Abstract
Rationale
Given that tetrahydrocannabinol (THC) and nicotine have similar effects on negative affect (NA), we hypothesized that a 7-mg nicotine patch (NP) would reduce NA-related cannabis (CAN) withdrawal symptoms in cannabis-dependent (CD) individuals who were not nicotine dependent.
Objective
We sought to determine whether NP reduces NA across 15 days of CAN abstinence in two groups: non-tobacco smokers (NTS) and light tobacco smokers (LTS).
Methods
CD participants (N = 127; aged 18–35) who used CAN at least 5 times/week for the past 12 + months were randomized to (1) NP or (2) a placebo patch (PP) and received $300 for sustained biochemically verified CAN abstinence. Of those randomly assigned, 52 of 63 NP, and 56 of 64 PP maintained biochemically verified CAN abstinence and 51 NP and 50 PP participants complied with all aspects of the study. Affect and other withdrawal symptoms were measured every 48 h across 15 days of CAN abstinence.
Results
After controlling for age, tobacco use, baseline THC concentration, and baseline measurements of the dependent variable, NP reduced NA symptoms across the 15-day treatment relative to PP. Differences in NA and CAN withdrawal symptoms were not moderated by tobacco user status.
Conclusions
The findings provide the first evidence that NP may be able to attenuate NA-related withdrawal symptoms in individuals with cannabis use disorder who are not heavy users of tobacco or nicotine.
Clinical trials registry
NCT01400243 http://www.clinicaltrials.gov
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis withdrawal symptoms (CWS) constitute an important syndrome that requires appropriate understanding, assessment, and treatment in various therapy settings. Cannabis use disorders (CUDs) are associated with significant health and mental health problems, as recognized by the DSM-5 (American Psychiatric Association 2013). CAN use and cannabis use disorders have increased dramatically over the past decade (Hasin et al. 2019). Because of the potential adverse effects of heavy CAN use, treatment for CUD was sought by 138,000 people in the USA in 2015 (Center for Behavioral Health Statistics and Quality 2015). Among the problem characteristics of CUD are its DSM-5-listed withdrawal symptoms, which include increases in negative affect (irritability-anger-aggression, nervousness-anxiety, and depressed mood), restlessness, sleep difficulty (i.e., insomnia, disturbing dreams), decreased appetite or weight loss, and at least one of a group of physical symptoms (abdominal pain, shakiness/tremors, sweating, fever, chills, or headache). It is believed by some that these symptoms make abstinence difficult in some of those attempting to abstain from CAN use (Budney et al. 2001). Though some small studies (e.g., Arendt et al. 2007) failed to find that CWS failed to predict relapse, there appear to be no well-powered, rigorous studies of the association of CWS severity to relapse.
Convergent evidence suggests that some use of MJ is motivated in part by attempts to decrease negative mood and emotional states, including those associated with CAN withdrawal. Large national surveys have demonstrated that individuals with social anxiety disorder and panic disorder are strongly disposed to CAN dependence (Agosti et al. 2002; Lynskey et al. 2002; Green et al. 2003). The use of CAN increased among Manhattan residents after the 9/11 terrorist attacks (Vlahov et al. 2002). Studies of daily CAN smokers have shown more overall anxiety, irritability, depressed mood, and decreased appetite compared with controls during a 28-day period of supervised abstinence (Kouri and Pope 2000; Budney et al. 2001, 2008). However, the effects of pharmacotherapies on CAN withdrawal symptoms have received relatively little attention.
Pharmacotherapy of withdrawal and promotion of abstinence is associated with improved treatment outcomes for nicotine (Jorenby et al. 2006) and opioid dependence (Kreek 2000). Haney and colleagues have conducted well-controlled trials assessing the effects of different pharmacotherapies on abstinence and withdrawal symptoms. For example, Haney et al. (2008) found that a combination of THC and lofexidine (an α2-adrenergic agonist that attenuates norepinephrine release) attenuated cannabis withdrawal and decreased relapse and found that nabilone (an FDA-approved synthetic analog of THC) decreased CWS and a laboratory measure of CAN relapse (Haney et al. 2013). Norepinephrine (NE) release is increased centrally during CAN withdrawal (Hart 2005) and other α2-adrenergic agonists can decrease extracellular NE levels in certain conditions, possibly by stimulation of autoreceptors that decrease functional adrenergic activity (Carter 1997). Clinical trials have shown that rimonabant (a cannabinoid type 1 receptor blocker) may be effective in curbing craving for food and nicotine (Gelfand and Cannon 2006) and pretreatment with rimonabant significantly decreased the physiological and psychological effects of CAN (Huestis et al. 2001). In contrast, a recent randomized clinical trial found that the combination of lofexidine and dronabinol (a partial cannabinoid receptor agonist) to be no more efficacious than placebo in individuals with CUD (Levin et al. 2016). Furthermore, preliminary evidence suggests that bupropion, a NE and dopamine reuptake inhibitor effective in the treatment of depression and tobacco smoking, may worsen NA associated with CAN abstinence likely because of its enhancing adrenergic functioning (Haney et al. 2001).
Though the data is mixed, evidence suggests that it is reasonable to continue to test the hypothesis that nicotine may reduce CAN withdrawal and promote abstinence by one of several potential mechanisms. Nicotine modulates functional NE release in the hippocampus (Barik and Wonnacott 2006) in a manner somewhat like the above-noted α2-adrenergic agonist, lofexidine (Haney et al. 2008). Nicotine can also reduce functional NE activity by stimulating α2 autoreceptors that in turn decreases overall NE release (Wonnacott 1997). Nicotine-induced decrease in NE release may desensitize NE receptors and thereby have functional effects similar to those associated with lofexidine (Sánchez-Merino et al. 1995). Scherma et al. (2016) reviewed preclinical evidence of several interactions between the endocannabinoid and nicotinic cholinergic systems, including findings suggesting that the α7 nicotinic-cholinergic receptor activation by nicotine may inhibit the development of CUD.
