Abstract
Background
Ethanol use disorders are a serious medical and public health problem in the world today. Acute ethanol intoxication can lead to cognitive dysfunction such as learning and memory impairment. Gamma oscillations (γ, 30–80 Hz) are synchronized rhythmic activity generated by population of neurons within local network, and closely related to learning and memory function. The hippocampus is a critical anatomic structure that supports learning and memory. On the grounds of structure and function, hippocampus can be divided into the intermediate (IH), the dorsal (DH), and ventral hippocampus (VH). The current study is the first to investigate the effects of acute ethanol on γ oscillations in these sub-regions of rat hippocampal slices.
Methods
The sustained γ oscillations were induced by 200 nM kainate (KA) in the CA3c of IH, DH, and VH. When KA-induced γ oscillation reached the steady state, ethanol (50 mM or 100 mM) was applied and the effects of ethanol on γ oscillation power was measured in the slices sequentially sectioned from ventral to dorsal hippocampus of adult rats.
Results
In the intermediate hippocampal slices, compared with control (KA only), ethanol (50 mM) caused 36.1 ± 3.9% decrease in γ power (p < 0.05, n = 10), while ethanol (100 mM) caused 55.3 ± 5.5% decrease in γ power (p < 0.001, n = 14). In the dorsal hippocampus, only ethanol (100 mM) caused 18.1 ± 8.6% decrease in γ power (p < 0.05, n = 12). However, in the ventral hippocampus, neither 50 mM nor 100 mM ethanol affected γ oscillation.
Conclusions
Our results demonstrate that ethanol may produce the differential suppression of γ oscillations in a dose-dependent manner in different sub-regions of hippocampus, suggesting that the modulation of ethanol on hippocampal γ oscillation is region-dependent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethanol is a substance with both fat- and water-soluble, easily goes through the blood-barrier, and has a strong affinity to brain tissue. Ethanol elicits depression, anxiety, euphoria, and dependence. Acute ethanol can cause neurotoxicity at a dose > 50 mM (Flentke et al. 2014). Hippocampus is closely related to the higher brain function such as learning, memory, and sensitive to the ethanol (Moselhy et al. 2001). Studies have shown that acute ethanol affects dendrites, synapses, receptors, and neurotransmitters, resulting in impairment of cognitive function (Harper 1998).
Hippocampal γ oscillation is generated by the precise interaction of interneurons and pyramidal neurons, provides time frame for the synchronization of firing of the neurons within the network (Fries et al. 2007), plays an important role in the integration of synaptic signals, and facilitates synaptic plasticity and the coordination of the multi-layered information. In vitro γ oscillation has been shown to be an accurate model reflecting the strength of in vivo γ, which are known to be closely related to spatial memory (Lu et al. 2011; Wulff et al. 2009).
The hippocampus can be divided into the dorsal, ventral, and the intermediate layer according to their different structures and functions (Bast et al. 2009; Fanselow and Dong 2010). The dorsal hippocampus is located in postural part of the hippocampus in primates, mainly related to cognitive function; the ventral hippocampus is located in the front portion of hippocampus in primates, participated in regulation of stress, emotion, and affect (Fanselow and Dong 2010); the intermediate hippocampus is a transition region between the dorsal and ventral hippocampus, its function is associated with translating cognitive and spatial information into motivation and action critical for survival (Bast et al. 2009), although the mechanism of the intermediate hippocampus in information processing is unknown.
Ethanol inhibits the glutamate N-methyl-d-aspartate receptors (NMDAR) (Ferrani-Kile et al. 2003) and synaptic plasticity (Izumi et al. 2005), but enhances GABAergic synaptic transmission (Ariwodola and Weiner 2004), leading to imbalance of excitation and inhibition and alteration of network function such as γ oscillations. Acute ethanol (80 mg/dl) increased the stimulus-induced γ oscillation amplitude and reduced peak frequency in human primary visual and motor cortex (Campbell et al. 2014). The recent study shows that ethanol (50-100 mM) decreased hippocampal γ oscillations involved in dopamine receptor (DR), NMDAR, and the intracellular kinases Akt and GSk3β activation (Wang et al. 2016). However, the effects of ethanol on different hippocampal regions have not been reported.
