Abstract
Rationale
Microglia are the main immune cells in the central nervous system and participate in neuroinflammation. When activated, microglia express increased levels of the translocator protein 18 kDa (TSPO), thereby making TSPO availability a marker for neuroinflammation. Using positron emission tomography (PET) scanning, our group recently demonstrated that smokers in the satiated state had 16.8% less binding of the radiotracer [11C]DAA1106 (a radioligand for TSPO) in the brain than nonsmokers.
Objectives
We sought to determine the effect of overnight smoking abstinence on [11C]DAA1106 binding in the brain.
Methods
Forty participants (22 smokers and 18 nonsmokers) completed the study (at one of two sites) and had usable data, which included images from a dynamic [11C]DAA1106 PET scanning session (with smokers having been abstinent for 17.9 ± 2.3 h) and a blood sample for TSPO genotyping. Whole brain standardized uptake values (SUVs) were determined, and analysis of variance was performed, with group (overnight abstinent smoker vs. nonsmoker), site, and TSPO genotype as factors, thereby controlling for site and genotype.
Results
Overnight abstinent smokers had lower whole brain SUVs (by 15.5 and 17.0% for the two study sites) than nonsmokers (ANCOVA, P = 0.004). The groups did not significantly differ in injected radiotracer dose or body weight, which were used to calculate SUV.
Conclusions
These results in overnight abstinent smokers are similar to those in satiated smokers, indicating that chronic cigarette smoking leads to global impairment of microglial activation which persists into early abstinence. Other explanations for study results, such as smoking leading to reduced numbers of microglia or smokers having more rapid metabolism of the radiotracer than nonsmokers, are also possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia are the main innate immune cells in the central nervous system (CNS) (Waisman et al. 2015). Under homeostatic conditions, they continuously monitor the surrounding environment for signs of infection or homeostasis-perturbing events (Gonzalez et al. 2014; Song et al. 2018). Microglia react to counteract such perturbations in order to protect neurons, which have a limited capacity to regenerate, leading to elevated levels of activated microglia in neurodegenerative diseases (Low and Ginhoux 2018; Schetters et al. 2017). In this context, activated microglia participate in various functions, such as clearance of apoptotic cells and extracellular pathogens, removal of degenerating neurons and extracellular proteins, and cytokine/chemokine production (Anthony and Pitossi 2012). When activated, microglial cellular morphology changes and expression of the translocator protein (TSPO) 18 kDa is increased, thereby making expression of TSPO a marker for neuroinflammation.
Epidemiological studies have shown that cigarette smokers have a lower risk of neurodegenerative diseases than the general population (Foucault-Fruchard and Antier 2017; Quik 2004), and much research has examined the mechanism by which smoking could diminish neuroinflammation, which contributes to neurodegenerative damage. Extensive pre-clinical work has shown that nicotine and/or other agonists at nicotinic acetylcholine receptors (nAChRs) protect against neuronal cell damage (Quik et al. 2015) via binding to α7 nAChRs and inhibition of microglial activation (Foucault-Fruchard and Antier 2017; Li et al. 2016; Noda and Kobayashi 2017; Shytle et al. 2004).
The radioligand N-(2,5-dimethoxybenzyl)-N-(5-fluoro-2-phenoxyphenyl) acetamide labeled with carbon-11 ([11C]DAA1106) has emerged as a reliable second-generation radiotracer for labeling TSPO (Maeda et al. 2004; Okubo et al. 2004; Zhang et al. 2003) with high affinity (Chaki et al. 1999; Chauveau et al. 2008; Venneti et al. 2007; Venneti et al. 2008) for positron emission tomography (PET) scanning in vivo. Specific binding of DAA1106 is reported to be greater than previously used ligands (e.g., PK11195) but less than at least one recently developed ligand (PBR28) (Owen et al. 2011). Specific binding of DAA1106 correlates with the presence of activated microglia identified by immunohistochemistry in situ (Venneti et al. 2008) and immunohistochemistry combined with autoradiography in brain tissue (Venneti et al. 2007). [11C]DAA1106 was chosen for use here because of these favorable properties and previous experience by our group with this radiotracer (Brody et al. 2014; Brody et al. 2017).