Supporting the possibility that nicotine patch (NP) or other forms of nicotine could prove to be an efficacious treatment of CUD, Solinas et al. (2007) presented evidence supporting their view that α7 nicotinic receptors may be an effective new target for the treatment of CUD and/or CWS. Their experimental evidence showed that blockade of α7 nicotinic receptors reversed the behavioral and neurochemical effects of cannabinoids. The literature review by Viveros et al. (2006) noted many parallels, contrasts, and interactions between nicotine and cannabinoids on brain and behavioral functioning. Both CAN (Corrigall et al. 1994) and nicotine (Gerdeman et al. 2003; Lupica and Riegel 2005) promote the release of mesolimbic dopamine contributing to the reinforcing properties of both substances. Consistent with the notion that a common biological disposition contributes to polysubstance abuse, individual differences in dopamine tone have been hypothesized to predict disposition to the abuse of many drugs (Blum et al. 2000; Lupica and Riegel 2005). These observations provide support for the view that there should be a high priority for testing the hypothesis that nicotine and other nicotinic agonists can reduce THC withdrawal symptoms and promote CAN abstinence in those attempting to quit using CAN. A finding that nicotine reduces CWS could have social and clinical benefits, possibly leading to a standard treatment of cannabis dependence and/or to the development of new cholinergic agonist therapies based on the observed efficacy of nicotine therapy.
In contrast to our hypothesis, a recent finding that, relative to normal nicotine cigarettes, low-nicotine cigarettes did not impact self-reported CAN intake (Pacek et al. 2016) could appear to be contrary to our hypotheses that nicotine promotes CAN abstinence. The study by Pacek et al. (2016) used participants who were not cannabis dependent (averaged 11.3 days of CAN use/past month). In contrast to the mixed findings in humans, studies using animal models have found interactions of nicotine with THC on CAN use-related behavior and brain mechanisms reviewed by Viveros et al. (2006), including dopamine release in the mesolimbic system, enhanced endogenous opioids release, stimulation of adrenocortical activity, anxiolytic, and other behavioral effects. There are also a number of behavioral similarities between THC and nicotine, as well as documented behavioral interactions between these two drugs (Viveros et al. 2006). For example, users report that both drugs produce relaxation, are used to cope with stress, and to more generally enhance positive and to attenuate negative affect (Green et al. 2003; Viveros et al. 2006; Martens and Gilbert 2008). Interactions of nicotine with THC include rodent studies indicating additive effects of the drugs reinforcing effects, THC potentially reducing nicotine withdrawal symptoms, but nicotine enhancing somatic THC withdrawal symptoms (Valjent et al. 2002; Viveros et al. 2006). In contrast, nicotine and THC have the opposite effects on hunger and on cognitive performance, with nicotine decreasing hunger and enhancing cognitive performance (reviewed by Viveros et al. 2006). However, no studies to date have assessed the aims of the current proposal, which are to assess the efficacy of nicotine in those with CUD who are not nicotine dependent and approximately half of whom have no history of nicotine use.
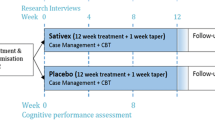
The study was not designed with the expectation that the methods we used would be used in normal clinical practice. Instead, it was designed to understand and provide a rigorous assessment of CAN withdrawal symptoms that may drive some individuals in real-world situations to relapse to chronic CAN use. The objective of this randomized controlled trial was to determine whether, relative to placebo patch (PP), low-dose (7 mg) NP reduces negative affect (NA) and CWS across 15 days of CAN abstinence in cannabis-dependent (CD) individuals who were either non-tobacco users or light tobacco smokers as defined by a baseline urine cotinine concentration < 150 ng/ml and self-reported by timeline follow-back assessments of no more than 5 cigarettes per day. Besides the initial orientation and consent sessions, participants completed four pre-quit monitoring sessions (twice per week, beginning 2 weeks immediately prior to quitting) during which they completed the below-noted mood and withdrawal measures, and eight post-quit monitoring sessions (on post-quit days 1, 3, 5, 7, 9, 11, 13, and 15 of abstinence). The four baseline sessions were used to help stabilize the downward drift of reported NA and CWS due to the repeated administration of questionnaires that are nearly universally found independent of interventions (Sharpe and Gilbert 1998; Arrindell 2001; Gilbert et al. 2019). For reasons noted in the methods, measures from the first day of abstinence were not included in the statistical analyses. We assessed heart rate and blood pressure because of the well-established fact that nicotine increases heart rate and in some cases blood pressure (Benowitz et al. 2002), while abstinence from THC has been reported to be associated with increases in blood pressure (Vandrey et al. 2011).
Methods
Participants
Individuals with DSM-5-assessed CUD, who reported on timeline follow-backs (the mean of four 3-month timelines, as well as the preceding month) 10 + CAN uses/week across the preceding year, were recruited from 2011 to 2014 by university postings and newspaper ads in Carbondale, IL, and surrounding communities. A great majority (98.6%) of uses were by smoking (joints, blunts, one-hitters, pipes, bongs, bowls, chillums). Female uses were predominantly via blunts (73.9%), while males’ use of blunts was lower (42.9%) due to their greater use of bowls and pipes (32.5% vs. 14.6% in females). Joints, bongs, vapes, and edibles in combination constituted approximately 10% of uses across both genders. Only one individual primarily vaped, and edibles were rarely used (0.3% of uses). Inclusionary criteria included CAN use on 10 + occasions per week, willingness to abstain from CAN and tobacco/nicotine for 15 days and a reported age between 18 and 50 years, baseline urine cotinine concentration assessed via NicAlert® test-strip value “3” (100–200 ng/ml) or lower (a concentration indicating minimal or no nicotine intake) during the nicotine-free baseline period (Blackford et al. 2006), and reported use of fewer than 5 tobacco cigarettes/day or equivalent tobacco/nicotine use during the past 2 years. Exclusionary criteria included weekly use of psychoactive drugs or medications (other than CAN, alcohol, or caffeine), tobacco smoking more than 5/day, alcohol use > 28 alcoholic drinks per week, age < 18 or > 50 years, non-English speaking, education < 12 years, atypical sleep cycles, pregnancy, body mass indices below 18.5 or above 35, use of creatinine-containing supplements, and current DSM-IV-TR assessed diagnoses of major depression or psychotic disorder. Participants received medical clearance by an in-house physician and procedures were approved by the local Institutional Review Board (IRB).
Verification of THC intake and abstinence
To establish a reliable pre-quit baseline and subsequent decreases in THC intake, a quantitative urine index of THC intake concentration was assessed by ExperTox® (Deer Park, TX) using gas chromatographic-mass spectroscopy of 11-nor-9-carboxy-9-tetrahydrocannabinol (THCCOOH), the primary THC metabolite. THCCOOH was normalized with urine creatinine concentration to obtain a THC metabolite-creatinine ratio (CN-THCCOOH). These normalized THC concentrations are presented in Fig. 1. The individual with the lowest baseline creatinine-normalized 11-nor-9-carboxy-Δ9-tetrahydrocannnabinol (CN-THCCOOH) concentration in our sample was 42 ng/mg, which is substantially greater than the approximately 30 ng/mg sample mean concentration observed in a cannabis treatment study by Schuster et al. (2016).
