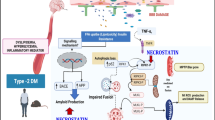
Abstract
The purpose of the present study was to elucidate the pharmacological effects of Geniposide (GEN) on high diet fed and streptozotocin (STZ)-caused diabetic cognitive impairment. The mice were fed with high fat diet (HFD) for 4 weeks and intraperitoneally injected with 60 mg/kg STZ for three times within 72 h. The mice with glucose level over 15 mmol/l were regarded as diabetic and selected for further studies. The animals were intragastrically treated with metformin or GEN once daily for 4 weeks. Afterwards, the animals were applied for Y maze, novel object recognition (NOR) test, step-through passive avoidance test, and Morris water maze (MWM) test. The blood glucose and body weight were examined. The SH-SY5Y cells were treated with GEN in the presence or absence of ibrutinib and stimulated with high-glucose culture medium. The tumor necrosis factor-a (TNF-α) and interleukin (IL)-6 in serum, hippocampus, and supernatant were measured using ELISA method. The protein expressions of Bruton’s tyrosine kinase (BTK), Toll-like receptor 4 (TLR4), myeloid differentiating factor 88 (MyD88), nuclear factor kappa-B (NF-κB), p-NF-κB, brain-derived neurotrophic factor (BDNF), cAMP-response element binding protein (CREB), p-CREB, and glucagon-like peptide-1 receptor (GLP-1R) were detected by western blot analyses. As a result, the GEN treatment notably attenuated the body weight, blood glucose, and cognitive decline. GEN also inhibited the generations of inflammatory cytokines. Furthermore, the administrations of GEN ameliorated the alterations of BTK, TLR4, MyD88, NF-κB, and BDNF in HFD + STZ–induced mice. With the application of ibrutinib, the selective inhibitor of BTK, it was also found that BTK/TLR4/NF-κB pathway was associated with the GEN treatment in high glucose–induced SH-SY5Y cells. In summary, the results suggested that GEN exerted the protective effect on STZ-induced cognitive impairment possibly through the modulation of BTK/TLR4/NF-κB signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, diabetes mellitus (DM) is widely acknowledged as a common metabolic dysfunction worldwide (Wu et al. 2017a). A variety of diabetic complications are observed in several organs both in peripheral and central nervous systems. Among them, the diabetic encephalopathy including brain atrophy and cognitive impairment frequently influences the life quality of patients (Infantegarcia et al. 2017). The cognitive function includes calculation, executive ability, memory, and spatial orientation (Geng et al. 2018). The diabetic patients suffer higher risk of cognitive decline than those without diabetes. The neurocognitive dysregulation–combined diabetes mellitus may deteriorate into Alzheimer’s disease (Wang et al. 2017a). The early diagnosis and treatment of cognitive impairment play a critical role for preventing the process in DM patients. Although mounting studies have studied diabetic-induced cognitive dysfunction during the past few decades, its etiology has not been fully understood. Additionally, there are seldom specific drugs treating this disorder without severe side effect in clinical. Thus, it is urgent to investigate the pathogenesis and explore the effective drug for diabetic cognitive dysfunction.
The long-term hyperglycemia initiates the degenerative processes which contribute to dysregulation of glucose metabolism, damage of nerve tissues, dysregulation of cognitive function, and overproduction of inflammatory mediators (Rasheed et al. 2018) in the brain. Streptozotocin (STZ), a compound leads to degeneration and necrosis of pancreatic β cells, has been widely used coupled with long-term high-glucose fat (HGF) diet to induce type 2 diabetic impairment (Wu et al. 2017b). Although the pathogenesis of the STZ-induced cognitive dysfunction has not been fully demonstrated, several studies confirmed that the high concentration of glucose promoted the expressions of inflammatory cytokines including interleukin (IL)-6 and tumor necrosis factor (TNF)-α (Liu et al. 2016).
Bruton’s tyrosine kinase (BTK), a multifunctional non-receptor tyrosine kinase, is a member of TEC kinase family. Kendall et al. elicited that BTK deficiency prevented diabetes (Kendall et al. 2009). It has been recently demonstrated that the inhibition of BTK prevents microglial activation and Alzheimer’s disease (Xia et al. 2018). BTK is also implicated with inflammatory reaction in intracerebral hemorrhage (Wang et al. 2018). Former investigators indicated that BTK could interact with Toll-like receptors including TLR4, which promoted the phosphorylation of NF-κB. NF-κB is the crucial transcriptional factor governing the generation of inflammatory cytokines (Chisato et al. 2015). Although seldom investigators reported the role of BTK in diabetic cognition, it was hypothesized that BTK was the therapeutic target for high fat diet (HFD)/STZ–induced cognitive decline in the present work.
Gardenia jasminoides (Cape jasmine) are long standing used for contusion, hepatic disease, brain injury, and inflammation in Traditional Chinese Medicine (Zhao et al. 2016). Geniposide (GEN), a bioactive iridoid glycoside isolated from the gardenia fruit, exhibits a broad spectrum of properties including anti-oxidative, anti-inflammatory, and anti-diabetic activities (Hu et al. 2018a). Recently, GEN has been suggested to attenuate cognitive deficiency in Alzheimer’s disease (Zhao et al. 2017). GEN also decreased the hippocampal content of Aβ1–42 in APP/PS1 transgenic mice (Liu et al. 2017a). However, scarce literatures reported the effect of GEN on HFD + STZ–induced cognitive impairment. The present study was carried out to evaluate the pharmacological effect of GEN on HFD + STZ–induced cognitive decline and investigate its potential mechanism.