Materials and methods
Animals
All animal use procedures were approved by the Ethics Committees at Xinxiang Medical University for the Care and Use of Laboratory Animals, and all efforts were made to minimize animal suffering and reduce the number of animals used. Electrophysiological studies were performed on hippocampal slices prepared from SD rats (male, 3–4 week old). For electrophysiology, the animals were anesthetized by intraperitoneal injection of sagatal (sodium pentobarbitone, 100 mg kg−1, Rhône Mérieux Ltd., Harlow, UK). When all pedal reflexes were abolished, the animals were perfused intracardially with chilled (5 °C) oxygenated artificial cerebrospinal fluid (ACSF) in which the sodium chloride had been replaced by isoosmotic sucrose. This ACSF (305 mosmol l−1) contained (in mM) 225 sucrose, 3 KCl, 1.25 NaH2PO4, 24 NaHCO3, 6 MgSO4, 0.5 CaCl2, and 10 glucose. For extracellular field recording, the hippocampal horizontal slices (400 μm) of rat brain were cut at 4–5 °C in the sucrose-ACSF, using a Leica VT1000S vibratome (Leica Microsystems UK, Milton Keynes, UK). The horizontal sequential section of whole rat brain from ventral to dorsal generated usually 5–6 slices per rat. The typical small “c,” big “C,” and “v” shape in dentate gyrus corresponding to VH, IH, and DH, respectively, can be visually identified.
Electrophysiological recording, data acquisition, and analysis
For extracellular field recordings, the two hippocampal slices were transferred to an interface recording chamber. The slices were maintained at a temperature of 32 °C and at the interface between ACSF and warm humidified carbon gas (95% O2–5% CO2). The ACSF contained (in mM) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 24 NaHCO3, 2 MgSO4, 2 CaCl2, and 10 glucose. The slices were allowed to equilibrate in this medium for 1 h prior to recording. Both channels of an Axoprobe 1A amplifier (Axon Instruments, Union City, CA, USA) were employed for extracellular field recordings, which were made using glass microelectrodes containing ACSF (resistance 2–5 MΩ). The γ oscillation from hippocampal CA3 was induced by perfusion of ACSF containing kainate (200 nM). Data were band-pass filtered online between 0.5 Hz and 2 kHz using the Axoprobe amplifier and a Neurolog system NL106 AC/DC amplifier (Digitimer Ltd., Welwyn Garden City, UK). The data were digitized at a sample rate of 5–10 kHz using a CED 1401 plus ADC board (Digitimer Ltd). Electrical interference from the main supply was eliminated from extracellular recordings online with the use of 50 Hz noise eliminators (HumBug; Digitimer Ltd).
Data were analyzed offline using software from Spike 2 (CED, Cambridge, UK). Power spectra were generated to provide a quantitative measure of the frequency components in a stretch of recording, where power, a quantitative measure of the oscillation strength, was plotted against the respective frequency. Power spectra were constructed for 60 s epochs of extracellular field recordings using a fast Fourier transform algorithm provided by Spike2. The parameters used for measuring the oscillatory activity in the slice were peak frequency (Hz) and area power (μV2). In the current study, area power was equivalent to the computed area under the power spectrum between the frequencies of 20 and 60 Hz.
All data statistics were performed using IBM SPSS Statistics 22 software (IBM, Armonk, NY, USA). Shapiro–Wilk test was used for testing the normality of the data. Parametric data were expressed as means ± standard error of the mean (SEM). The paired or unpaired Student’s t tests were used to compare two groups of parametric data. One or two way ANOVA or repeated measure (RM) ANOVA was used to compare three or more group means. Non-parametric data were expressed as the median ± interquartile range. The Wilcoxon rank sum test and the Wilcoxon signed ranks test were used for the comparison of the two groups of non-parametric data. One or two way ANOVA on ranks or RM ANOVA on ranks were used for three or more group comparison. Effects were considered significant if P < 0.05.