To verify continuous THC abstinence during the quit phase, we used the THC abstinence criteria algorithm developed by Schwilke et al. (2011) that estimates the probability of new THC use from CN-THCCOOH values at a given number of days of THC abstinence with the values at the pre-quit baseline. The algorithm also uses different abstinence criteria as a function of different baseline CN-THCCOOH concentrations. We used the 95% confidence interval of a CN-THCCOOH concentration as indicating a new use of THC during the abstinence period. Only three of the 101 included participants failed to meet the criteria across all assessments beginning with day 5 of abstinence. All participants included in our statistical analyses of the effects of abstinence on withdrawal symptoms and affect met the Schwilke abstinence criteria across each of the latter assessment days (7, 9, 11, 13, and 15). We included the two participants not meeting the CN-THCCOOH criteria on day 5, because they met the criteria on all subsequent days and because 2–3 individuals would be expected to have false positive value indicating CAN use when there, in fact, was no use, given the 95% threshold and concentrations indicating clear compliance from day 7 onward. Urine specimens were also tested for psychoactive drugs (cocaine, cannabis, opiates, amphetamines, methamphetamine, phencyclidine, benzodiazepine, methadone, barbiturate, tricyclic antidepressants, oxycodone, and propoxyphene with built-in adulteration strips for creatinine, oxidants, and pH, including a temperature strip in the back of each cup) using the qualitative ICup™ 12 panel Urine Drug Test. Specimens were also evaluated with a Urine Check 7-drug alteration test for the detection of diluted or adulterated urine. For those in the PP group, subjects were judged nicotine-abstinent from tobacco if urine cotinine levels were below a cutoff of 50 ng/ml. Results showed that 51 of the 63 individuals in the NP group and 50 of the 64 in the PP group maintained biochemically verified CAN abstinence and complied with all other aspects of the study.
As outlined in the study flow chart (Online Resource), of the 1596 individuals who inquired about the study and were screened for potential eligibility by phone, 567 were eligible for in-person screening interviews, 420 showed up for in-person interviews, and of these, 146 were consented. Of the 146, 19 dropped out prior to the randomization to treatment/abstinence phase, leaving 127 individuals who entered abstinence with patch treatment. Of those not meeting eligibility criteria, 751 failed to use CAN > 10 times/week across 5 + days or CAN for less than a year, 186 used tobacco/nicotine more than 35 times/week, 95 had serious medical or psychiatric conditions, 14 had BMIs in excess of 35, 6 drank more than 35 alcoholic drinks/week, 35 used illicit drugs routinely, and 2 were not available for lab sessions. Most (101/127) of those entering treatment complied with study requirements, including meeting the above-noted CAN abstinence criteria throughout the full 15 days of abstinence (i.e., without reported or CN-THCCOOH ratio-assessed slips), and used the nicotine patch continuously throughout the 15 days as assessed by urinary cotinine values using NicAlert® test strips (nicotine patch group mean test-strip value = 5.42 [0.88 SD] reflecting cotinine values between 500 and 2000 ng/ml, placebo patch group value = 0.53 [0.67 SD] reflecting cotinine values between below 10 ng/ml). Test-strip values of less than “4” (200–500 ng/ml) on any one or more post-quit assessment sessions were considered to reflect the failure to comply with patch use requirements in the nicotine group. In the PP group, values of greater than “2” (30–100 ng/ml) were used as indicators of nicotine use, though no participant exceeded this value. Of the 26 participants excluded from the present treatment effects analysis, 19 clearly did not comply with CAN abstinence as indicated by the Schwilke et al. (2011) algorithm, while 6 participants had cotinine assays suggesting a large degree but not full compliance with the study protocol for patch use. Only individuals unambiguously complying with study requirements were included in the statistical analyses of the effects of treatment.
Structured interview for DSM-IV-TR (SCID-research version)
The SCID is a semi-structured interview designed for use by trained lay interviewers for the lifetime assessment of mental disorders (First et al. 2002) using DSM-IV-TR criteria (American Psychiatric Association 2000). The DSM-IV-TR research version of the SCID was administered to all participants during the screening and orientation sessions by an interviewer with extensive training and experience with the SCID. The interviewer’s work and diagnoses were closely reviewed by a highly experienced licensed clinical psychologist. The research version of the SCID-IV-TR assesses 11 of the 12 criteria used in the DSM-5 version to diagnose CUD. The one item not assessed was “craving” something that we assessed using the CAN version of the Questionnaire for Smoking Urges (Tiffany and Drobes 1991). Thus, we were able to assure that all participants had a DSM-5 diagnosis of CUD and also were able to assess the current severity of CUD on the DSM-5 scale of mild (presence of 2–3 of the 12 items), moderate (4–5 items), and severe (6 or more items). The 12 CUD items cover areas assessing tolerance, withdrawal, use despite social/interpersonal/legal problems, neglected major roles, and repeated attempts to quit or control use over the past 12 months.
All consented individuals met the DSM-5 criteria for CUD, with the mean number of symptoms being 5.78 (SD = 1.1) and mean CUD severity of 2.66 (SD = 0.5) on the DSM-5 three-point severity scale. The characteristics of the study sample are shown in Table 1. Study completers included roughly equal numbers of African Americans and European Americans (42 and 49, respectively, with 11 other ethnicities). However, consistent with gender distributions of cannabis-dependent individuals, 75 of the study completers were male and 26 were female. Similar proportions of males and females were consented (M = 99; F = 47). Mean pre-quit baseline CAN uses per week were slightly greater for the nicotine (19.96) than for the placebo group (16.00), P < 0.05.
Questionnaires
Mood, withdrawal, and personality questionnaires included the following: The Profile of Mood States (POMS), (McNair et al. 1971), including the anger-irritability, sadness-depression, and tension-anxiety scales; the Marijuana Withdrawal Checklist (MWC), (Budney et al. 2001); and the Marijuana Problems Scale (Stephens et al. 1994) which assesses problems related to the use of CAN in the past 90 days using a list of 19 negative psychological, social, occupational, and legal consequences. Endorsements of items are counted to create indices of the total number of problems (range = 0–19; alpha = 0.90).