Methods
Regents
Geniposide (purity > 98%; Fig. 1) was produced by Xi’an Kailai Biological Engineering Co., Ltd. (Xi’an, China). Metformin hydrochloride (Met) was purchased from Jiangsu Suzhong Pharmaceutical Group (Taizhou, China). Ibrutinib was purchased from MCE (NJ, USA). All the drugs were dissolved in sterilized PBS and DMSO [with DMSO concentration less than 0.1% (v/v)]. BCA protein concentration determining kit was provided by Beyotime Biotech Co., Ltd. (Wuhan, China). The enzyme-linked immunosorbent assay (ELISA) detecting kits for TNF-α and IL-6 were supplied from Elabscience Biotech. Co., Ltd. (Wuhan, Hubei, China). Streptozocin (STZ) and DMSO were obtained from Sigma (St. Louis, USA). High-glucose Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were produced by GIBCO-BRL (Grand Island, USA). The corresponding primary antibodies including anti-TLR4 (#14358), anti-MyD88 (#4283), anti-p-NF-κB (#3039), anti-NF-κB (#8242), anti-BDNF (#4201), anti-CREB (#9197), anti-p-CREB (#9198) and anti-GAPDH (#5174) antibodies were obtained from Cell Signaling Technology (Danvers, USA). Anti-GLP-1R (ab108443), Anti-BTK (ab208937) and anti-pY223 BTK (p-BTK, ab68217) were provided by abcam (Cambridge, UK).
Animals
Male ICR mice, 8–10 weeks old, were purchased from Qinglongshan Experimental Animal Breeding Farm (Nanjing, China) 5 days prior to the experiment for adaption. The animals were maintained under controlled condition with a 12-h light/dark cycle at 25 ± 2 °C. All the mice consumed food pellets and had free access to water. All animal experiments were conducted according to the National Institutes of Health Guidelines.
Experimental design
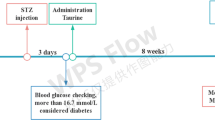
The mice were randomly assigned to vehicle group (n = 10) and diabetic group. The vehicle mice were fed with standard diets throughout the experiment, whereas the mice in diabetic group were fed with high fat diet (HFD, 77% regular diet, 15% lard, 5% white sugar, 2% cholesterol, 0.7% salt and 0.3% sodium cholate) at the same time. After 4 weeks feeding, the mice in diabetic group were fasted with free access to water for 12 h and intraperitoneally injected three times with 60 mg/kg STZ (dissolved in citrate buffer, pH 4.2) solution within 72 h. Meanwhile, the vehicle mice intraperitoneally received citrate buffer at the same volume. Three days later, the body weight was recorded and fasting blood plasma glucose concentration in a tail-vein sample was measured using a glucose monitor. A mouse with glucose level over 15 mmol/l was regarded as diabetic and selected for further studies (with 4 mice excluded from experiment). The diabetic mice were randomly divided to four groups (n = 12): STZ + HFD group, STZ+ HFD + metformin (Met, 200 mg/kg) group, STZ + HFD + GEN (10 mg/kg) group, STZ + HFD + GEN (40 mg/kg) group. The animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Afterwards, the animals were applied for Y maze, novel object recognition (NOR) test, open field test (OFT), passive avoidance test, and Morris water maze (MWM) test. Finally, the blood samples were harvested from the orbital sinus and mice were sacrificed. After centrifugating at 3000×g for 10 min, the serum samples were stored at − 80 °C for the further examination. The brain tissues were immediately collected for biochemical and western bolt analyses. Some hippocampus tissues were quickly isolated on ice and then maintained at − 80 °C until pending analyses. The 10% hippocampus homogenate was dissolved in normal saline (0.9% sodium chloride); the supernatant was prepared after the centrifugation at 10,000×g for 10 min at 4 °C. All the efforts were made to minimize the animals’ suffering.
Behavioral tests for cognitive evaluation
Y maze
The apparatus was comprised of three compartments (34 cm long, 8 cm wide, and 14.5 cm high) connected with passages (8 cm × 8 cm × 8 cm) at 120° angles from each other. The wall and floor of Y maze were constructed of black plastic. Twenty-four hours before the test, the mice were placed in one of the arms to adapt the environment. The mouse was slightly placed in one compartment facing the dark wall; then, the sequence and number of arm entries were monitored and calculated during the 8-min test. The percentage alternation = [(Number of alternations)/(Total arm entries − 2)] × 100%. Seventy-five v/v ethanol was used to clean the apparatus to remove odors and residues after each trail.
Novel object recognition test
The NOR test is a commonly used behavioral test for memory and recognition using a plastic chamber (50 cm × 50 cm × 50 cm) with the illumination placed at the center of the arena. Object A (5 cm × 5 cm × 5 cm, a black plastic cube) and object B (3 cm diameter, 6 cm height) were used in NOR test. The mice were allowed to habituated for 5 min to two object A (the familiar object) placed in the field with 35 cm spacing. Twenty-four hours later, one object A was replaced with an object B (the novel object) and the mice were allowed to explore for 5 min. Object recognition was defined as orienting their nose toward the object at a distance of no more than 2 cm, sniffing or touching the object. The discrimination index (DI) = (novel object exploration time − familiar object exploration time)/(total exploration time).