Results
Ethanol inhibition of γ oscillation in the intermediate hippocampus at both 50 mM and 100 mM
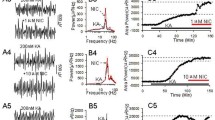
Figure 1 shows a diagraph which illustrates three hippocampal regions (dorsal, ventral, intermediate) and the example photos taken from different regions by stereo- microscope (×20). The sustained γ oscillation was induced by 200 nM kainate (KA) in the CA3c region of intermediate hippocampus. When γ power reached a steady state after KA application for 90 min, and ethanol (50 mM and 100 mM) containing ACSF was perfused into brain slices, a significant, concentration-dependent inhibition of γ oscillation was found, γ power can be partially or fully restored after washout of ethanol (Fig. 2c).
The diagraph and the example photos illustrate three hippocampal regions (dorsal, ventral, intermediate). a The hippocampus can be divided into the dorsal (DH), ventral (IH), and intermediate (VH) parts, and the example photos were taken from different regions by stereo-microscope (×20). DG, Dentate gyrus. b The extracellular field potential (1 s) was induced by 200 nM KA in the CA3 region of DH. c The power spectrum of field potentials corresponding to the conditions before (left panel) and after application of KA (right panel). d Time-effect curve of γ power before and after application of KA
The effects of ethanol (50 mM, 100 mM) on hippocampal γ oscillation in CA3 area of the intermediate hippocampal slice. a The example extracellular field traces of γ oscillation before and after application of ethanol. b The power spectrum of field potentials corresponding to the conditions shown in (A). c The time-effect curve of normalized γ power before and after application of ethanol. d The percent change of γ power before and after application of ethanol (* p < 0.05, *** p < 0.001, compared with control, one-way RM ANOVA, n = 10)
On average, 50 mM ethanol caused 36.1 ± 3.9% decrease in γ power (KA + ethanol 50 mM, 1354.61(662.04, 2296.09) μV2 vs. KA control, 2766.07 (1061.65, 3374.45) μV2, * p < 0.05, RM ANOVA following by post-hoc Tukey test, n = 10, Fig. 2d); 100 mM ethanol caused 55.3 ± 5.5% decrease in γ power (KA + ethanol 100 mM: 927.50 (617.45, 1701.72) μV2 vs. KA control, ** p < 0.01, RM ANOVA following by post-hoc Tukey test, n = 10, Fig. 2d). These results indicate that ethanol dose-dependently inhibits KA-induced γ oscillation in intermediate hippocampus.
Ethanol inhibition of γ oscillation in the dorsal hippocampus at 100 mM
When KA-induced γ oscillation reached the steady state, 50 mM ethanol containing ACSF was perfused into the slice of dorsal hippocampus. Ethanol, 50 mM, did not significantly affect γ power (0.3 ± 7.3%, KA + ethanol 50 mM: 731.97 (513.34, 2568.29) μV2 vs. KA control, 734.60 (570.43, 2763.96) μV2, p > 0.05, RM ANOVA on ranks following by post-hoc Tukey test, n = 14, Fig. 3d). Ethanol, 100 mM, caused, however, 18.1 ± 8.6% decrease in γ power (KA + ethanol 100 mM, 552.34 (437.971, 632.69) μV2 vs. KA control or 50 mM ethanol *p < 0.05, RM ANOVA on ranks following by post-hoc Tukey test, n = 14, Fig. 3d). γ power can be partially or fully restored after the washout of ethanol.