Procedure
An IRB-approved consent form was signed by each participant. Participants received $300 for biochemically verified abstinence and for completing twelve mood and urine monitoring sessions. All participants were asked to and were biochemically verified (cotinine) to have refrained from any tobacco use (other than that associated with smoking blunts) beginning at least 2 weeks prior to initiating study procedures and to continue to refrain throughout the study. Low concentrations of cotinine in the NP group would indicate failure to reliably use the NP during the quit phase. Abstinence was verified by urine cotinine concentrations and a completed pre-quit baseline phase that included assessments of mood, breath carbon monoxide (CO) concentration, and urine/saliva cotinine monitoring days. It should be noted that CO cannot distinguish CAN from tobacco smoking, but since both were prohibited, elevated (5 + ppm) CO was used for validation. Subjects were then randomly assigned by an urn technique without replacement approach, to one of the two treatment groups (NP or PP). Participants received counseling based on the guide “Getting Out of It: How to Cut Down or Quit Cannabis” (Mentha 2001).
Patches
In order to minimize the adverse effects of nicotine (primarily nausea and lightheadedness), we used the low-dose (7 mg) nicotine patch (Habitrol®) that has a slower nicotine blood rise time than other brands of patches (Gupta et al. 1993). This slow rise time greatly lessens the adverse effects of nicotine in non-tobacco users relative to fast blood-rise patches such as NicoDerm CQ® (Gilbert et al. 2003). Placebo patches were manufactured and blinded by Rejuvenation Labs (Salt Lake City, UT). All patches were obtained with NIDA funding and were not supplied or sponsored in any way and without any consultation, influence, or input from a drug or other company.
Data analysis
General linear mixed models (GLMM) (Koller and Stahel 2011) were calculated in order to determine the effect of the treatment (PP or NP) on NA (model 1), CAN withdrawal symptoms based on a total score for the MWC (model 2), and CAN craving based on a single item from the MWC (model 3) across 7 measurements within the 15-day time period (days 3, 5, 7, 9, 11, 13, and 15 of abstinence). Day 1 of abstinence was excluded from analysis because participants had been on the patch for only 4–8 h at the time of the first monitoring session and it takes several hours for maximal blood nicotine concentration to be achieved (Gupta et al. 1993); thus, much or most of the participants’ first day of abstinence up to the time of the day 1 assessment was spent with very low doses of blood nicotine. Each model included the following fixed independent variables: treatment group assignment (1 = NP, 2 = PP), time (1–7), baseline CN-THCCOOH measurements (continuous), tobacco user status (“0” = not a tobacco user, “1” = tobacco user), baseline measurements (mean of the final two pre-quit baseline) of the dependent variable (continuous), age, and a two-way interaction for time and treatment group assignment. Although we considered including random slopes in the models, our final presented models do not contain random slopes because (a) our sample size was small and (b) the variance on random slopes in our models were near zero. Analyses were conducted using the “robustlmm” package in R Studio (Koller 2016). Though the study was a double-blinded, randomized controlled trial, it differs from traditional RCTs in that the outcomes/dependent variables were not a dichotomous abstinent vs. relapsed category, but instead were based on continuous variables (symptom severities) analyzed only on the great majority of participants who maintain abstinence and who complied fully with study requirement. Thus, traditional intent-to-treat analyses were not appropriate or specified in our NIDA proposal for this study because the study focus was on CWS, not on the maintenance of abstinence. Finally, because “age” and “smoker status” were not significant in any of the analyses reported below, statistics related to these variables are included only in the Online Resource, where the full set of analytic values are presented for CWS and negative affect.
Results
Verification of THC abstinence
Mean post-abstinence CN-THCCOOH concentrations decreased during each of the seven assessments from the value of the preceding assessment. The CN-THCCOOH abstinence criteria algorithm developed by Schwilke et al. (2011) demonstrated 95% confidence that there were no uses after the first point of algorithm validity (day 5), except in three subjects where this criterion was not reached until day 7 of abstinence.
Verification of NP use and tobacco abstinence
NicAlert® dipsticks (JANT Pharmaceutical Corporation) were used to assess nicotine intake level prior (pre-quit) and on days 1, 3, 5, 9, 11, 13, and 15 after the quit date. The NicAlert® test yields a semiquantitative measure of nicotine’s major metabolite cotinine, using a colorimetric immunoassay reaction. The assay displays seven zones reflecting urine cotinine concentrations from “0” (0–10 ng/ml) to “6” (> 1000 ng/ml). There was a mean value of 5.42 (corresponding to an estimated 1000 ng/ml cotinine) in the NP group, relative to a value of 0.70 (estimated 10 ng/ml, nonsmoker range) in the placebo group, F(1,100) = 1259.07, p < 0.001. Six individuals in the NP group were not included in the study analyses because of low cotinine concentrations (< 100–200 ng/ml) on the NicAlert® 6-point scale during one or more of the seven post-quit phase assessments.
Expired breath CO concentrations were measured with a MiniCO™ meter (Vitalograph, Lenexa, Kansas). CO concentrations of < 5 ppm were used to help validate tobacco and CAN smoking abstinence. No individual from either group had a CO concentration of 5 ppm or greater during the abstinence period, something that helped validate our use of the three participants with slightly greater than expected day-5 CN-THCCOOH concentrations. Urine was also tested during each session for cannabis, antidepressants, cocaine, benzodiazepines, opioids, and methamphetamine using the qualitative ICup™ 10-panel Urine Drug Test (Instant Technologies, Norfolk, VA). No one was excluded after random assignment to treatment because of drug use. Chain-of-custody procedures with urine temperature measurement were used to assure valid sample use.
Effects of NP on heart rate and blood pressure
NP, relative to placebo (PP), had the expected cardiovascular activating effects on resting heart rate (HR) and blood pressure (BP). Relative to pre-quit-pre-patch baseline, NP was associated with an 8.5 BPM (1.29 SE) increase in mean HR, while the PP group had an increase of 1.02 BPM, (1.30 SE), F(1,99) = 16.78, p < 0.001, pη2 = 0.145, ePower = 0.982. NP also increased diastolic BP (M = 6.9 mmHg, 0.99 SE) relative to PP (M = 1.5 mmHg, 0.99 SE), F(1,99) = 14.87, p < 0.001, pη2 = 0.126, ePower = 0.963. The trend for NP to increase systolic BP relative to placebo did not achieve statistical significance, p = 0.107. There were no main effects for time or for time × patch type for HR or BP across the seven post-quit sessions statistically analyzed.
Blindness to patch assignment
Blindness of patch assignment was assessed with a questionnaire at the end of the abstinence day 15 assessment session. Those on the PP thought there was a 48.7% chance that they were on a nicotine patch with a difference from 0.5 chance (t, 49, p = 0.77, two-tailed), while those on NP thought that there was a 56.6% chance that they were on a nicotine patch with a difference from 0.5 chance (t, 50, p = 0.097, two-tailed). While these differences approached statistical significance in the NP group, they also indicate that neither group was accurate in guessing whether their patch contained nicotine or not. Thus, the drug-condition blind was largely maintained given the 50% chance of random guessing.