Passive avoidance test
The passive avoidance test was considered as a simple method for testing memory using a step-through test cage consisting of an illuminated compartment and black compartment separated by a sliding door. The floor of the black room was consisted of stainless steel rods at 1 cm spacing apart. The guillotine door was raised in the training phase; each mouse was individually placed in the light compartment and left to explore freely for 10 s. After the mice completely entered into the dark room, it received a mild foot shock of 0.2 mA for 3 s and went back to its compartment. Twenty-four hours later, the probe test was performed and the mice were placed in the light room. The step-through latency and the error number to enter the dark compartment were recorded.
The Morris water maze test
The Morris water maze (MWM) test was applied to evaluate the long-term spatial learning and memory functions for a total 5 days. The MWM consists of a circular pool (80 cm in radius and 45 cm high) with black-painted sidewall and an escape platform below the 25 °C water. The pool was divided into four quadrants according to the markers for location. A 12-cm-diameter circular escape platform was immobilized 1 cm below the water surface with a flag in the target quadrant. The orientation navigation tests were carried out four times per day with a constant 1 h interval for 4 consecutive days. The mouse was individually placed on the platform for 15 s prior to the first trail and swim for 90 s. The mice unable to find the safety island were gently put on the platform for a 15-s rest. For the other trails in orientation navigation tests, the mice were randomly put in water facing the pool wall at four quadrants entry points. The animals were given a maximum of 90 s to reach the platform. No matter the mouse found or failed to find the escape platform, it was allowed to rest on the platform for 30 s after arriving. It was noteworthy that the flag was removed on trails of 3rd and 4th days. On the fifth day, the platform was removed and a 120 s probe trial was carried out. The escape latency to reach the platform, the numbers of target crossings over the previous location of the target platform, and the time spent in the target quadrant were monitored by the video analysis-management equipment (Viewer 2 Tracking Software, Ji Liang Instruments, China).
Cell culture and drug treatment
The SH-SY5Y cell line was purchased from cell bank of the Chinese Academy of Sciences, (Shanghai, China). The cells were cultured in DMEM/F12 culture medium complemented with 10% FBS, 100 IU/ml streptomycin, and 100 IU/ml penicillin. The SH-SY5Y cells were assigned to: vehicle group, high glucose group, high glucose + GEN group, vehicle + ibrutinib group, high glucose + ibrutinib group, and high glucose + ibrutinib + GEN group. GEN and ibrutinib were dissolved in DMSO (less than 0.1% (v/v)) and high-glucose (HG) DMEM/F12 (33 mM glucose, 2% FBS). In brief, the SH-SY5Y cells in 80% confluency were seeded at 4 × 105/ml. Twenty-four hours later, the culture medium was removed and serum-deprived normal medium was added for 4 h. Afterwards, the cells were treated with GEN (20 μM) dissolved in DMSO and high-glucose DMEM/F12 as described in previous literature (Yin et al. 2015) for 2 h; ibrutinib (0.5 μM) was incubated with cells. After another incubation for 22 h, the cell and supernatant were collected for measurement.
Determination of cytokines in serum and hippocampus using ELISA method
The levels of IL-6 and TNF-α in serum and hippocampus of STZ-induced mice were examined by commercial available ELISA kits. The experimental procedures were conducted in accordance with the manufacturer’s instructions.
Western blot analysis
The hippocampus tissues were collected, chopped, and homogenized with 1 ml of RIPA and 10 μl PMSF. The protein of hippocampal samples and SH-SY5Y cells was determined using BCA kit produced by Beyotime (Nanjing, China). The protein samples were subjected to 10% SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. After the blockage with 5% milk for 2 h, the membranes were washed for three times with TBST. The blots were incubated with the corresponding primary antibodies at 4 °C for overnight. On the next day, the membranes were washed and balanced for 1 h. Thereafter, the HRP-labeled secondary antibodies were added to the membranes for another 2 h. Consequently, the immunoblots were observed using the Beyotime ECL detection system (Nanjing, China) and a gel imaging system.
Statistical analysis
All the results were presented as means ± SDs. Data were analyzed by analysis of variance (ANOVA) with Tukey multiple comparison tests or two-way repeated measures ANOVA with Bonferroni multiple comparison tests. A value less than 0.05 was considered significant.
Results
Effect of GEN on blood glucose levels
The concentration of blood glucose was measured to estimate the anti-diabetic property of GEN. Before GENadministration, the 4 model (STZ + HFD) groups showed notable increased glucose levels as compared with those in normal mice (p < 0.001). After the GEN (40 mg/kg, p < 0.01) or metformin (p < 0.01) administrations, the glucose content was significantly reduced compared with that in STZ + HFD group. The GEN (10 mg/kg, p < 0.05) group also relieved the hyperglycemia condition which was less potent than GEN (40 mg/kg) group.
In addition, there seemed no obvious difference of body weight between the five groups before drug treatment. It was statistically satisfied that STZ + HFD stimulation decreased the body weight versus the vehicle group. By contrast, the administrations of metformin (p < 0.001), GEN (40 mg/kg, p < 0.001), and GEN (10 mg/kg, p < 0.05) attenuated the body weight reduction as compared with that in model group. Our results suggested that the diabetic murine model was established successfully and GEN might attenuate the symptom (Fig. 2).