Our group recently used [11C]DAA1106 with PET scanning to compare smokers who had smoked to satiety (~ 15 min prior to scanning) with nonsmoking controls (Brody et al. 2017). The groups differed in whole brain standardized uptake values (SUVs) for the radiotracer, with smokers having 16.8% lower values than nonsmokers (and lower mean SUVs in menthol- than nonmenthol-cigarette smokers). Smokers also had lower SUVs (by 14.6–19.7%) in a range of smaller brain volumes of interest. In addition, whole-brain SUV was negatively correlated with participant-reported cigarettes per day. These study findings were consistent with much prior research demonstrating that smokers have less inflammatory functioning than nonsmokers.
For the study presented here, we sought to determine if the reduction in [11C]DAA1106 SUV (the marker for neuroinflammation) found in satiated smokers was still present in early (overnight) smoking abstinence. In order to study smokers in a state with potentially significant differences from nonsmoking controls, overnight (> 12 h) abstinence from smoking was chosen as the time point of interest because nicotine would be expected to be recently eliminated from the body at that time (plasma half-life of ~ 2 h; Benowitz et al. 2009) and withdrawal symptoms (e.g., urge to smoke and anxiety/irritability) would be expected to be elevated (Schuh and Stitzer 1995; Ward et al. 2001). As in our prior study (Brody et al. 2017), we hypothesized that smoker vs. nonsmoker effects would occur globally, based on prior research demonstrating that cigarette smoke is rapidly absorbed (Hukkanen et al. 2005) and results in saturation (or near saturation) of nicotinic acetylcholine receptors throughout the brain (Brody et al. 2006a; Brody et al. 2009a; Brody et al. 2011; Esterlis et al. 2010). We also sought to examine the effect of menthol, because menthol smoking is common (~ 1/3 of US smokers) (SAMHSA 2009), menthol smokers have more trouble quitting in standard treatment programs than nonmenthol-cigarette smokers (Gandhi et al. 2009; Okuyemi et al. 2007; Pletcher et al. 2006), menthol smoking has been found to lead to elevated serum nicotine/cotinine and exhaled carbon monoxide levels (Williams et al. 2007) (though not all studies agree on this point; Heck 2009; Werley et al. 2007), and menthol smokers had lower mean [11C]DAA1106 SUVs than nonmenthol smokers in our prior study of smokers in satiety (Brody et al. 2017).
Method
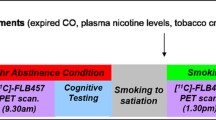
Forty participants (22 cigarette smokers and 18 nonsmokers) completed the study and had usable data. These participants underwent telephone and in-person screening, overnight smoking abstinence prior to the PET session (for the smoker group), a bolus [11C]DAA1106 PET scanning session, a blood draw to determine TSPO affinity genotype, and a structural magnetic resonance imaging (MRI) scan, as described below. Four additional participants were enrolled, but were excluded because they were homozygous for the low affinity TSPO genotype (Fig. 1). All participants provided written informed consent on forms approved by the Institutional Review Boards of either the VA San Diego Healthcare System or VA Greater Los Angeles Healthcare System. A subset of the nonsmoker group in the analysis presented here consisted of participants who were included in our previous report (n = 13) (Brody et al. 2017).