Adverse events
Adverse events (AEs) were assessed in all 127 individuals who initiated patch use. There were no serious adverse events, and as seen in Online Resource Table 1, the number of non-serious AEs was small and not systematically related to either the active patch or the placebo patch, except for more frequent reports of itchiness (NP = 14; PP = 6) and nausea (NP = 10; PP = 4), (p < 0.05, based on chi-square).
Effects of nicotine patch therapy on negative affect and cannabis withdrawal
POMS negative affect
Results (Table 2, Fig. 2) showed a significant interaction of treatment with time for POMS negative affect, b = 209.13e-5 [95% CI = 1.09e-5, 417.17e-5] such that the attenuation of NA by NP tended to be greater at later points in time (days 7, 11, 13, 15). The mean negative affect score for the NP group was different than for the PP group at days 7 (Cohen’s d = 0.2), 11 (Cohen’s d = 0.2), 13 (Cohen’s d = 0.2), and 15 (Cohen’s d = 0.2). Also, differences in NA did not differ by tobacco use status for all subjects, b = − 215.03e-5 [95% CI = − 1632.46e-5, 1202.40e-5]. A separate linear trend analysis of the decrease in POMS NA scores across the four pre-quit baseline days, prior to CAN abstinence and the onset of treatment, revealed a significant downward slope, t = − 19.44, p < 0.001. The treatment main effect across all points in time was not significant.
Negative affect (NA) decreased significantly across the four pre-quit baseline days and then increased beginning the third day of CAN abstinence. Note that the patches were applied beginning on the first day of quitting (post-quit day 1). The four baseline sessions occurred across the 2 weeks prior to the simultaneous initiation of abstinence and patch use at 2- to 4-day intervals. The decrease in NA across the four baseline days is consistent with previous studies demonstrating large downward drift testing effects on measures of NA. Asterisks indicate a significant small patch effect size (Cohen’s d = 0.2) at that time point. Across post-quit days 3–15, NA scores were lower in the NP than in the PP group. However, when examined day by day, on post-quit days 7, 11, 13, and 15 were lower in the NP group than in the PP group. The dependent variable was transformed with a log10 + 100 transformation because model residuals were not normally distributed
Marijuana withdrawal checklist
Another model (Table 3) showed that NP was associated with higher CAN withdrawal symptoms overall, b = − 67.72e-4 [95% CI = − 124.08e-4, − 11.36e-4]; however, there was a significant interaction effect for time and treatment group (“Patch”) on CAN withdrawal symptoms, b = 26.57e-4 [95% CI = 8.97e-4, 44.18e-4]. Measurements on the MWC, taken every 48 h across a 15-day time period for the PP and NP groups, are shown in Fig. 3. The NP group had greater values on post-quit day 3 (Cohen’s d = 0.3), day 5 (Cohen’s d = 0.2), and day 11 (Cohen’s d = 0.2). Differences in CAN withdrawal symptoms total score did not differ between the light and nonsmoker groups, b = − 16.80e-4 [95% CI = − 100.16e-4, 66.56e-4]. A separate analysis of the decrease in total MWC scores across the four pre-quit baseline days revealed a significant downward drift, t = − 14.97, p = 0.001.
Marijuana Withdrawal Checklist withdrawal symptoms decreased across the baseline days and then increased with the onset of abstinence, followed by a gradual decrease in craving. Note that the four baseline days (B1, B2, B3, B4) occurred prior to the onset of cannabis abstinence and simultaneous treatment onset. Asterisks indicate a significant small patch effect size (Cohen’s d = 0.2) at that time point. The increase in post-quit increase in withdrawal symptoms on post-quit days 3, 5, and 11 largely reflected increased nausea that was significantly greater in the nicotine than in the placebo group (see Table 2)
Marijuana withdrawal checklist craving item
A final model (Table 4) showed that treatment was not associated with CAN craving symptoms overall across the 15 days of abstinence; however, there was a significant interaction effect for time and treatment group (“Patch”) on CAN craving symptoms, b = 0.02e-1 [95% CI = 0.01e-1, 0.04e-1]. Measurements on the MWC craving are shown in Fig. 4. CAN craving symptoms decreased during the 15-day abstinence period to a greater degree for the NP group than the PP group, especially as measured on day 13 of cannabis abstinence. The mean MWC craving score for the NP group was different than for the PP group at baseline day 4 (Cohen’s d = 0.2) and on day 13 (Cohen’s d = 0.2).
CAN craving decreased across the baseline days and then dramatically further decreased with the onset of abstinence and did not differ between groups at any point after abstinence onset, except on day 13. Note that the four baseline days (B1, B2, B3, B4) occurred prior to the onset of cannabis abstinence and simultaneous treatment onset. Asterisks indicate a significant small patch effect size (Cohen’s d = 0.2) at that time point
Also, differences in CAN craving symptoms did not differ by tobacco use status for either treatment group, b = − 0.07e-1 [95% CI = − 5.76e-1, 5.61e-1]. A separate linear trend analysis of the decrease in CAN craving scores across the four pre-quit baseline days revealed a significant effect, t = − 15.30, p = 0.001. The large decrease in craving across the four baseline sessions was followed by a subsequent large decrease in craving with the onset of the abstinence period, t = − 3.25, p = 0.001.
Analyses of finding with previously dependent tobacco smokers excluded
Our nonsmoker group included only three former smokers (individuals who smoked more than 5 cigarettes/day [all prior to the past 2 years]). We ran follow-up analyses without these three individuals and found that the analyses were nearly identical, with interpretations on key variables remaining the same as our analysis including the 3 former smokers. As seen in our Online Resource Tables 2, 3 and 4, tobacco user status did not significantly influence outcome measures.
Discussion
Our findings support our primary hypothesis that low-dose (7 mg) NP therapy reduces negative affect-related CAN withdrawal symptoms as assessed by the well-validated Profile of Mood States (McNair et al. 1971) beginning 7 days after initiation of patch use and through at least 15 days of use in individuals with CUD, independent of whether they are light tobacco smokers or nonsmokers. Other important findings include (1) NP did not reduce total CAN withdrawal symptom scores assessed by the MWC and in fact increased nausea, which in turn increased total MWC score at several points in time, (2) NP had no reliable effects on MWC CAN craving scores, (3) the only frequent and statistically significant adverse effects of NP were nausea and itchiness near the patch application site, and (4) there were large downward drifts in withdrawal symptom scores, craving, and NA across the four pre-quit pretreatment baseline sessions, something that may have important implications for the present findings and future research assessing NA-related and other symptoms of CAN withdrawal severity. As detailed below, before concluding that NP-induced attenuation of NA-related CWSs is beneficial as a treatment aid, these findings need to be replicated and the relative importance of reductions in NA assessed in longer-term studies without large incentives for CAN abstinence so that the effects of NP on abstinence maintenance can be assessed in more natural conditions with typical high-relapse rates. Also, in some individuals wanting to quit their CAN use, the benefits of NA reduction may be offset by the increase in AEs and/or worsening of other CWSs. The present finding suggests that nausea and patch-site itchiness caused by NP may offset any potential beneficial effects of NP on NA, something that might be even more problematic in future studies using nicotine doses in excess of the presently used 7 mg.