The effects of ethanol (50 mM, 100 mM) on hippocampal γ oscillation in CA3 area of the dorsal hippocampal slice. a The example extracellular field traces of γ oscillation before and after application of ethanol. b The power spectrum of field potentials corresponding to the conditions shown in (A). c The time-effect curve of normalized γ power before and after application of ethanol. d The percent change of γ power before and after application of ethanol (*p < 0.05, versus control or 50 mM ethanol, RM ANOVA on ranks, n = 14)
Ethanol did not affect γ oscillation of the ventral hippocampus at either 50 mM or 100 mM
When KA-induced γ oscillation in CA3 area of the ventral hippocampal slices reached the steady state, application of 50 mM ethanol did not reduce but rather increased slightly γ power (13.3 ± 8.5%, KA + ethanol 50 mM, 509.20 (248.66, 1497.59) μV2 vs. KA control, 560.08 (227.10, 1528.12) μV2, p > 0.05, RM ANOVA on ranks, n = 12, Fig. 4d). Further application of 100 mM ethanol did not y cause a significant decrease in γ power (2.1 ± 8.4%, KA + ethanol 100 mM, 559.12(179.84, 1246.18) μV2 vs. control or 50 mM ethanol, p > 0.05, RM ANOVA on ranks, n = 12, Fig. 4d). γ power can be partially or fully restored after the washout of ethanol.
The effects of ethanol (50 mM, 100 mM) on hippocampal γ oscillation in CA3 area of the ventral hippocampal slice. a The example extracellular field traces of γ oscillation before and after application of ethanol. b The power spectrum of field potentials corresponding to the conditions shown in (A). c The time-effect curve of normalized γ power before and after application of ethanol. d The percent change of γ power before and after application of ethanol (2.1 ± 8.4%, p > 0.05, versus control or 50 mM ethanol, RM ANOVA on ranks, n = 12)
Comparison of γ powers among IH (2766.07 (1061.65, 3374.45) μV2, n = 10), DH (734.60 (570.43, 2763.96) μV2, n = 14), and VH (560.08 (227.10, 1528.12) μV2, n = 12) in control conditions, there was only a statistically significant difference in γ powers between IH and VH (*P < 0.05, Kruskal–Wallis one way ANOVA on Ranks followed by post-hoc test, Dunn’s Method). A two-way non-parametric ANOVA for γ powers in three different sub-regions of hippocampal slices before and after application of different concentrations of ethanol revealed a significant main effect of the region (F(2,33) = 21.272, P < 0.001) and a significant main effect of ethanol concentrations (F(2,33) = 14.171, P < 0.001). There was a significant interaction effect between region and ethanol concentrations (F(4,33) = 5.624, P < 0.001).
Discussion
Our results show that acute ethanol exposure affect γ oscillation of CA3 area of hippocampus in a region-dependent manner: significant inhibition of γ oscillation was seen in the intermediate hippocampus for both low and high concentration of ethanol, the inhibition was only seen in the dorsal hippocampus at high concentration of ethanol and no inhibition can be seen in ventral hippocampus even at a high concentration of ethanol. Acute ethanol intoxication impaired mental abilities at the blood concentrations > 0.25% (~54 mM), which is in line with inhibition of γ oscillations seen in dorsal and intermediate hippocampus.
The intermediate and dorsal hippocampus are involved in the spatial information encoding and execution (Kenney and Manahan-Vaughan 2013). The dorsal hippocampal CA1 riches in dense place cell coding spatial location; subiculum of dorsal hippocampus contains a lot of “head direction” cell coding head space location (Taube 2007). The ventral hippocampus mainly involved in non-spatial information learning and memory, encoding and storage of emotion, and affection information (Fanselow and Dong 2010). Acute ethanol can lead to spatial memory deficits, without prejudice, and even facilitate non-spatial memory, suggesting that ethanol may not affect ventral hippocampal function (Hoffmann and Matthews 2001; Matthews and Silvers 2004). In consistent with these studies, our results show that acute ethanol exposure inhibited the intermediate and dorsal hippocampal γ oscillation, which is consistent with the distinctive function of these two hippocampal regions: the involvement of spatial learning and memory formation and that acute ethanol have no significant effect on γ oscillation in ventral hippocampus.