The effect of GEN on plasma glucose levels before drug treatment (a), blood glucose levels after drug treatment (b), body weight before drug treatment (c), and body weight after drug treatment (d). The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. ###p < 0.001 compared with vehicle group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with STZ + HFD group
Effect of GEN on Y-maze test
Y-maze test was applied for estimating the function of GEN on impairment of working memory and cognition. As illustrated in Fig. 3a, STZ + HFD challenge markedly reduced the spontaneous alternation as compared with that in vehicle group (p < 0.01), whereas the reduction of spontaneous alternation behavior was attenuated by the treatment with GEN (10, 40 mg/kg) and metformin (p < 0.001). The results demonstrated that the administration of GEN attenuated the STZ + HFD–related cognitive deficiency.
Effect of GEN on alteration in Y maze test (a), and discrimination index (b), and discrimination time (c) in novel object recognition test. The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. #p < 0.05, ##p < 0.01 compared with vehicle group. *p < 0.05, **p < 0.01 compared with STZ + HFD group
The effect of GEN on novel object recognition test
The discrimination index (DI) and the discrimination time in NOR test are the classic indices for reflecting recognition memory. As depicted in Fig. 3b, c, the vehicle mice distinguished the novel object from the familiar object, whereas the mice in STZ + HFD group failed to distinguish them. The administrations of GEN (10, 40 mg/kg) and metformin notably increased the exploration time for novel object (p < 0.05) and decreased the exploration time for familiar object (p < 0.05) in STZ + HFD group. Additionally, the exposure to STZ + HFD reduced the discrimination index versus the vehicle group, while the treatment with GEN (10, 40 mg/kg) and metformin effectively increased the discrimination index (p < 0.05) compared with that in model group. The obtained data displayed the protective effect of GEN on cognition and memory.
The effect of GEN on passive avoidance test
The aversive memory performance was evaluated by passive avoidance test. The fear-motivated memory retention was implicated with the latency to enter the dark room with electric stimulation. The STZ + HFD challenge remarkably shortened the passive avoidance latency (p < 0.01) and increased error number as compared with those in vehicle animals. On the contrary, the GEN (40 mg/kg) and metformin treatment significantly increased the latency (p < 0.05) and reduced the error times compared with those in STZ + HFD group. The data displayed that the administration of the GEN could attenuate cognitive decline caused by STZ + HFD (Fig. 4).
Effect of GEN on representative images of moving paths (a), error number (b), and latency (c) in passive avoidance test. The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. #p < 0.05, ##p < 0.01 compared with vehicle group. *p < 0.05, **p < 0.01 compared with STZ + HFD group
Effect of GEN on Morris water maze
Next, the spatial learning and memory ability was evaluated by Morris water maze test and the notable spatial learning ability. During navigation training, the average escape latencies in STZ/HFD group were obviously increased compared with that in vehicle group (4 trials/mice/day for 5 days; effect of day, F (4, 79) = 29.95, p < 0.01; effect of group, F (4, 79) = 49.40, p < 0.01; effect of group-by-day interaction, F (4, 79) = 1.22, p > 0.05), which indicated the spatial learning ability deficiency. Nonetheless, the metformin or GEN (40 mg/kg) group notably decreased the escape latency.
The mice were carried out with the probe trial after the navigation training. The mice suffered STZ/HFD challenge passed through the location for fewer times compared with that in vehicle mice (p < 0.01), whereas the administration of metformin or GEN (40 mg/kg) effectively increased the platform crossing times (p < 0.01). Besides, the exposure to STZ/HFD reduced the time in target quadrant (p < 0.01). The treatment with GEN (40 mg/kg, p < 0.01), GEN (10 mg/kg, p < 0.05) and metformin (p < 0.01) presented more preference for the target quadrant compared with that in model group. The analyzed results displayed that GEN ameliorated the spatial learning-memory ability in HFD/STZ-diabetic mice (Fig. 5).
Effect of GEN on representative images of the swimming paths in the probe trial (a), escape latency during the 4-day place navigation training (b), and the platform crossing (c); the percentage of time in the target quadrant during the probe trial test of MWM test. The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then, the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. ##p < 0.01 compared with vehicle group. *p < 0.05, **p < 0.01 compared with STZ + HFD group
Effects of GEN on inflammatory cytokines in serum and hippocampus
The anti-inflammatory property of GEN was detected by ELISA method. As shown in Fig. 6, the dramatical augments of TNF-α and IL-6 contents in serum (p < 0.05) were observed in STZ + HFD–exposed mice. The treatments with GEN (40 mg, p < 0.01), GEN (10 mg, p < 0.05), and metformin (p < 0.01) markedly decreased the elevated serum levels of inflammatory cytokines in cognitive impairment mice. Moreover, the exposure to STZ + HFD also reduced the levels of TNF-α (p < 0.05) and IL-6 (p < 0.01) in hippocampus. In contrast, the GEN (40 mg/kg) and metformin decreased the concentrations of inflammatory mediators compared with those in model group (p < 0.05). The results suggested that GEN was capable of suppressing the generation of inflammatory modulators.
Effect of GEN on inflammatory cytokines IL-6 and TNF-α in serum (a, b) and hippocampus (c, d). The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then, the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. #p < 0.05, ##p < 0.01 compared with vehicle group. *p < 0.05, **p < 0.01 compared with STZ + HFD group
Effects of GEN on the protein expressions of BTK/TLR4/NF-κB pathway and BDNF/CREB signaling in hippocampus
To investigate the ameliorating mechanism of GEN on cognitiveimpairment, the protein expressions of p-BTK, BTK, TLR4, MyD88, p-NF-κB, NF-κB, BDNF, p-CREB, CREB were detected. As illustrated in Figs. 7 and 8, the expressions of BTK, TLR4, MyD88, and p-NF-κB were strikingly upregulated in the STZ + HFD–induced group compared with those in vehicle group (p < 0.01). Nevertheless, the expressions were considerably downregulated in both the metformin and GEN (40 mg/kg) treated groups. GEN (10 mg/kg) group also inhibited the phosphorylation of NF-κB.