We assessed NA, craving, and other “withdrawal” symptoms on four different days prior to the onset of treatment and abstinence initiation so that we could assess and attenuate post-quit downward drifts in these symptoms that are inherent to repeated assessments of NA. Downward drifts in NA the control groups consisting of ex-CAN users and non-CAN users have been observed in previous studies of CAN withdrawal symptoms (Kouri and Pope 2000; Budney et al. 2003), but these and other CAN studies have used only a single baseline assessment point and thus were not able to detect tendencies for downward drift in their CAN quit groups or controls prior to abstinence initiation. The limitations of single time point baseline is underscored by numerous investigations that have shown downward drifts in NA in non-abstinent general populations (Sharpe and Gilbert 1998) and during tobacco smokers prior to quitting (Gilbert et al. 2002, 2019). The importance of using extended pre-quit baselines in the assessment of abstinence symptoms can been seen when it is realized that the severity and duration of withdrawal symptoms have been shown to be greatly underestimated when only a single (initial) pre-quit session is used as the baseline because of the inherent tendency of NA-related scores to decrease independent of any intervention (Gilbert et al. 1998, 2002, 2019; Gilbert and Pergadia 2017). The use of multiple baselines allowed us to partly attenuate the effects of downward drift; as can be seen, if one compares, what would have happened to our NA and CWS severity estimates had we used the first two, rather than the last two baseline sessions to compute our change scores—the interpretation would have been that NA, craving, and other CWS were less severe and of lesser duration.
An even stronger experimental design to more accurately assess CWS and NA symptom severity would be to combine multiple baselines with a randomized delayed-quit no-medicated control group that would perform all of the assessments across the same time periods as used in the randomized NP and PP groups. Use of such a delayed-quit group would allow the characterization of inherent measurement drift across the full period of abstinence, not just the baseline. A number of tobacco withdrawal symptom studies have been conducted using delayed-quit group designs and the most recent of these studies concluded that “…the results strongly suggest that controlled trials using randomized delayed-quit groups with incentivized abstinence contingencies are as close as one can reasonably expect to get to a gold standard for scientific research on NA SAS [smoking abstinence symptom] trajectories, and that returning to control-group levels, rather than to baseline symptoms levels, is best practice when examining smoking abstinence-symptom resolution.” (Gilbert et al. 2019). The present findings suggest that randomized delayed-quit groups with incentivized abstinence contingencies are also as close as one can reasonably expect to get to a gold standard for scientific research on the severity and duration of CAN withdrawal symptoms.
Future studies attempting to characterize the severity of CAN abstinence symptoms, including craving, NA, and general withdrawal symptoms, would clearly benefit from the randomization of CUD individuals to one of three groups: (1) delayed-quit controls, (2) placebo controls, or to (3) active treatment. Such designs would allow that assessment of continued downward drift in self-report indices independent of abstinence. Another very important limitation of the study is that there was not a group of individuals randomized to a delayed-quit control group that continued to smoke and complete the NA and CWS questionnaires at the same intervals as the NP and PP groups abstained for 15 days. The effects of NP on NA and CWS could have been larger in groups motivated to quit CAN use. A delayed-quit group would have provided a control for downward drift and other variabilities in the NA and CWS scores across time and repeated testing, something that has proven to be critical in studies of tobacco abstinence symptoms (Gilbert et al. 1998, 2002, 2019).
Study limitations and future directions
There are many limitations to this investigation. First, the modest sample size limited the ability to precisely characterize how the effects of patch type may be modified by personality traits and individual differences in CAN, tobacco, and other drug use histories. Participant selection factors limit the generalizability of study findings. The study excluded individuals with current psychiatric disorders and frequent psychoactive drug use other than CAN, alcohol, and low-level tobacco use. The use of a single nicotine dose (7 mg) is a further limitation. Larger doses, possibly with a step-up from the 7-mg dose to avoid nausea and other adverse effects, may have proven to be more efficacious. Given that we only had three previously dependent tobacco smokers, we had no power to assess their differences in response to patch treatment from those of other participants. However, our statistical analyses revealed that our light tobacco smoker versus nonsmoker classification did not affect the measured outcomes. Females were greatly under-represented in the current sample, providing minimal power to detect sex differences. Nonetheless, our results may lack generalizability as many cannabis users are heavy smokers (Badiani et al. 2015). The time-demanding nature of the study also resulted in self-selection bias. While study funding limited the assessment to only 15 days of abstinence, a longer period of observation and study, with measurements at 30, 45, 60, and 90 days might provide a more convincing demonstration of the potential efficacy of NP. Additionally, the results will not necessarily generalize to the group of CAN-dependent smokers who are also nicotine dependent. It is not clear what the effect of NP would be on NA-related CWS or other CWS symptoms in nicotine-dependent tobacco smokers. It has been found that nontreatment-seeking tobacco smokers benefit less from well-established drug treatments for tobacco cessation than do treatment-seeking individuals desiring to quit (Perkins et al. 2006). Our use of individuals who were not treatment-seeking could have minimized or otherwise altered the effect of NP. Despite study limitations, the current results of this randomized controlled trial provide evidence that NA-related CAN withdrawal symptoms may be attenuated by 7-mg nicotine patch therapy relative to placebo in both light users and nonusers of tobacco. Finally, given the modest effect size for NA, one could question whether the effect size is clinically meaningful—something that only future research can answer.
In conclusion, it is important to emphasize that the large monetary contingencies assured that a great majority of the participants maintained biochemically verified abstinence. This high and non-differential rate of abstinence between the NP and PP groups was the goal of the study so that selective dropout/relapse would not bias focus of the study—characterization of the effect of NP on NA-related CWS severity as a function of treatment in a highly controlled situation. Thus, the present investigation was not designed to detect the efficacy of NP vs. PP on the maintenance of CAN abstinence, and our findings should not be viewed as suggesting anything concerning the efficacy of NP therapy on CAN abstinence success. However, the present findings suggest that NP may attenuate CAN abstinence-induced NA. Gaining an understanding of the mechanisms underlying this NA attenuation could lead to potential enhanced treatments for CUD.