Participants were recruited through posted flyers and the Internet (e.g., Craigslist). Inclusion criteria were healthy adult (18 to 65 years old) daily cigarette smokers (range 2–25 cigarettes/day and evidence of tobacco dependence on the Fagerström test for nicotine dependence (FTND)) or nonsmokers (< 100 cigarettes lifetime and none within the past year), smoking primarily (> 80%) either menthol or nonmenthol cigarettes (for the smoker group), ability to give voluntary informed consent, and an exhaled CO > or < 8 ppm (and urine cotinine > or < 200 ng/mL) during the study screening visit to support smoking or nonsmoking status, respectively. Exclusion criteria were any psychiatric diagnosis (including mood, anxiety, psychotic, and substance abuse disorders other than tobacco use disorder) within the past 6 months; any current medication or history of a medical condition that might affect the central nervous system at the time of scanning (e.g., current treatment with a psychotropic medication, or history of severe head trauma with loss of consciousness, epilepsy, or other neurological diseases); regular use (> 1×/week) of anti-inflammatory medication, such as steroidal or nonsteroidal anti-inflammatory medications (e.g., corticosteroids, ibuprofen, naproxen, aspirin, or celecoxib (Celebrex®)); unstable cardiovascular disease, severe liver disease, or renal insufficiency, which might make tolerating study procedures difficult; or pregnancy. Occasional drug/alcohol use not meeting criteria for abuse or dependence was not exclusionary, but participants were instructed to abstain from drug/alcohol use for at least 48 h prior to PET scanning.
For the telephone screening, a thorough smoking history, including age of first cigarette, maximum smoking habit, menthol- or nonmenthol-cigarette use, length and dates of abstinence periods, previous treatments used, and current smoking habit, was obtained. A brief medical, psychiatric, and substance use history was also obtained. During a subsequent in-person visit, eligibility criteria were confirmed and general demographics, smoking history, and symptom ratings were obtained with the FTND (Fagerstrom 1978; Heatherton et al. 1991) (to assess severity of nicotine dependence), Spielberger State Trait Anxiety Index (STAI) (Spielberger 1983), and Beck Depression Inventory (BDI) (Beck et al. 1996) (to confirm the absence of potentially confounding psychiatric symptoms) for all participants, and Smoker’s Profile Form (Brody et al. 2006a; Brody et al. 2013) and Shiffman–Jarvik Withdrawal Scale (SJWS) (Shiffman and Jarvik 1976) (to measure craving and withdrawal) for participants scanned at UCSD. A brief medical review of systems and chart review were also performed by a study physician, along with an exhaled carbon monoxide (CO) measurement (Micro+ Smokerlyzer Breath CO Monitor; Bedfont Scientific, Ltd., UK), urine cotinine screen (The Accutest® NicAlert™; Jant Pharmacal Corp., Encino, CA), breathalyzer (AlcoMatePro), urine toxicology screen (Test Country I-Cup Urine Toxicology Kit), and urine pregnancy test (Test Country Cassette Urine Pregnancy Test), in order to determine if participants met inclusion/exclusion criteria.
Participants who met inclusion/exclusion criteria and wished to participate underwent a [11C]DAA1106 PET scanning session 1 week later at either the University of California at San Diego (UCSD) Center for Molecular Imaging (n = 15; 10 overnight abstinent smokers and 5 nonsmokers) or VA Greater Los Angeles Healthcare System (VAGLAHS) PET Center (n = 25; 12 overnight abstinent smokers and 13 nonsmokers), using a procedure similar to the one developed in previous studies (Ikoma et al. 2007; Takano et al. 2010; Yasuno et al. 2008; Yasuno et al. 2012). Smokers were instructed to abstain from cigarettes and other nicotine-containing products from prior to midnight on the night before PET scanning. The PET session was initiated in the afternoon with participants undergoing a brief clinical interview, breathalyzer, exhaled CO level, and urine toxicology and pregnancy screens, in order to verify continued meeting of inclusion/exclusion criteria (including drug abstinence at the time of scanning). An exhaled CO of < 6 ppm was considered consistent with overnight abstinence. Following these procedures, participants were positioned on the PET scanning bed and a venous line was placed. They then received a bolus injection of 350 (± 53) MBq of [11C]DAA1106 and underwent dynamic PET scanning of the brain for the next 90 min. PET scans were obtained using either an ECAT HR+ PET scanner (CTI PET systems, Knoxville, TN) (UCSD) or a Philips Gemini TruFlight PET Scanner (Koninklijke Philips Electronics N.V., Eindhoven, the Netherlands) (VAGLAHS). [11C]DAA1106 was prepared by an established method (Wang et al. 2012). All scans consisted of eighteen 5-min frames. For the UCSD scanner, a 20-min transmission scan from a Ge-68 rod source was performed for attenuation correction following the dynamic scan. The reconstruction used a manufacturer’s OSEM algorithm with 4 iterations and 16 subsets. For the VAGLAHS scanner, CT scanning was performed for attenuation correction following the dynamic scan, and the manufacturer’s RAMLA algorithm was used for image reconstruction. Investigational new drug (IND) approvals from the Food and Drug Administration (INDs 133984 (UCSD) and 122041 (VAGLAHS)) were obtained to use the radiotracer [11C]DAA1106 for the studies described here.