Effect of GEN on the protein expressions of p-BTK/TLR4/NF-κB pathway in hippocampus. The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. ##p < 0.01 compared with vehicle group. *p < 0.05, **p < 0.01 compared with STZ + HFD group
Effect of GEN on the protein expressions of BDNF/CREB signaling in hippocampus. The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. #p < 0.05, ##p < 0.01 compared with vehicle group. *p < 0.05, **p < 0.01 compared with STZ + HFD group
Furthermore, the stimulation with STZ + HFD remarkably suppressed the protein expressions of BDNF (p < 0.05) and p-CREB (p < 0.01). On the contrary, the GEN (40 mg/kg) and metformin treatments restored the protein level of BDNF (p < 0.05). The administrations of GEN (40 mg/kg, p < 0.01), GEN (10 mg/kg, p < 0.05), and metformin (p < 0.01) compromised the upregulation of CREB phosphorylation. These findings indicated that the ameliorated activity of GEN on STZ-induced cognitive impairment mice might be attributed the BTK/TLR4/NF-κB pathway and BDNF/CREB signaling.
Effect of GEN on p-BTK/TLR4/p-NF-κB pathway in high glucose–induced SH-SY5Y cells
We further explored the potential pathogenesis of GEN on high glucose (HG)–induced SH-SY5Y cells. As presented in Fig. 10, the HG stimulation pronouncedly elevated the expressions of p-BTK (p < 0.01), TLR4 (p < 0.001), and p-NF-κB (p < 0.01). By contrast, the GEN treatment effectively reduced the protein levels of p-BTK, TLR4, and p-NF-κB (p < 0.05). In the presence of selective inhibitor ibrutinib, the HG incubation also promoted the expressions of p-BTK (p < 0.05), TLR4 (p < 0.01), and p-NF-κB (p < 0.05), while the GEN treatment suppressed the upregulation of p-BTK (p < 0.05) and TLR4 (p < 0.05). It was noteworthy that the p-BTK (p < 0.05) and TLR4 (p < 0.05) protein expressions in HG + GEN + ibrutinib group were inhibited compared with those in HG + GEN group. Notably, the expressions of p-BTK, TLR4, and p-NF-κB in HG + ibrutinib group were downregulated compared with those in HG group (p < 0.05). The experimental results reflected the role of GLP-1 receptor agonist GEN in p-BTK/TLR4/p-NF-κB pathway in HG-induced SH-SY5Y cells.
Effect of GEN on the protein expression of GLP-1R in hippocampus. The mice were fed with HFD and intraperitoneally injected three times with 60 mg/kg STZ. Then, the animals were intragastrically treated with metformin (Met, 200 mg/kg) or GEN (10, 40 mg/kg) once daily for 4 weeks. Data were presented as means ± SDs. ##p < 0.01 compared with vehicle group. *p < 0.05, **p < 0.01 compared with STZ + HFD group
Effect of GEN on the protein expressions of BTK/TLR4/NF-κB pathway in HG-induced SH-SY5Y cells. SH-SY5Y cells were treated with GEN (20 μM) dissolved in high-glucose DMEM/F12 for 2 h; then, ibrutinib (0.5 μM) was added to the cells. After another incubation for 22 h, the cell and supernatant were collected for measurement. Data were presented as means ± SDs. @p < 0.05 compared with HG + GEN group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with other group
Effect of GEN on the protein expression of GLP-1R in HG-induced SH-SY5Y cells. SH-SY5Y cells were treated with GEN (20 μM) dissolved in high-glucose DMEM/F12 for 2 h, then ibrutinib (0.5 μM) was added to the cells. After another incubation for 22 h, the cell and supernatant were collected for measurement. Data were presented as means ± SDs. @p < 0.05 compared with HG + GEN group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with other group
Effect of GEN on GLP-1R expressions in hippocampus and high glucose–induced SH-SY5Y cells
As GEN also served an agonist of GLP-1 receptor (GLP-1R), and NF-κB drove the signaling of GLP-1R, we detected the expressions of GLP-1R in hippocampus. As illustrated in Fig. 9, the exposure to HFD+STZ markedly downregulated the expression of GLP-1R (p < 0.01). In contrast, the administration of metformin (p < 0.05) and GEN (p < 0.01) evidently restored the GLP-1R expressions.
In addition, the HG stimulation dramatically downregulated the GLP-1R expression compared with vehicle group in the absence of BTK selective inhibitor ibrutinib (p < 0.05), whereas the treatment with GEN markedly elevated the protein level of GLP-1R (p < 0.05). Besides, in the presence of ibrutinib, HG challenge caused the reduction of GLP-1R level compared with that of vehicle group (p < 0.01). However, the GEN treatment effectively elevated the level of GLP-1R (p < 0.05). Of note, the GLP-1R level of HG + ibrutinib group was significantly higher than that of HG group (p < 0.05). Our data indicated the critical role of BTK in the GEN-mediated GLP-1R expression (Fig. 11).
Discussion
STZ/HFD–induced diabetes is a well-established and widely used murine model of type 2 diabetes which results in marked hyperglycemia. The model of diabetes mellitus is likely to alter motor cognitive performance and has been commonly used for screening potential therapeutic candidate and exploring the underlying mechanism for diabetic cognitive decline.
GEN has been proved to exhibit beneficial effect on various diabetic complications including diabetic nephropathy (Hu et al. 2017), diabetic depression (Wang et al. 2016), and diabetic Alzheimer’s disease (Gao et al. 2014). Although seldom investigation reported, we assumed that GEN might be efficient for diabetic cognitive impairment. As the anti-inflammatory properties of GEN were acknowledged in microglial cells (Wang et al. 2017b) and ischemic stroke (Li et al. 2016), it was also hypothesized that GEN attenuated worsened diabetic cognitive performance via the anti-inflammatory effect. The present study imparted the novel insight for the protective effect of GEN on STZ/HFD–induced cognitive impairment. GEN obviously relieved the impaired performance of in Y maze test, passive avoidance test, novel object recognition test, and Morris water maze test caused by STZ/HFD stimulation. GEN prevented the inflammatory reactions occurring in the brain tissues during the progression of cognitive deficiency via the BTK/TLR4/NF-κB cascade and BDNF/CREB signaling.
Numerous evidences illustrated that GEN functioned as an agonist of GLP-1R. GLP-1R was considered to be expressed in various brain regions, such as hypothalamus, frontal cortex, substantia nigra, and hippocampus (Athauda and Foltynie 2016). The activation of GLP-1R mediated the neurotrophic modulator and played a neuroprotective role in brain dysfunction. The promotion of GLP-1R by GEN influenced insulin secretion and attenuated pancreatic β cell apoptosis in type 2 diabetes mellitus (Liu et al. 2012). Clinical trials also confirmed that GLP-1R agonist was capable of ameliorating cognitive impairment in type 2 diabetes patients (Grant et al. 2011). The present experiment might provide a novel insight of GEN in diabetic cognitive impairment. The augmentation of GLP-1R attenuated NF-κB signaling activation in neuroinflammation (Lee et al. 2018). Our results demonstrated that GLP-1R was successfully restored by GEN, which further indicated the protective effect of GEN on diabetes and cognitive decline. The in vitro study also displayed the critical role of BTK in the GEN-mediated GLP-1R expression.
The inflammatory process is a crucial element during the etiology of diabetes-accompanied cognitive impairment. TNF-α and IL-6 are the key pro-inflammatory cytokines initiating and promoting the inflammatory process which accelerates the type 2 diabetes caused by HFD/STZ (Ma et al. 2017). The excessive inflammatory cytokines also exacerbate diabetes-induced behavioral and neurochemical deficits (Jawale et al. 2016). The intrahippocampal microinjection of STZ contributes to the augments of inflammatory modulators including TNF-α (Zhang et al. 2016). The amyloid β–induced cognitive impairment in STZ-induced diabetic rats is also relieved by the inhibition of TNF-α (Chun et al. 2014). The role of IL-6 in the neurocognitive disorder was also elucidated in the former literature (Hu et al. 2018b). Our results confirmed that GEN treatment could suppress the generation and secretion of TNF-α and IL-6 in both serum and hippocampus, which indicated that GEN exhibited anti-inflammatory properties in HFD/STZ-stimulated cognitive deficiency.
PCI-32765, also named ibrutinib, irreversibly inhibits the phosphorylation of BTK at Tyr223 and represses the activity to phosphorylate or activate downstream events. With the application of ibrutinib, BTK was proved to be expressed in numerous inflammatory disorders (Miklos et al. 2017). BTK has been recently confirmed to be expressed in brain tissues and modulate the behavior alteration (Keaney et al. 2019). It was displayed that BTK possessed anti-diabetic property associated with Notch2 (Case et al. 2015). Ibrutinib was also reported to decrease the overproduction of pro-inflammatory mediators in response to high glucose (Fan et al. 2018). As there was limited research focused on the effect of BTK in diabetes combined with cognitive impairment, we assumed that BTK might be a target for the treatment of diabetic cognitive decline. A number of literatures proposed that ibrutinib suppressed the inflammatory progression in a battery of diseases including pulmonary injury (Liu et al. 2017b), trauma-hemorrhagic shock (Liu et al. 2017b), and ischemic stroke (Ito et al. 2015). Nam et al. proposed that ibrutinib suppressed LPS-induced neuroinflammatory responses in microglial cells (Nam et al. 2018). It was hypothesized that the protective effect of BTK might be associated with the inhibition of inflammatory cytokine generation in the present study.
BTK is a versatile signaling protein interacting with several immune receptors including TLR2 and TLR4. MyD88 is also an essential intracellular adaptor protein in the downstream molecular of TLR4 (Paracha et al. 2014). MyD88 phosphorylates NF-κB and drives the inflammatory reaction by promoting the expressions of pro-inflammatory cytokines including TNF-α and IL-6, which was involved in cognitive impairment (Keaney et al. 2019). Ibrutinib treatment could significantly downregulated the activation of NF-κB (Bercusson et al. 2018). BTK associated and phosphorylated IκB-α at Y289 and Y305 and then promote the phosphorylation and activation of NF-κB (Pontoriero et al. 2019). It was also indicated that GEN performed its protective effect via NF-κB signaling pathway as a GLP-1 receptor agonist. BDNF-CREB neurotrophic axis is highly related to the cognitive deficiency, synaptic plasticity, and insulin resistance (Lao-Peregrín et al. 2017). Our data indicated that the GEN treatment could attenuate BTK, TLR4, MyD88, NF-κB, BDNF, and CREB expressions. With the inhibition of ibrutinib, it was suggested that BTK/TLR4/NF-κB pathway was involved in the GEN-mediated diabetic cognitive decline.
In summary, the present study provided evidence that GEN exhibited protective effects on HFD/STZ–induced diabetic cognitive impairment by inhibiting inflammatory response through BTK/TLR4/NF-κB signaling. Further researches with transgenic mice are warranted before clinical application.
References
Athauda D, Foltynie T (2016) Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol 145-146:98–120
Bercusson A, Colley T, Shah A, Warris A, Armstrong-James D (2018) Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood 132:1985–1988
Case JB, Bonami RH, Nyhoff LE, Steinberg HE, Sullivan AM, Kendall PL (2015) Bruton’s tyrosine kinase synergizes with Notch2 to govern marginal zone B cells in nonobese diabetic mice. J Immunol 195:61
Chisato S, Mitsuru S, Takato T, Hiroshi K (2015) Specific binding of the WASP N-terminal domain to Btk is critical for TLR2 signaling in macrophages. Mol Immunol 63:328–336
Chun Y, Bin Z, Jie D, Zhi-Gang W (2014) Isoflurane anesthesia aggravates cognitive impairment in streptozotocin-induced diabetic rats. Int J Clin Exp Med 7:903–910
Fan Z, Wang Y, Xu X, Wu Y (2018) Inhibitor of Bruton’s tyrosine kinases, PCI-32765, decreases pro-inflammatory mediators’ production in high glucose-induced macrophages. Int Immunopharmacol 58:145–153
Gao C, Liu Y, Jiang Y, Ding J, Li L (2014) Geniposide ameliorates learning memory deficits, reduces tau phosphorylation and decreases apoptosis via GSK3β pathway in streptozotocin-induced alzheimer rat model. Brain Pathol 24:261–269
Geng J, Wang L, Zhang L, Qin C, Song Y, Ma Y et al (2018) Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Front Aging Neurosci 10:129
Grant P, Lipscomb D, Quin J (2011) Psychological and quality of life changes in patients using GLP-1 analogues. J Diabetes Complicat 25:244–246
Hu X, Zhang X, Jin G, Shi Z, Sun W, Chen F (2017) Geniposide reduces development of streptozotocin-induced diabetic nephropathy via regulating nuclear factor-kappa B signaling pathways. Fundam Clin Pharmacol 31:54–63
Hu X, Yu D, Zhuang L, Zhou M, Shi Z, Jin G, Zhang X (2018a) Geniposide improves hepatic inflammation in diabetic db/db mice. Int Immunopharmacol 59:141–147
Hu J, Feng X, Valdearcos M, Lutrin D, Uchida Y, Koliwad SK et al (2018b) Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br J Anaesth 120:537–545
Infantegarcia C, Ramosrodriguez JJ, Hierrobujalance C, Ortegon E, Pickett E, Jackson R et al (2017) Antidiabetic polypill improves central pathology and cognitive impairment in a mixed model of Alzheimer’s disease and type 2 diabetes. Mol Neurobiol 55:1–15
Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A et al (2015) Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun 6:7360
Jawale A, Datusalia AK, Bishnoi M, Sharma SS (2016) Reversal of diabetes-induced behavioral and neurochemical deficits by cinnamaldehyde. Phytomedicine. 23:923–930
Keaney J, Gasser J, Gillet G, Scholz D, Kadiu I (2019) Inhibition of Bruton’s tyrosine kinase modulates microglial phagocytosis: therapeutic implications for Alzheimer’s disease. J NeuroImmune Pharmacol 14:448–461
Kendall PL, Moore DJ, Chrys H, Hoek KL, Khan WN, Thomas JW (2009) Reduced diabetes in btk-deficient nonobese diabetic mice and restoration of diabetes with provision of an anti-insulin IgH chain transgene. J Immunol 183:6403–6412
Lao-Peregrín C, Ballesteros JJ, Fernández M, Zamora-Moratalla A, Saavedra A, Gómez LM et al (2017) Caffeine-mediated BDNF release regulates long-term synaptic plasticity through activation of IRS2 signaling. Addict Biol 22:1706–1718
Lee CH, Jeon SJ, Cho KS, Moon E, Sapkota A, Jun HS, Ryu JH, Choi JW (2018) Activation of glucagon-like peptide-1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Mol Neurobiol 55:3007–3020
Li F, Li W, Li X, Li F, Zhang L, Wang B, Huang G, Guo X, Wan L, Liu Y, Zhang S, Kang S, Ma J (2016) Geniposide attenuates inflammatory response by suppressing P2Y 14 receptor and downstream ERK1/2 signaling pathway in oxygen and glucose deprivation-induced brain microvascular endothelial cells. J Ethnopharmacol 185:77–86
Liu J, Yin F, Xiao H, Guo L, Gao X (2012) Glucagon-like peptide 1 receptor plays an essential role in geniposide attenuating lipotoxicity-induced beta-cell apoptosis. Toxicol in Vitro 26:1093–1097
Liu X, Mo Y, Gong J, Li Z, Peng H, Chen J, Wang Q, Ke Z, Xie J (2016) Puerarin ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Metab Brain Dis 31:417–423
Liu Z, Zhang Y, Liu J, Yin F (2017a) Geniposide attenuates the level of Aβ 1–42 via enhancing leptin signaling in cellular and APP/PS1 transgenic mice. Arch Pharm Res 40:1–8
Liu X, Zhang J, Han W, Wang Y, Liu Y, Zhang Y et al (2017b) Inhibition of BTK protects lungs from trauma-hemorrhagic shock-induced injury in rats. Mol Med Rep 16:192–200
Ma C, Yu H, Xiao Y, Wang H (2017) Momordica charantia extracts ameliorate insulin resistance by regulating the expression of SOCS-3 and JNK in type 2 diabetes mellitus rats. Pharm Biol 55:2170–2177
Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, Flowers ME, Logan AC, Nakamura R, Blazar BR, Li Y, Chang S, Lal I, Dubovsky J, James DF, Styles L, Jaglowski S (2017) Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 130:2243–2250
Nam HY, Nam JH, Yoon G, Lee JY, Nam Y, Kang HJ et al (2018) Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J Neuroinflammation 15:271
Paracha RZ, Ali A, Ahmad J, Hussain R, Niazi U, Muhammad SA (2014) Structural evaluation of BTK and PKC δ mediated phosphorylation of MAL at positions Tyr86 and Tyr106. Comput Biol Chem 51:22–35
Pontoriero M, Fiume G, Vecchio E, de Laurentiis A, Albano F, Iaccino E et al (2019) Activation of NF-kappaB in B cell receptor signaling through Bruton’s tyrosine kinase-dependent phosphorylation of IkappaB-alpha. J Mol Med (Berl) 97:675–690
Rasheed NOA, El-Sayed NS, El-Khatib AS (2018) Targeting central β2 receptors ameliorates streptozotocin-induced neuroinflammation via inhibition of glycogen synthase kinase3 pathway in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 86:S0278584618301258
Wang J, Duan P, Cui Y et al (2016) Geniposide alleviates depression-like behavior via enhancing BDNF expression in hippocampus of streptozotocin-evoked mice. Metab Brain Dis 31:1113–1122
Wang Q, Yuan J, Yu Z, Lin L, Jiang Y, Cao Z, et al. FGF21 attenuates high-fat diet-induced cognitive impairment via metabolic regulation and antiinflammation of obese mice. Mol Neurobiol.Mol Neurobiol. 2018 Jun;55(6):4702-4717.
Wang J, Chen L, Liang Z, Li Y, Yuan F, Liu J et al (2017b) Genipin inhibits LPS-induced inflammatory response in BV2 microglial cells. Neurochem Res 42:1–8
Wang G, Guo Z, Tong L, Xue F, Krafft PR, Budbazar E, Zhang JH, Tang J (2018) TLR7 (Toll-like receptor 7) facilitates heme scavenging through the BTK (Bruton tyrosine kinase)-CRT (calreticulin)-LRP1 (low-density lipoprotein receptor-related protein-1)-Hx (hemopexin) pathway in murine intracerebral hemorrhage. Stroke 49:3020–3029
Wu YJ, Lin CC, Yeh CM, Chien ME, Tsao MC, Tseng P et al (2017a) Repeated transcranial direct current stimulation improves cognitive dysfunction and synaptic plasticity deficit in the prefrontal cortex of streptozotocin-induced diabetic rats. Brain Stimul. 2017 Nov - Dec;10(6):1079-1087.
Wu D, Yan ZB, Cheng YG, Zhong MW, Liu SZ, Zhang GY, et al. 2017b Deactivation of the NLRP3 inflammasome in infiltrating macrophages by duodenal-jejunal bypass surgery mediates improvement of beta cell function in type 2 diabetes. Metabolism Clinical & Experimental. :S0026049517303049.
Xia J, Lu Z, Feng S, Yang J, Ji M (2018) Different effects of immune stimulation on chronic unpredictable mild stress-induced anxiety- and depression-like behaviors depending on timing of stimulation. Int Immunopharmacol 58:48–56
Yin Z, Yu H, Chen S, Ma C, Ma X, Xu L, Ma Z, Qu R, Ma S (2015) Asiaticoside attenuates diabetes-induced cognition deficits by regulating PI3K/Akt/NF-κB pathway. Behav Brain Res 292:288–299
Zhang CT, Jing RL, Feng W, Ghosh A, Su ST, Mei H et al (2016) Montelukast ameliorates streptozotocin-induced cognitive impairment and neurotoxicity in mice. Neurotoxicology. 57:214–222
Zhao C, Lv C, Li H, Du S, Liu X, Li Z et al (2016) Geniposide protects primary cortical neurons against oligomeric A[beta].sub.1-42-induced neurotoxicity through a mitochondrial pathway. PLoS One 11:e0152551
Zhao C, Zhang H, Li H, Lv C, Liu X, Li Z, Xin W, Wang Y, Zhang W (2017) Geniposide ameliorates cognitive deficits by attenuating the cholinergic defect and amyloidosis in middle-aged Alzheimer model mice. Neuropharmacology. 116:18–29
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (No. 2632017PY14) and National Natural Science Foundation (No.81673434)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All animal experiments were conducted according to the National Institutes of Health Guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Zheng, M., Li, Y. et al. The protective effect of Geniposide on diabetic cognitive impairment through BTK/TLR4/NF-κB pathway. Psychopharmacology 237, 465–477 (2020). https://doi.org/10.1007/s00213-019-05379-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05379-w