References
Agosti V, Nunes E, Levin F (2002) Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse 28:643–652. https://doi.org/10.1081/ADA-120015873
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, fourth edition, text revision (DSM-IV-TR). American Psychiatric Association, Arlington, VA
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®), 5th edn. American Psychiatric Association, Washington, DC
Arendt M, Rosenberg R, Foldager L, Sher L, Munk-Jørgensen P (2007) Withdrawal symptoms do not predict relapse among subjects treated for cannabis dependence. Am J Addict 16:461–467. https://doi.org/10.1080/10550490701640985
Arrindell W (2001) Changes in waiting-list patients over time: data on some commonly-used measures. Beware! Behav Res Ther 39:1227–1247. https://doi.org/10.1016/S0005-7967(00)00104-2
Badiani A, Boden JM, De Pirro S et al (2015) Tobacco smoking and cannabis use in a longitudinal birth cohort: evidence of reciprocal causal relationships. Drug Alcohol Depend 150:69–76. https://doi.org/10.1016/j.drugalcdep.2015.02.015
Barik J, Wonnacott S (2006) Indirect modulation by α7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol 69:618–628. https://doi.org/10.1124/mol.105.018184
Benowitz NL, Jacob P III, Ahijevych K et al (2002) Biochemical verification of tobacco use and cessation. Nicotine Tob Res 4:149–159. https://doi.org/10.1080/14622200210123581
Blackford AL, Yang G, Hernandez-Avila M et al (2006) Cotinine concentration in smokers from different countries: relationship with amount smoked and cigarette type. Cancer Epidemiol Biomark Prev 15:1799–1804. https://doi.org/10.1158/1055-9965.EPI-06-0427
Blum K, Braverman ER, Holder JM et al (2000) The reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. J Psychoactive Drugs 32:1–112. https://doi.org/10.1080/02791072.2000.10736099
Budney AJ, Hughes JR, Moore BA, Novy PL (2001) Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry 58:917–924. https://doi.org/10.1001/archpsyc.58.10.917
Budney AJ, Moore BA, Vandrey RG, Hughes JR (2003) The time course and significance of cannabis withdrawal. J Abnorm Psychol 112:393–402. https://doi.org/10.1037/0021-843X.112.3.393
Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z (2008) Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abus Treat 35:362–368. https://doi.org/10.1016/j.jsat.2008.01.002
Carter AJ (1997) Hippocampal noradrenaline release in awake, freely moving rats is regulated by alpha-2 adrenoceptors but not by adenosine receptors. J Pharmacol Exp Ther 281:648–654
Center for Behavioral Health Statistics and Quality (2015) Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health
Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653:278–284. https://doi.org/10.1016/0006-8993(94)90401-4
First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition. 94–101
Gelfand EV, Cannon CP (2006) Rimonabant: a cannabinoid receptor type 1 blocker for management of multiple cardiometabolic risk factors. J Am Coll Cardiol 47:1919–1926. https://doi.org/10.1016/j.jacc.2005.12.067
Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci 26:184–192. https://doi.org/10.1016/S0166-2236(03)00065-1
Gilbert DG, Pergadia ML (2017) Nicotine withdrawal and depression. In: Hall FS, Young JW, Der-Avakian A (eds) Negative affective states and cognitive impairments in nicotine dependence. Elsevier, Philadelphia, pp 289–310
Gilbert DG, McClernon FJ, Rabinovich NE et al (1998) Effects of smoking abstinence on mood and craving in men: influences of negative-affect-related personality traits, habitual nicotine intake and repeated measurements. Pers Individ Dif 25:399–423. https://doi.org/10.1016/S0191-8869(98)00003-8
Gilbert DG, McClernon FJ, Rabinovich NE et al (2002) Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. J Consult Clin Psychol 70:142–152. https://doi.org/10.1037/0022-006X.70.1.142
Gilbert DG, Rabinovich NE, Rosenberger S (2003) Effects of fast and slow blood-rise nicotine patches on nausea and feeling states in nonsmokers. In: Ninth Annual Scientific Sessions of the Society for Research on Nicotine. New Orleans, LA
Gilbert DG, Rabinovich NE, Gilbert-Matuskowitz EA et al (2019) Smoking abstinence symptoms across 67 days compared with randomized controls—moderation by nicotine replacement therapy, bupropion, and negative-affect traits. Exp Clin Psychopharmacol. https://doi.org/10.1037/pha0000278
Green B, Kavanagh D, Young R (2003) Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev 22:453–460. https://doi.org/10.1080/09595230310001613976
Gupta SK, Benowitz NL, Jacob P III et al (1993) Bioavailability and absorption kinetics of nicotine following application of a transdermal system. Br J Clin Pharmacol 36:221–227. https://doi.org/10.1111/j.1365-2125.1993.tb04221.x
Haney M, Ward AS, Comer SD, Hart CL, Foltin RW, Fischman MW (2001) Bupropion SR worsens mood during marijuana withdrawal in humans. Psychopharmacology 155:171–179. https://doi.org/10.1007/s002130000657
Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW (2008) Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology 197:157–168. https://doi.org/10.1007/s00213-007-1020-8
Haney M, Cooper ZD, Bedi G, Vosburg SK, Comer SD, Foltin RW (2013) Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsychopharmacology 38:1557–1565. https://doi.org/10.1038/npp.2013.54
Hart CL (2005) Increasing treatment options for cannabis dependence: a review of potential pharmacotherapies. Drug Alcohol Depend 80:147–159. https://doi.org/10.1016/j.drugalcdep.2005.03.027
Hasin DS, Shmulewitz D, Sarvet AL (2019) Time trends in US cannabis use and cannabis use disorders overall and by sociodemographic subgroups: a narrative review and new findings. Am J Drug Alcohol Abuse 45:1–21. https://doi.org/10.1080/00952990.2019.1569668
Huestis MA, Gorelick DA, Heishman SJ, et al (2001) Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58:322. https://doi.org/10.1001/archpsyc.58.4.322
Jorenby DE, Hays J, Rigotti N, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study Group (2006) Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63. https://doi.org/10.1001/jama.296.1.56
Koller M (2016) Robustlmm : an R package for robust estimation of linear mixed-effects models. J stat Softw 75:1–24. https://doi.org/10.18637/jss.v075.i06
Koller M, Stahel WA (2011) Sharpening wald-type inference in robust regression for small samples. Comput Stat Data Anal 55:2504–2515. https://doi.org/10.1016/j.csda.2011.02.014