Different regions of hippocampal formation differ in the local circuit. The intermediate hippocampus receive the input from the entorhinal cortex intermediate band (Burwell 2000) and amygdala nucleus (Petrovich et al. 2001; Pitkanen et al. 2000) and project to the amygdala (Pitkanen et al. 2000; Kishi et al. 2006); the dorsal hippocampus send projections to the subiculum (Traub et al. 2004), cingulate gyrus (Cenquizca and Swanson 2007; Van Groen and Wyss 2003), mammillary nuclei (Ishizuka 2001), and septal nucleus and receive inputs from these structures (Risold et al. 1997); the ventral hippocampus project to the olfactory bulb/cortex, accumbens nucleus, and amygdala (Kishi et al. 2006; Cenquizca and Swanson 2007) and receive fibers from the above nucleus (Petrovich et al. 2001; Pitkanen et al. 2000). The above results indicate that the different local circuit in different hippocampal regions may result in distinctive responsiveness to ethanol. However, most of the connection from outside of hippocampus was disrupted for a hippocampal slice in vitro; it is unlikely that our results are related to the circuit connectivity.
The difference of receptor and intracellular kinase levels or function in three areas of hippocampus may contribute to the region-related difference in the sensitivity of ethanol on γ oscillations. NMDA receptor mediates excitatory synaptic transmission and plays an essential role in learning and memory. Acute ethanol inhibits NMDA receptor-mediated currents (Lovinger et al. 1990; Proctor et al. 2006; Xu and Woodward 2006) and NMDA receptor-dependent long-term potentiation (LTP) (Blitzer et al. 1990). Recent study show that the pre-blocking NMDA receptor enhanced ethanol suppression of γ oscillation, suggesting that NMDA receptor is involved in ethanol inhibition of γ oscillation (Wang et al. 2016). Interestingly, compared with dorsal hippocampus, ventral hippocampus showed lower levels of mRNA and protein expression for NR2A and NR2B subunits (Pandis et al. 2006), which probably explain the less effect of ethanol on γ oscillation of ventral hippocampal slice due to less availability of NMDAR.
Acute ethanol (50–100 mM) is known to evoke dopamine release and activates type 1 dopamine receptor (D1R), adenylate cyclase (AC), and cAMP-dependent protein kinase A (PKA) (Ferrani-Kile et al. 2003; Rabin et al. 1992; Moonat et al. 2010; Coller and Hutchinson 2012; Lovinger 2002). In line with this, the recent study demonstrate that ethanol suppressed γ oscillation in intermediate hippocampal slices via D1R but not D2R activation (Wang et al. 2016). Interestingly, D1R not D2R in dorsal hippocampus play a role in social learning (Matta et al. 2017). The role of dorsal D1R on social learning or the role of intermediate D1R on γ oscillations may be related to abundant expression of D1R in dorsal and intermediate regions of hippocampus, and/or the less amounts of D2R expression or the reduced inhibitory role of D2R on AC and cAMP production, as it is known that D1-class receptors stimulate AC activity and the D2-class receptors have an opposite effect on AC activity. Indeed, D2R expression is lower in dorsal than in ventral hippocampus (Dubovyk and Manahan-Vaughan 2019). A low expression of D2R may trigger D1R hypersensitivity to ethanol and ultimately leading to compulsive-like drinking (Bocarsly et al. 2019).
In addition, acute ethanol exposure also affects intracellular kinases such as Akt/Gsk3β signaling. Acute ethanol activates Akt/protein kinase B and glycogen synthase kinase 3beta (GSK3β) (Zeng et al. 2012). Interestingly, compared with dorsal area, GSK3β activity is reduced in ventral hippocampus, in agreement with GSK3β findings; Akt is more active ventrally than dorsally (Fuster-Matanzo et al. 2011). Because acute ethanol suppression of γ oscillation in intermediate hippocampus was involved in GSk3β activation (Wang et al. 2016), the more active GSK3β in dorsal than ventral hippocampus may well explain the region-related difference in the sensitivity of γ oscillation to ethanol seen in this study.
Thus, ethanol suppression of γ oscillation in dorsal and intermediate hippocampus may be related to the D1R-PKA activation, lower D2R expression and GSK3β activity. On the other hand, no effect of ethanol on ventral hippocampal γ oscillation is likely related to the reduced NMDAR expression, lower GSK3β activity, and higher D2R expression.