A 5-ml blood sample was drawn prior to the initiation of PET scanning for genotyping of each individual’s TSPO affinity subtype (high (C/C), medium (C/T), or low (T/T)), because these affinity subtypes affect radiotracer binding of all currently used radiotracers determining TSPO availability (Owen et al. 2011; Owen et al. 2012; Yoder et al. 2013). Genomic DNA was extracted from whole blood using QiaAmp DNA Blood Mini Kits (Qiagen, Valencia, CA) (by E.N. and L.S.) and TSPO single-nucleotide polymorphism (rs6971) genotyping using the TaqMan Allelic Discrimination (Thermo Fisher Scientific, Canoga Park, CA) platform was performed in duplicate, according to the manufacturer’s specified protocol. Quality control was ensured by perfect concordance of replicate samples, expected minor allele frequencies, and adherence to Hardy–Weinberg equilibrium. Only scans from participants with the high- or medium- affinity genotypes, present in > 90% of North Americans (Mizrahi et al. 2012), were included in study analyses, in order to avoid a potential confound. The exclusion of homozygous low-affinity binders from data analysis is standard practice in recent research in this field (Hafizi et al. 2016; Hannestad et al. 2013; Koshimori et al. 2015; Zurcher et al. 2015).
Within 1–2 weeks of PET scanning, an MRI scan of the brain was obtained, in order to facilitate localization of regions on the PET scans. At UCSD, high-resolution T1-weighted 3D MRIs were obtained on a Siemens 3 T Skyra scanner (Erlangen, Germany), with the following specifications: TR = 2300 ms, TE = 3 ms, 9° angle, 2 acquisitions, and 160 × 256 × 256 matrix. Participants who received PET scans at VAGLAHS had MRI scans with the following specifications: 3 T GE Medical Systems Signa scanner (Milwaukee, WI) three-dimensional Fourier-transform (3DFT) spoiled-gradient-recalled acquisition with TR = 30 ms, TE = 7 ms, 30° angle, 2 acquisitions, and 256 × 192 view matrix.
As in previous research by our group (Brody et al. 2002; Brody et al. 2004; Brody et al. 2006a; b; Brody et al. 2009a; b), MRI/PET co-registration was performed using Statistical Parametric Mapping software (FIL Methods Group, UK), and automated volumes of interest (VOIs) were determined on MRI using FSL tools for structural MRI. These automated VOIs were transferred from each participant’s MRI to his/her co-registered PET scan and visually inspected using PMOD (PMOD Technologies Ltd., Zurich, Switzerland). The primary VOI was whole brain (including gray and white matter) for reasons noted above. However, since automated volumes are easily attained and regional differences are possible, VOIs were also determined for the amygdala, caudate, hippocampus, nucleus accumbens, putamen, and thalamus, similar to VOIs obtained in prior research (Takano et al. 2010; Yasuno et al. 2012).
In order to obtain semi-quantitative measurements of radiotracer binding to TSPO in brain, SUVs were calculated using the standard definition of SUV = mean tissue activity concentration (Bq/mL)/(injected dose (Bq)/body weight (g)). Mean tissue activity from 20 to 40 min post-injection was used, based on time-activity curves from our previous study (Brody et al. 2017) demonstrating stable activity during this time period. SUV was used as the primary outcome measure because it avoids invasive arterial blood sampling.
For the statistical analysis of data, demographic and rating scale variables were compared between groups using Student t tests for continuous variables and Chi-square tests for categorical variables, in order to determine if groups were similar on these variables. For the central study analysis, an analysis of covariance (ANCOVA) was performed, with whole-brain SUV as the measure of interest and both group (overnight abstinent smoker vs. nonsmoker) and TSPO genotype (heterozygous or homozygous for the high affinity allele) as between-subject factors (as in Setiawan et al. 2015; Varrone et al. 2015). Research site (UCSD vs. VAGLAHS) was included in the model as a nuisance covariate, in order to control for possible systematic differences in SUVs related to the different PET scanners or other differences in methodology at the two sites (which were somewhat, but not significantly, different). To determine if group differences were due to regional effects, a multivariate ANCOVA (MANCOVA), using the smaller automated VOIs, was performed with the same structure as the preceding ANCOVA, followed by univariate ANCOVAs for the individual VOIs. The use of an overall MANCOVA for determination of main effect of group (nonsmoker vs. overnight abstinent smoker) controls for multiple comparisons. For the smaller VOIs, means of left and right SUVs were used. For descriptive purposes, percent difference between study groups for both sites was calculated as: 100 * (SUVnonsmokers − SUVsmokers) / SUVnonsmokers. Based on prior research reporting greater brain exposure to cigarette smoke in menthol- than nonmenthol-cigarette smokers, we also performed an ANCOVA for whole brain SUV with the same structure as the above test, using nonsmoker vs. menthol- vs. nonmenthol-cigarette preference as a between-subject factor, followed by the same analyses for smaller automated VOIs as for the preceding analysis. For the exploration of associations between PET data and smoking-related behavioral symptoms, correlations were determined for the smoker group between whole brain SUV and smoking-related ratings (for smokers with completed rating scales), while controlling for genotype. For exploratory associations between SUVs and demographic variables, correlations or tests for associations with the same structure as in the preceding analyses were performed for age, sex, and race/ethnicity for the whole study sample. Statistical tests were performed using the statistical software program SPSS version 24 (SPSS Inc., Chicago, IL).
Results
The nonsmoker and overnight abstinent smoker groups did not differ significantly in age (P = 0.24), sex (P = 0.71), race/ethnicity (P = 0.50), height (P = 0.18), weight (P = 0.25), depression (P = 0.16) or anxiety (P = 0.94) levels, or caffeine (P = 0.23), alcohol (P = 0.27), or marijuana (P = 0.29) use (Table 1). On average, participants were middle-aged, mostly male, and had generally low levels of depression/anxiety and drug/alcohol use. On the scan day, participants had low levels of exhaled CO (1.9 ± 0.8 ppm for the nonsmoker group and 3.3 ± 1.4 ppm for smokers), and smokers had a mean of 17.9 ± 2.3 h abstinence at the time of radiotracer injection (due to overnight abstinence and availability of PET scanning for research at our institutions in the mid-to-late afternoon).
PET data analysis comparing overnight abstinent smokers and nonsmokers revealed a significant effect of group for whole brain SUVs (ANCOVA, P = 0.004), due to the overnight abstinent smokers having lower values than nonsmokers. Mean whole brain SUVs were 0.87 for nonsmokers and 0.73 for overnight abstinent smokers at UCSD and 1.00 for nonsmokers and 0.83 for overnight abstinent smokers at VAGLAHS (15.5% lower at UCSD and 17.0% lower at VAGLAHS). Consistent with these global findings, a significant multivariate effect of group was found (MANCOVA, P = 0.002) for the smaller VOIs, with all VOIs having a significant between-group effect on univariate analysis (range of P values < 0.0005 to 0.026), due to overnight-abstinent smokers having lower SUV values than nonsmokers (range 6.8 to 29.5%) in all VOIs studied.
For the three-group comparison (nonsmokers vs. nonmenthol-cigarette smokers vs. menthol-cigarette smokers), the whole brain SUV comparison was significant (ANCOVA, P = 0.001), due to nonsmokers having the highest values, followed by nonmenthol-cigarette smokers, and then menthol-cigarette smokers. In the multivariate analysis of smaller VOIs, a significant effect of group was found (MANCOVA, P < 0.0005), with all VOIs having a significant between-group effect, due to the range (from high to low) of SUV values from smokers to nonmenthol smokers to menthol smokers. In comparing only the nonmenthol- with the menthol-cigarette smokers, the whole brain SUV comparison was significant (ANCOVA, P = 0.02), and similar results were found for the smaller VOIs (ANOVAs; P = 0.02 to 0.07), possibly less highly significant than the overall analysis due to the smaller samples used for comparing the nonmenthol- with the menthol-cigarette smoker subgroups. In comparing results from the two study sites, ratios of smoker to non-smoker SUVs were similar (Table 2).
In the exploratory analysis of smoking-related behavioral variables, a significant inverse relationship was found between depth of inhalation of cigarettes (as rated by participants on the smoker’s profile form) and whole brain SUV (correlation = −0.74, P = 0.02), suggesting that higher levels of cigarette smoke exposure were associated with lower levels of TSPO availability (Fig. 2). No significant associations were found for cigarettes per day, FTND, SJWS scores, age, sex, or race/ethnicity.
Discussion
Cigarette smokers who underwent overnight abstinence had less [11C]DAA1106 binding than nonsmokers throughout the brain, indicating less TSPO availability (and less neuroinflammatory function). Furthermore, menthol-cigarette smokers had lower levels of [11C]DAA1106 binding than nonmenthol-cigarette smokers. These findings are remarkably similar to those of our previous study of smokers in the satiated state (Brody et al. 2017), and indicate that chronic cigarette smoking leads to reduced TSPO availability found in satiety and persisting into early (overnight) abstinence. In addition, the global (rather than localized) effect found here is consistent with prior research demonstrating global effects of smoking on other molecules (such as nAChRs) that are located throughout the brain (Brody et al. 2006a; Brody et al. 2009a; Brody et al. 2011; Brody et al. 2013; Cosgrove et al. 2009; Staley et al. 2006).
A straightforward explanation for the central study finding is that chronic cigarette smoking leads to global impairment of microglial activation in early abstinence. This explanation is consistent with much prior research demonstrating that smokers have impaired inflammatory functioning in other parts of the body, an effect that leads to compromised wound healing (Goncalves et al. 2011; Towler 2000) and lasts for weeks (Pluvy et al. 2015; Rinker 2013). It is also consistent with basic science research demonstrating that microglial cells express nAChRs (Shytle et al. 2004) and that pre-treatment with nicotine (or acetylcholine) inhibits microglial activation (De Simone et al. 2005; Park et al. 2007; Shytle et al. 2004). Similarly, in an animal study of autoimmune encephalomyelitis, nicotine administration was found to decrease microglial activation (Gao et al. 2014), although other condensate of cigarette smoke was found to increase it. In addition, the inverse correlation in this study between [11C]DAA1106 binding and participant self-reports of depth of inhalation indicates that severity of impaired microglial activation may be related to the amount of exposure to cigarette smoke (since depth of inhalation is known to affect absorbed constituents of tobacco smoke (Benowitz et al. 2009)).
Other explanations for study findings are possible. For example, cigarette smoking has been shown to reduce numbers of resident microglial cells (Shi et al. 2009), which would presumably lead to less radiotracer binding. Cigarette smokers are also known to have higher metabolism of some medications than nonsmokers (Li and Shi 2015), which (if applicable to [11C]DAA1106) would be expected to lessen radiotracer uptake and binding. In addition, cigarette smoking may result in inflammation in parts of the body other than brain (Rom et al. 2013), which could lead to sequestration of the radiotracer and less binding in brain.
In addition to the overall difference between smokers and nonsmokers, the menthol cigarette smoker subgroup had less [11C]DAA1106 binding than the nonmenthol-cigarette smoker subgroup. This finding is consistent with prior research by our group (Brody et al. 2013) showing greater up-regulation of nicotinic acetylcholine receptors throughout almost all brain regions in menthol- than nonmenthol-cigarette smokers, along with a similar finding for TSPO availability in our prior study with [11C]DAA1106 PET and smokers in satiety (Brody et al. 2017). Also, research by others demonstrates that menthol-cigarette smoking is associated with more severe biological abnormalities in some (Williams et al. 2007), but not all (Abobo et al. 2012; Muscat et al. 2009), studies that have examined this issue. Therefore, as in prior research, the present finding may be due to greater brain exposure to cigarette smoke (leading to greater impairment of microglial activation) in menthol-cigarette smokers, a direct effect of menthol flavoring or some other mechanism.
The primary limitation of this study is the absence of arterial blood sampling, precluding the determination of total distribution volume (Vt), which is the gold standard outcome measure for this type of research. Vt may control for the potential confounds of between-subject differences in radiotracer metabolism and binding to vascular endothelium and plasma protein (Koshimori et al. 2015; Rizzo et al. 2014; Turkheimer et al. 2015). While Vt is a common outcome measure in PET studies examining TSPO in conditions other than tobacco dependence (Colasanti et al. 2016; Haarman et al. 2016; Narendran et al. 2014), the SUV measure was used because it is less invasive than methods that include arterial blood sampling and arterial sampling was not feasible. Other similar studies have used pseudo-reference regions for PET data analysis (Colasanti et al. 2016; Coughlin et al. 2014; Hamelin et al. 2016; Kreisl et al. 2016; Lyoo et al. 2015; Zurcher et al. 2015) to minimize potential confounds, but this method would not have been appropriate here due to the hypothesized and confirmed effect of smoking throughout the brain. Additional limitations included scanning at two sites with different model scanners (though results were similar at the two sites), a modest sample size, the absence of measurement of brain nAChR levels, and the fact that smokers were scanned in early abstinence, such that we did not determine whether TSPO availability normalizes with prolonged abstinence. Associations between TSPO availability and brain nAChR availability as determined with PET or single photon emission computed tomography imaging (as we and others have done in prior research (Brody et al. 2013, Brody et al. 2016, Cosgrove et al. 2009; Staley et al. 2006)) or evaluation of normalization of TSPO availability with prolonged abstinence could be addressed in future research.
In summary, cigarette smokers in the overnight abstinent state have lower TSPO availability than nonsmoking controls, similar to our recent finding in satiated smokers. The effect was global and greater for menthol- than nonmenthol-cigarette smokers. Future research could build upon this and our previous (Brody et al. 2017) initial studies and directly compare TSPO availability in smokers in satiety with those undergoing short-term and prolonged abstinence. This approach could provide direct information about the time course of normalization of available TSPO levels in smokers who maintain abstinence. Additional future studies could focus on the interplay between smoking, neuroinflammation, and the progression of diseases thought to be mediated by neuroinflammation.
References
Abobo CV, Ma J, Liang D (2012) Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res 14:801–808
Anthony DC, Pitossi FJ (2012) Special issue commentary: the changing face of inflammation in the brain. Mol Cell Neurosci
Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck depression inventories-IA and -II in psychiatric outpatients. J Pers Assess 67:588–597
Benowitz NL, Hukkanen J, Jacob P, 3rd (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 29–60
Brody AL, Mandelkern MA, London ED, Childress AR, Bota RG, Ho ML, Lee GS, Saxena S, Baxter LR, Madsen D, Jarvik ME (2002) Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59:1162–1172
Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA (2004) Smoking-induced ventral striatum dopamine release. Am J Psychiatr 161:1211–1218
Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG (2006a) Cigarette smoking saturates brain alpha4beta2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63:907–915
Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT (2006b) Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry 63:808–816
Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG (2009a) Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. IntJNeuropsychopharmacol 12:305–316
Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED (2009b) Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology 34:282–289
Brody AL, Mandelkern MA, London ED, Khan A, Kozman D, Costello MR, Vellios EE, Archie MM, Bascom R, Mukhin AG (2011) Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Arch Gen Psychiatry 68:953–960
Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA, Mamoun MS, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern MA (2013) Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol 16:957–966
Brody AL, Okita K, Shieh J, