 0.013 in Fig. 2c). The increased levels of IL-1β and IL-6 were also significantly reduced by imipramine treatment (q = 4.727, p = 0.02 in Fig. 2a; q = 4.295, p = 0.04 in Fig. 2b). However, acute minocycline injection only showed a decreased trend in the levels of IL-1β (Fig. 2a, q = 2.868, p = 0.282), IL-6 (Fig. 2b, q = 2.909, p = 0.269), and TNF-α (Fig. 2c, q = 3.182, p = 0.194). Similar with the previous study (Zhang et al. 2019), our findings implied that chronic minocycline exerted the antidepressant effect possibly by reducing the neuroinflammation.
Chronic minocycline treatment alters species abundance of gut microbiota of CUMS mice
It has been reported that gut microbiota imbalance is closely related to depression (Dash et al. 2015; Wong et al. 2016; Yu et al. 2017), so we believe that the antibiotic effect of minocycline plays an important role in its antidepressant effect. Hence, the fecal microbiota community diversity was analyzed. Because of the ineffective antidepressant effect of acute minocycline treatment, chronic minocycline treatment was chosen in the following experiments. Based on the analysis of α-diversity, we use Chao index and Ace index to reflect species richness (Fig. 3a, b), while Shannon index and Simpson index to reflect species diversity (Fig. 3c, d). The results showed that there was no significant difference in the number of species of bacteria in feces after CUMS induction or chronic minocycline treatment (Fig. 3a–d). But LDA effect size (LEfSe) analysis revealed the significant differences in species abundance between groups (Fig. 3e). Each small circle on different circle layers represents a classification at this level, and the diameter of the small circle is proportional to the relative abundance of the classification (Fig. 3f). Comparative analysis showed that the abundance of Proteobacteria and Spirochaetae in CUMS group was increased at the phylum level, but was decreased by chronic minocycline treatment (Fig. 3g). Additionally, significant differences in the abundance at the genus level were shown (Fig. 3h). The abnormal enrichment of Bacteroides, Helicobacter, Alloprevotella, Erysipelatoclostridium, Parabacteroides, and Brachyspira in the CUMS group was reversed by chronic minocycline treatment. All of these indicated that chronic minocycline treatment altered the imbalance of gut microbiota induced by CUMS.
Chronic minocycline treatment alters the imbalance of gut microbiota metabolites induced by CUMS
Furthermore, high-throughput metabolites in cecum and proximal colon of mice were analyzed by GC/MS. A total of 1236 compounds were detected. By comparing with the peak shape of gas chromatography/mass spectrometry database, 206 metabolites with a matching factor of over 80% were selected for identification, including short-chain fatty acids, sugars, amino acids, and neurotransmitters (see Online Resource). Next, we compared the metabolite components in different samples. Partial least square-discriminant (PLS-DA) analysis established the relationship between groups (Y) and metabolites (X), which was used to find differences between groups and obtain differential metabolites. From the scoring chart, the cohesiveness of intra-group samples was acceptable, and the samples of three groups had a tendency to separate, indicated that the metabolic situation between groups was quite different (Fig. 4a). In the PLS-DA load diagram, some metabolites were far from the central point, which indicated that they mainly contributed to distinguish the different groups (Fig. 4b). Based on the importance of variables and their contribution to sample differentiation, the variable importance in project (VIP) chart showed the top 15 differential metabolites (Fig. 4c), and the chemical names can be looked up for in the supplementary data. Then in the unit statistical analysis, three groups of metabolites were analyzed by one-way ANOVA to screen the metabolites with VIP value greater than 1, which was considered to be significant (default P = 0.05 was the threshold) (Fig. 4d). Together with Fig. 4c, d, three metabolites which were decreased in CUMS group and increased by chronic minocycline were screened, including phosphoric acid (AlignID: 143), butyrate (AlignID: 402), and 4-methyl-5-hydroxyethyl-thiazole (AlignID: 411). In addition, two metabolites which were increased in CUMS group and decreased by chronic minocycline treatment were screened, including sarcosine (AlignID: 23) and 2-amino-isobutanoic acid, (AlignID: 61).
Chronic minocycline treatment altered the content of bacterial metabolites in cecum and proximal colon. a The PLS-DA scoring map showed the cohesion of samples in the same group; the trend of separation of the three groups of samples was observed. The ellipse represented the 95% confidence interval of the sample and the probability distribution of the sample. b The PLS-DA load map reflected the contribution of metabolites to the differentiation of sample groups. The farther away from the center, the greater the contribution to the differentiation of sample groups. c Based on the VIP chart of PLS-DA, the top 15 metabolites were counted to analyze their importance and contribution in sample differentiation. The right color coding reflected the concentration of this metabolite in different groups. The greater the VIP value, the greater their contribution to the sample differentiation. Variables with VIP greater than 1 are considered to have significant differences. d In one-way ANOVA, each colored dot represents a metabolite. The gray line indicates p = 0.05 threshold line, and the metabolite of p > 0.05 is labeled with gray, indicating that there is no significant difference between groups in ANOVA test. p < 0.05, the metabolite was labeled in red, indicating that there was a significant difference between groups in ANOVA test. The blue name labeled in the figure is metabolites with VIP values greater than 1 from PLS-DA analysis. CUMS, unpredictable chronic mild stress; MIN, minocycline
Chronic minocycline treatment ameliorates intestinal barrier disruption induced by CUMS
As the most direct contact with the gut microbiota and the metabolites, the intestinal mucosal barrier is the first station and the most basic part of the communication between the microbes and the brain-gut axis. Then we tested the effects of minocycline on the integrity of intestinal mucosal barrier. HE staining showed that CUMS caused significant pathological changes in the ileum, including mucosal and submucosal swelling, congestion, hemorrhage, shedding of some villi, inflammatory infiltration, and mucosal erosion (Fig. 5a). However, chronic minocycline treatment resulted in observable recovery in the villi compared with the CUMS mice and exhibited minimal changes in the villi similar to control group (Fig. 5a).
Chronic minocycline treatment alleviated ileal pathological damages. a Representative histological changes of distal ileum acquired from different groups (HE, ×100). n = 3 mice in each group; Bar = 100 μm. b Representative western blots showed the expression of tight-junction proteins (TJPs) in the distal ileum. c–e Quantitative analysis of cumulative western blot experiments. Immunoreactivities of total ZO-1 (c), Occludin (d), and Claudin-1 (e) were normalized to β-actin. n = 6 mice from three independent experiments. CUMS, unpredictable chronic mild stress; MIN, minocycline. **p < 0.01 versus control group; #p < 0.05 versus model group
Tight junction proteins (TJPs), the most important components of the physical paracellular barrier, play a critical role in the maintenance of barrier function (Guo et al. 2015). Previous studies have shown that the expression of TJPs, such as zonula occludens-1 (ZO-1) and Occludin decreased after the intestinal permeability, increased (Lam et al. 2010). Then we investigated the changes of TJPs expression in the ileum by western blot analysis. One-way ANOVA showed significant effects of treatments on the levels of ZO-1 (F2,15 = 14.97, p < 0.01), Claudin-1 (F2,15 = 23.01, p < 0.01), and Occludin (F2,15 = 12.91). Further post hoc tests showed that the levels of ZO-1 (Fig. 5b, c, q = 7.729, p < 0.01), Claudin-1 (Fig. 5b, d, q = 9.521, p < 0.01), and Occludin (Fig. 5b, e, q = 5.403, p < 0.01) were all decreased in CUMS group (Fig. 5b). Compared to the CUMP group, chronic minocycline promoted the expression of ZO-1 (q = 4.178, p = 0.025) and Occludin (q = 5.78, p < 0.01), although it did not change the reduced level of Claudin-1 protein (Fig. 5e, q = 1.403, p = 0.593).
When the intestinal mucosal barrier is damaged, the increased permeability can lead to gut microbiota metabolites and pathogen-related molecules entering the blood circulation, which may affect the whole system (Kelly et al. 2015). To further confirm the protective effects of minocycline on imbalance of gut microbiota and the metabolites-induced inflammation, we measured the bacteriological indexes in peripheral blood. One-way ANOVA showed significant effects of treatments on the levels of DAO (F2,15 = 11.67, p < 0.01), CRP (F2,15 = 5.43, p = 0.016), endotoxin (F2,15 = 9.904, p < 0.01), and D-Lac (F2,15 = 11.17, p < 0.01). Further post hoc tests showed that CUMS resulted in a notable increase in plasma DAO (Fig. 6a, q = 6.006, p < 0.01), CRP (Fig. 6b, q = 4.280, p = 0.021), endotoxin (Fig. 6c, q = 6.214, p < 0.01), and D-Lac (Fig. 6d, q = 6.673, p < 0.01) compared with control mice. Compared to the CUMS group, chronic treatment with minocycline significantly reduced the levels of DAO (q = 5.824, p < 0.01), CRP (q = 3.737, p = 0.045), and endotoxin (q = 3.977, p = 0.033). Although there were no significant differences, the decreased trend of D-Lac was found in chronic minocycline-treated group (q = 2.999, p = 0.1191).
Chronic minocycline treatment decreased the levels of bacteriological indexes in the plasma. Plasma levels of DAO (a), CRP (b), endotoxin (c), and D-lactate (d) after different treatments were tested. n = 6 mice in each group. CUMS, unpredictable chronic mild stress; MIN, minocycline; DAO, diamine oxidase; D-lac, D-lactate; CRP, C-reaction protein. ** P < 0.01 versus control group; # P < 0.05, ## P < 0.01 versus CUMS group
Taken together, the above results collectively suggested that chronic minocycline treatment improved intestinal barrier disruption and inhibited the inflammation in peripheral system caused by CUMS.
Discussion
It has been recognized that gut microbiota appears to significantly interact with the nervous system through the gut–brain axis, a communication channel between gut and central nervous system (Forsythe et al. 2010; Schmidt 2015). Here, we report that chronic minocycline treatment, not acute treatment, improves the depression-like behaviors caused by CUMS. Further studies find that chronic minocycline treatment not only inhibits the neuroinflammation, but also alters the imbalance of gut microbiota and the metabolites and ameliorates intestinal barrier disruption induced by CUMS. Taken together, all the findings demonstrate that minocycline exerts its antidepressant effect and inhibits neuroinflammation and modulates gut microbiota.
Several studies have demonstrated that CUMS activated the microglia, which were the primary inflammatory mediator in the central nervous system and main source of cytokine (Kreisel et al. 2014a; Srinivasan et al. 2016). Microglia-induced neuroinflammation can result in abnormal behaviors, including depression (Yirmiya et al. 2015). Therefore, microglia antagonist minocycline distinctly improved depression-like symptoms through its anti-inflammatory effects (Zhang et al. 2019). In the present study, we found that both acute and chronic administration of minocycline ameliorated CUMS-triggered behavioral disorders, also reduced the levels of TNF-α, IL-1β, and IL-6 in the hippocampus. Thus, these findings together with previous reports indicate that chronic minocycline treatment can inhibit inflammation-mediated neurotoxicity and decrease abnormal behavior under chronic stress. However, another interesting finding was that indexes of bacterial infection in the peripheral blood, including DAO, D-Lac, CRP, and endotoxin, were also affected by CUMS and minocycline treatment, as the inflammatory cytokines in the brain. More importantly, CUMS disrupted the tissue structure and tight junction proteins (ZO-1, Claudin-1, and Occludin) in the intestinal mucosal barrier, which were also rectified by chronic minocycline treatment. These results suggest that in addition to inhibiting central inflammation, chronic minocycline treatment also alters intestinal inflammatory infiltration into the peripheral circulation through its antibacterial activity.
The composition and diversity of gut microbiota is strongly associated with depression (Rogers et al. 2016; Zheng et al. 2016). It is reported that compared to the normal population, Bifidobacteria and Lactobacillus in fecal were significantly reduced in patients with depression (Aizawa et al. 2016). Extensive studies have shown that probiotics supplementation can alleviate depressive symptoms and even achieve similar effects as traditional antidepressants and improve cognition and metabolism (Peirce and Alvina 2019; Zamani et al. 2017). Lactic acid bacteria, such as Lactobacillus casei (Zamani et al. 2017), Lactobacillus helveticus (Chiba et al. 2012), and Bifidobacteria (Abildgaard et al. 2017), have been widely reported. Additionally, stress exposure increased the Proteobacteria population in the gut microbiota composition with elevated neuroinflammation and gut membrane permeability (Campos et al. 2016; Jang et al. 2018; Jang et al. 2019). These consistent findings suggest a predominance of Proteobacteria possibly is harmful to the mood, and could serve as a biomarker for evaluating depression susceptibility. Furthermore, the increased Parabacteroides and Bacteroides of CUMS group at the genus level were also reproducible in major depressive disorder (MDD) patients (Cheng et al. 2019; Jiang et al. 2015). Through 16S RNA gene sequencing and analysis of fecal microbiota, our study identified the abundances of several bacterial species which were altered by CUMS and could be corrected by chronic minocycline treatment. At phylum level, we found that CUMS group had increased levels of Proteobacteria, which was also observed in MDD patients (Jiang et al. 2015). The increased level of Spirochaetae in CUMS group was also found in our study at the first time, which may be a new bacterium involving in depression. Bacteroides, a genus, has a particularly rich armamentarium for metabolizing more complex carbohydrates including glycans of human mucin (Hehemann et al. 2012); the abundance trend in different depression patients were divergent, but the level of Parabacteroides is higher in MDD patients (Cheung et al. 2019; Jiang et al. 2015). Bacteroides were increased in the responded-MDD group and were significantly decreased in active-MDD group (Jiang et al. 2015). Our results showed that the abnormal enrichment of Bacteroides, Parabacteroides, Helicobacter, Alloprevotella, and Erysipelatoclostridium in the CUMS group were reversed by chronic minocycline treatment in the CUMS group. In addition, similar with our findings, Helicobacter pylori infection was reported to be related to depressive symptoms in the general adult population (Gu et al. 2019). However, the roles of Alloprevotella, Erysipelatoclostridium, and Brachyspira in depression have not been seen. Taken together, the antidepressant effect of chronic minocycline treatment is closely related to the improvement of dysregulated gut microbiota. Further studies are warranted to elucidate the relationships between the specific gut microbiota and depression.
Gut microbiota can affect the central nervous system in various ways; altering intestinal microbial metabolites is one of them (De Vadder et al. 2014; Iannone et al. 2019). SCFAs are some of the most significant functioning metabolites synthesized by the gut microbiota. And they are reported to be critical modulators in the gastrointestinal tract capable of regulating the pro-inflammatory and anti-inflammatory responses during autoimmune neuroinflammation (Haghikia et al. 2015). Through metabonomic analysis of cecum and proximal colon, we found five distinct metabolites, the quantities of which were altered by CUMS and were reversed by chronic minocycline treatment. Even though the amount of each metabolite varied greatly in an individual, the ratio of each component remained stable. Butyrate, one of the identified metabolites by us, has displayed the anti-inflammation and antidepressant profiles in animal models (Koh et al. 2016; Makki et al. 2018; Valvassori et al. 2014). It has been reported that several gut microbiotas, which have abnormal abundance in our findings, play important roles in butyrate production. In a study about the role of the gut microbiome in the pathogenesis of type 1 diabetes in children, the result showed that Bacteroides-dominated subgroups were associated with decreased potential of butyrate production via the co-fermentation of acetate (Endesfelder et al. 2016). Another study showed that 3 weeks of a Galacto-oligosaccharides diet induced significantly enrichment in Alloprevotella, Bacteroides, and Parasutterella, but decreased the abundance of butyrate-producing bacteria (Cheng et al. 2018). Although there is no exact association between butyrate and Helicobacter, but butyrate has a bactericidal effect on Helicobacter Pylori (Yonezawa et al. 2012). However, it is still unknown that whether Erysipelatoclostridium, Parabacteroides, and Brachyspira modulate butyrate production, more detailed studies are needed to elucidate the effects of other metabolites we found, such as phosphoric acid, 4-methyl-5-hydroxyethyl-thiazole, sarcosine, and 2-amino-isobutanoic acid.
More importantly, minocycline has good tolerability in depression treatment, though it has a lot of second targets. During our study, there was no adverse events occurred. Recently, a systematic review and meta-analysis of clinical trials showed that a large antidepressant effect was observed for minocycline compared to placebo with good tolerability. But the potential adverse effect cannot be completely ruled out, and longer trials might be required.
To sum up, our findings confirm that chronic minocycline treatment exerts antidepressant effect, while it inhibits neuroinflammation and modulating gut microbiota. All of these imply that the antidepressant mechanism of chronic minocycline treatment is maybe due to the combined actions of neuroinflammation and gut microbiota modulation, which need further prospective studies.
References
Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S (2017) Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 79:40–48
Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H (2016) Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 202:254–257
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55:541–555
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151
Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. P Natl Acad Sci USA 108:16050–16055
Burke NN, Kerr DM, Moriarty O, Finn DP, Roche M (2014) Minocycline modulates neuropathic pain behaviour and cortical M1-M2 microglial gene expression in a rat model of depression. Brain Behav Immun 42:147–156
Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF (2017) Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatry 82:472–487
Campos AC, Rocha NP, Nicoli JR, Vieira LQ, Teixeira MM, Teixeira AL (2016) Absence of gut microbiota influences lipopolysaccharide-induced behavioral changes in mice. Behav Brain Res 312:186–194
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801
Cheng W, Lu J, Lin W, Wei X, Li H, Zhao X, Jiang A, Yuan J (2018) Effects of a galacto-oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice. Food Funct 9:1612–1620
Cheng S, Han B, Ding M, Wen Y, Ma M, Zhang L, Qi X, Cheng B, Li P, Kafle OP, Liang X, Liu L, Du Y, Zhao Y, Zhang F (2019) Identifying psychiatric disorder-associated gut microbiota using microbiota-related gene set enrichment analysis. Brief Bioinform
Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME (2019) Systematic review of gut microbiota and major depression. Front Psychiatry 10:34
Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H (2012) Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro-Psychopharmacol Biol Psychiatry 39:112–119
Choe S, Woo SH, Kim DW, Park Y, Choi H, Hwang BY, Lee D, Kim S (2012) Development of a target component extraction method from GC-MS data with an in-house program for metabolite profiling. Anal Biochem 426:94–102
Dash S, Clarke G, Berk M, Jacka FN (2015) The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry 28:1–6
De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G (2014) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457
Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM (2001) Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A 98:14669–14674
Endesfelder D, Engel M, Davis-Richardson AG, Ardissone AN, Achenbach P, Hummel S, Winkler C, Atkinson M, Schatz D, Triplett E, Ziegler AG, zu Castell W (2016) Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome 4: 17
Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J (2010) Mood and gut feelings. Brain Behav Immun 24:9–16
Gao M, Hu P, Cai Z, Wu Y, Wang D, Hu W, Xu X, Zhang Y, Lu X, Chen D, Chen Z, Ma K, Wen J, Wang H, Huang C (2019) Identification of a microglial activation-dependent antidepressant effect of amphotericin B liposome. Neuropharmacology 151:33–44
Garrido-Mesa N, Zarzuelo A, Galvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169:337–352
Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, Flynn I, Khochanskiy D, Moya-Perez A, Peterson V, Rea K, Murphy K, Makarova O, Buravkov S, Hyland NP, Stanton C, Clarke G, Gahan CGM, Dinan TG, Cryan JF (2017) Microbiota-related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 24:166–178
Gu Y, Zheng L, Kumari S, Zhang Q, Liu L, Meng G, Wu H, Bao X, Yao Z, Sun S, Wang X, Zhou M, Jia Q, Song K, Niu K (2019) The relationship between Helicobacter pylori infection and depressive symptoms in the general population in China: the TCLSIH cohort study. Helicobacter:e12632
Guo S, Nighot M, Al-Sadi R, Alhmoud T, Nighot P, Ma TY (2015) Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88. J Immunol 195:4999–5010
Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad DA, Grohme DA, Kleinewietfeld M, Kempa S, Thone J, Demir S, Muller DN, Gold R, Linker RA (2015) Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43:817–829
Han A, Sung YB, Chung SY, Kwon MS (2014) Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology 81:292–302
Hehemann JH, Kelly AG, Pudlo NA, Martens EC, Boraston AB (2012) Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc Natl Acad Sci U S A 109:19786–19791
Honda K, Littman DR (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535:75–84
Iannone LF, Preda A, Blottiere HM, Clarke G, Albani D, Belcastro V, Carotenuto M, Cattaneo A, Citraro R, Ferraris C, Ronchi F, Luongo G, Santocchi E, Guiducci L, Baldelli P, Iannetti P, Pedersen S, Petretto A, Provasi S, Selmer K, Spalice A, Tagliabue A, Verrotti A, Segata N, Zimmermann J, Minetti C, Mainardi P, Giordano C, Sisodiya S, Zara F, Russo E, Striano P (2019) Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev Neurother:1–14
Jang HM, Lee HJ, Jang SE, Han MJ, Kim DH (2018) Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol 11:1386–1397
Jang HM, Lee KE, Kim DH (2019) The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 11
Jiang HY, Ling ZX, Zhang YH, Mao HJ, Ma ZP, Yin Y, Wang WH, Tang WX, Tan ZL, Shi JF, Li LJ, Ruan B (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behavior and Immunity 48:186–194
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP (2015) Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9:392
Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4:e525
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345
Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R (2014a) Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19:699–709
Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R (2014b) Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry 19:699–709
Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH (2010) Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One 7:e34233
Liu Z, DeSantis TZ, Andersen GL, Knight R (2008) Accurate taxonomy assignments from 16S rRNA sequences produced by highly parallel pyrosequencers. Nucleic Acids Res 36:e120
Makki K, Deehan EC, Walter J, Backhed F (2018) The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23:705–715
Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, Janssen WG, Labonte B, Parise EM, Lorsch ZS, Golden SA, Heshmati M, Tamminga C, Turecki G, Campbell M, Fayad ZA, Tang CY, Merad M, Russo SJ (2017) Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20:1752–1760
Moller T, Bard F, Bhattacharya A, Biber K, Campbell B, Dale E, Eder C, Gan L, Garden GA, Hughes ZA, Pearse DD, Staal RG, Sayed FA, Wes PD, Boddeke HW (2016) Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia 64:1788–1794
Murrough JW, Huryk KM, Mao X, Iacoviello B, Collins K, Nierenberg AA, Kang G, Shungu DC, Iosifescu DV (2018) A pilot study of minocycline for the treatment of bipolar depression: effects on cortical glutathione and oxidative stress in vivo. J Affect Disord 230:56–64
Ostaff MJ, Stange EF, Wehkamp J (2013) Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med 5:1465–1483
Peirce JM, Alvina K (2019) The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res 97:1223–1241
Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S (2016) From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 21:738–748
Rosenblat JD, McIntyre RS (2018) Efficacy and tolerability of minocycline for depression: a systematic review and meta-analysis of clinical trials. J Affect Disord 227:219–225
Schmidt C (2015) Thinking from the gut. Sci Am 312:S12–S15
Srinivasan K, Friedman BA, Larson JL, Lauffer BE, Goldstein LD, Appling LL, Borneo J, Poon C, Ho T, Cai F, Steiner P, van der Brug MP, Modrusan Z, Kaminker JS, Hansen DV (2016) Untangling the brain’s neuroinflammatory and neurodegenerative transcriptional responses. Nat Commun 7
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21:2580–2588
Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J, Reus GZ, Quevedo J (2014) Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res 11:359–366
Wang Y, Qian PY (2009) Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One 4:e7401
Wong ML, Inserra A, Lewis MD, Mastronardi CA, Leong L, Choo J, Kentish S, Xie P, Morrison M, Wesselingh SL, Rogers GB, Licinio J (2016) Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry 21:797–805
Yirmiya R, Rimmerman N, Reshef R (2015) Depression as a microglial disease. Trends Neurosci 38:637–658
Yonezawa H, Osaki T, Hanawa T, Kurata S, Zaman C, Woo TDH, Takahashi M, Matsubara S, Kawakami H, Ochiai K, Kamiya S (2012) Destructive effects of butyrate on the cell envelope of helicobacter pylori. J Med Microbiol 61:582–589
Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, Zou Z (2017) Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal 138:231–239
Zamani B, Farshbaf S, Golkar HR, Bahmani F, Asemi Z (2017) Synbiotic supplementation and the effects on clinical and metabolic responses in patients with rheumatoid arthritis: a randomised, double-blind, placebo-controlled trial. Br J Nutr 117:1095–1102
Zhang C, Zhang YP, Li YY, Liu BP, Wang HY, Li KW, Zhao S, Song C (2019) Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res 356:348–357
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P (2016) Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796
Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM (2002) Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417:74–78
Zhu S, Wang J, Zhang Y, Li V, Kong J, He J, Li XM (2014) Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res 1576:81–90
Funding
This research was supported by National Natural Science Foundation of China (81870893 and 81503046) and National Postdoctoral Program for Innovative Talents (BX20160025 and 45416).
Author information
Authors and Affiliations
Contributions
Q.Y. and L.L. designed and performed the experiments, collected and analyzed data, and drafted the manuscript. L.F.C and T.S. substantially contributed to the experimental design and proofed the manuscript. H.Y.L. performed animal experiments. Y.W. performed histological examination and immunofluorescence staining. L.Y. and A.L. put together the entire data and interpreted data. Q.Q.L and Y.W. analyzed the data. M.G.Z contributed to the experimental design and the critical argument. B. F and S.X.W designed and supervised the study and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 23 kb) Online Resource. The list of 206 metabolites with a matching factor of over 80%. High-throughput metabolites in cecum and proximal colon of mice were analyzed by gas chromatography/mass spectrometry (GC/MS). A total of 1236 compounds were detected. By comparing with the peak shape of GC/MS database, 206 metabolites with a matching factor of over 80% were selected for identification, and their information was listed
Rights and permissions
About this article
Cite this article
Yang, Q., Luo, L., Sun, T. et al. Chronic minocycline treatment exerts antidepressant effect, inhibits neuroinflammation, and modulates gut microbiota in mice. Psychopharmacology 237, 3201–3213 (2020). https://doi.org/10.1007/s00213-020-05604-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05604-x