Kouri EM, Pope HG (2000) Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol 8:483–492. https://doi.org/10.1037/1064-1297.8.4.483
Kreek MJ (2000) Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann N Y Acad Sci 909:186–216
Levin FR, Mariani JJ, Pavlicova M, et al (2016) Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebocontrolled trial. Drug Alcohol Depend 159:53–60. https://doi.org/10.1016/j.drugalcdep.2015.11.025
Lupica CR, Riegel AC (2005) Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48:1105–1116. https://doi.org/10.1016/j.neuropharm.2005.03.016
Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG (2002) Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol Med 32:195–207. https://doi.org/10.1017/S0033291701005062
Martens KM, Gilbert DG (2008) Marijuana and tobacco exposure predict affect-regulation expectancies in dual users. Addict Behav 33:1484–1490. https://doi.org/10.1016/j.addbeh.2008.07.002
McNair DM, Lorr M, Droppleman LF (1971) Manual for the POMS. Educ. Ind. Test. Serv
Mentha H (2001) Getting out of it: how to cut down or quit cannabis. Australia: Inner East Community Health Service, Hawthorn
Pacek LR, Vandrey R, Dermody SS, Denlinger-Apte RL, Lemieux A, Tidey JW, McClernon F, Bangdiwala AS, Drobes DJ, al'Absi M, Strasser AA, Koopmeiners JS, Hatsukami DK, Donny EC (2016) Evaluation of a reduced nicotine product standard: moderating effects of and impact on cannabis use. Drug Alcohol Depend 167:228–232. https://doi.org/10.1016/j.drugalcdep.2016.08.620
Perkins KA, Stitzer M, Lerman C (2006) Medication screening for smoking cessation: a proposal for new methodologies. In: Psychopharmacology
Sánchez-Merino JA, Marin J, Balfagón G, Ferrer M (1995) Involvement of α2-adrenoceptors and protein kinase C on nicotine-induced facilitation of noradrenaline release in bovine cerebral arteries. Gen Pharmacol Vasc Syst 26:827–833. https://doi.org/10.1016/0306-3623(94)00259-P
Scherma M, Muntoni AL, Melis M, Fattore L, Fadda P, Fratta W, Pistis M (2016) Interactions between the endocannabinoid and nicotinic cholinergic systems: preclinical evidence and therapeutic perspectives. Psychopharmacology 233:1765–1777. https://doi.org/10.1007/s00213-015-4196-3
Schuster RM, Hanly A, Gilman J, Budney A, Vandrey R, Evins AE (2016) A contingency management method for 30-days abstinence in non-treatment seeking young adult cannabis users. Drug Alcohol Depend 167:199–206. https://doi.org/10.1016/j.drugalcdep.2016.08.622
Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, Pope HG, Huestis MA (2011) Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction 106:499–506. https://doi.org/10.1111/j.1360-0443.2010.03228.x
Sharpe JP, Gilbert DG (1998) Effects of repeated administration of the beck depression inventory and other measures of negative mood states. Pers Individ Dif 24:457–463. https://doi.org/10.1016/S0191-8869(97)00193-1
Solinas M, Scherma M, Fattore L et al (2007) Nicotinic 7 receptors as a new target for treatment of cannabis abuse. J Neurosci. https://doi.org/10.1523/jneurosci.0027-07.2007
Stephens RS, Curtin L, Simpson EE, Roffman RA (1994) Testing the abstinence violation effect construct with marijuana cessation. Addict Behav 19:23–32. https://doi.org/10.1016/0306-4603(94)90048-5
Tiffany ST, Drobes DJ (1991) The development and initial validation of a questionnaire on smoking urges. Addiction 86:1467–1476. https://doi.org/10.1111/j.1360-0443.1991.tb01732.x
Valjent E, Mitchell JM, Besson M-J et al (2002) Behavioural and biochemical evidence for interactions between Δ9-tetrahydrocannabinol and nicotine. Br J Pharmacol 135:564–578. https://doi.org/10.1038/sj.bjp.0704479
Vandrey R, Umbricht A, Strain EC (2011) Increased blood pressure after abrupt cessation of daily cannabis use. J Addict Med 5:16–20. https://doi.org/10.1097/ADM.0b013e3181d2b309
Viveros M-P, Marco EM, File SE (2006) Nicotine and cannabinoids: parallels, contrasts and interactions. Neurosci Biobehav Rev 30:1161–1181. https://doi.org/10.1016/j.neubiorev.2006.08.002
Vlahov D, Galea S, Resnick H, Ahern J, Boscarino JA, Bucuvalas M, Gold J, Kilpatrick D (2002) Increased use of cigarettes, alcohol, and marijuana among Manhattan, New York, residents after the September 11th terrorist attacks. Am J Epidemiol 155:988–996. https://doi.org/10.1093/aje/155.11.988
Wonnacott S (1997) Presynaptic nicotinic ACh receptors. Trends Neurosci 20:92–98. https://doi.org/10.1016/S0166-2236(96)10073-4
Acknowledgments
We thank Quincy Scott, our study physician, John D. Lindt, and the dozens of undergraduate and graduate research assistants who helped conduct this study, and without whom, it would have been impossible to complete.
Funding
The study was supported by NIH grant R01DA031006 awarded to David Gilbert.
Author information
Authors and Affiliations
Contributions
The study was designed by DGG and NER. The data were acquired by DGG and NER. The analyses were conducted by DGG and JTM. The manuscript was drafted by DGG, NER, and JTM. Each author contributed to critical revisions and approval of the final version of the manuscript. Funding was obtained by DGG. Supervision of the study was done by DGG and NER.
Corresponding author
Ethics declarations
DGG, NER, and JTM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
David Gilbert ended grant funding and all other connections with or benefits from R. J. Reynolds Tobacco Company in 1997 and has not received funding from any source other than the National Institutes of Health since that time. Prior to 2000 the first author received free nicotine and placebo patches from Glaxo-Smith Kline and received a consultation fee in 2003 and co-authorship on two manuscripts from Pfizer in 2004 and 2007 dealing with tobacco smoking withdrawal symptoms. Norka Rabinovich worked with several grant projects from R. J. Reynolds prior to the termination of funding from RJR in 1997. Justin McDaniel has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 195 kb)
Rights and permissions
About this article
Cite this article
Gilbert, D.G., Rabinovich, N.E. & McDaniel, J.T. Nicotine patch for cannabis withdrawal symptom relief: a randomized controlled trial. Psychopharmacology 237, 1507–1519 (2020). https://doi.org/10.1007/s00213-020-05476-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05476-1