The ethanol suppression of γ oscillation in intermediate and dorsal hippocampus indicate that ethanol may impair IH-related fast learning and the short-term spatial memory (Bast et al. 2009; Bast 2007) and DH-related long-term spatial memory formation (Kemp and Manahan-Vaughan 2007). No effect of γ oscillation in ventral hippocampus may suggest that VH-related emotional activity will be less affected by ethanol.
In vivo hippocampal γ band activity can be divided into slow (25–55 Hz) and fast γ (60–100 Hz) oscillations, which are proposed to correlate with distinct functional states in hippocampal network (Jiang et al. 2020; Colgin 2016). However, in vitro hippocampal γ band is limited to the slow γ band activity (25–55 Hz) due to the nature that the connections from the outside are disrupted. Though there are multiple peaks seen in the power spectra in our results (Figs. 2, 3, and 4b), the higher frequency peaks are less likely a real event of oscillation but likely the harmonic, as these peaks are exactly two or three times of the first peak.
In summary, our results demonstrate that the modulation of ethanol on γ oscillation is hippocampal region-dependent, further confirm the distinctive function in different hippocampal regions among DH, IH, and VH.
References
Ariwodola OJ, Weiner JL (2004) Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci 24(47):10679–10686
Bast T (2007) Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci 18:253–282
Bast T, Wilson IA, Witter MP, Morris RGM (2009) From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol 7(4):e1000089
Blitzer RD, Gil O, Landau EM (1990) Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res 537(1–2):203–208
Bocarsly ME, da Silva e Silva D, Kolb V, Luderman KD, Shashikiran S, Rubinstein M, Sibley DR, Dobbs LK, Alvarez VA (2019) A mechanism linking two known vulnerability factors for alcohol abuse: heightened alcohol stimulation and low striatal dopamine D2 receptors. Cell Rep 29(5):1147–1163.e5
Burwell RD (2000) The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci 911:25–42
Campbell AE, Sumner P, Singh KD, Muthukumaraswamy SD (2014) Acute effects of alcohol on stimulus-induced gamma oscillations in human primary visual and motor cortices. Neuropsychopharmacology 39(9):2104–2113
Cenquizca LA, Swanson LW (2007) Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 56(1):1–26
Colgin LL (2016) Rhythms of the hippocampal network. Nat Rev Neurosci 17(4):239–249
Coller JK, Hutchinson MR (2012) Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 134(2):219–245
Dubovyk V, Manahan-Vaughan D (2019) Gradient of expression of dopamine D2 receptors along the dorso-ventral axis of the hippocampus. Front Synaptic Neurosci 11:28
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65(1):7–19
Ferrani-Kile K, Randall PK, Leslie SW (2003) Acute ethanol affects phosphorylation state of the NMDA receptor complex: implication of tyrosine phosphatases and protein kinase a. Brain Res Mol Brain Res 115(1):78–86
Flentke GR, Garic A, Hernandez M, Smith SM (2014) CaMKII represses transcriptionally active beta-catenin to mediate acute ethanol neurodegeneration and can phosphorylate beta-catenin. J Neurochem 128(4):523–535
Fries P, Nikolic D, Singer W (2007) The gamma cycle. Trends Neurosci 30(7):309–316
Fuster-Matanzo A et al (2011) Different susceptibility to neurodegeneration of dorsal and ventral hippocampal dentate gyrus: a atudy with transgenic mice overexpressing GSK3β. PLoS One 6(11):e27262
Harper C (1998) The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol 57(2):101–110
Hoffmann SE, Matthews DB (2001) Ethanol-induced impairments in spatial working memory are not due to deficits in learning. Alcohol Clin Exp Res 25(6):856–861
Ishizuka N (2001) Laminar organization of the pyramidal cell layer of the subiculum in the rat. J Comp Neurol 435(1):89–110
Izumi Y, Nagashima K, Murayama K, Zorumski CF (2005) Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136(2):509–517
Jiang H et al (2020) Distinct directional couplings between slow and fast gamma power to the phase of theta oscillations in the rat hippocampus. Eur J Neurosci 51(10):2070–2081
Kemp A, Manahan-Vaughan D (2007) Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci 30(3):111–118
Kenney J, Manahan-Vaughan D (2013) Learning-facilitated synaptic plasticity occurs in the intermediate hippocampus in association with spatial learning. Front Synaptic Neurosci 5:10
Kishi T, Tsumori T, Yokota S, Yasui Y (2006) Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol 496(3):349–368
Lovinger DM (2002) NMDA receptors lose their inhibitions. Nat Neurosci 5(7):614–616
Lovinger DM, White G, Weight FF (1990) NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 10(4):1372–1379
Lu CB, Jefferys JGR, Toescu EC, Vreugdenhil M (2011) In vitro hippocampal gamma oscillation power as an index of in vivo CA3 gamma oscillation strength and spatial reference memory. Neurobiol Learn Mem 95(3):221–230
Matta R, Tiessen A, Choleris E (2017) The role of dorsal hippocampal dopamine D1-type receptors in social learning, social interactions, and food intake in male and female mice. Neuropsychopharmacology 42(12):2344–2353
Matthews DB, Silvers JR (2004) The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem 82(3):299–308
Moonat S, Starkman BG, Sakharkar A, Pandey SC (2010) Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci 67(1):73–88
Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36(5):357–368
Pandis C, Sotiriou E, Kouvaras E, Asprodini E, Papatheodoropoulos C, Angelatou F (2006) Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience 140(1):163–175
Petrovich GD, Canteras NS, Swanson LW (2001) Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev 38(1–2):247–289
Pitkanen A et al (2000) Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 911:369–391
Proctor WR, Diao L, Freund RK, Browning MD, Wu PH (2006) Synaptic GABAergic and glutamatergic mechanisms underlying alcohol sensitivity in mouse hippocampal neurons. J Physiol 575(Pt 1):145–159
Rabin RA, Edelman AM, Wagner JA (1992) Activation of protein kinase A is necessary but not sufficient for ethanol-induced desensitization of cyclic AMP production. J Pharmacol Exp Ther 262(1):257–262
Risold PY, Thompson RH, Swanson LW (1997) The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev 24(2–3):197–254
Taube JS (2007) The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30:181–207
Traub RD, Bibbig A, LeBeau FEN, Buhl EH, Whittington MA (2004) Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci 27:247–278
Van Groen T, Wyss JM (2003) Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463(3):249–263
Wang J et al (2016) Acute ethanol inhibition of γ oscillations is mediated by Akt and GSK3β. Front Cell Neurosci 10:189
Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, Both M, Tort ABL, Kopell NJ, Wisden W, Monyer H (2009) Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A 106(9):3561–3566
Xu M, Woodward JJ (2006) Ethanol inhibition of NMDA receptors under conditions of altered protein kinase a activity. J Neurochem 96(6):1760–1767
Zeng T, Zhang CL, Song FY, Zhao XL, Yu LH, Zhu ZP, Xie KQ (2012) PI3K/Akt pathway activation was involved in acute ethanol-induced fatty liver in mice. Toxicology 296(1):56–66
Funding
This study was supported by the National Natural Science Foundation of China (NSFC, grant numbers: 81271422; U1804170; 81100953; 1070938), International Collaboration of Henan Province Science-Technique Bureau (134300510040), Natural Science Foundation of Hebei Province (H2012203067) and Key Program for Applied Basic Research of Hebei Key Program for Applied Basic Research of Hebei Province (12966119D).
Author information
Authors and Affiliations
Contributions
ZRL and LCB designed and supervised the experiments; LZH, YB, and GFL performed the experiments; RF, ZHX, ZXY, LZH, and LCB analyzed the data. LCB, LZH, and ZRL wrote and revised the manuscript; All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Ren, F., Yang, B. et al. Region-dependent regulation of acute ethanol on γ oscillation in the rat hippocampal slices. Psychopharmacology 237, 2959–2966 (2020). https://doi.org/10.1007/s00213-020-05584-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05584-y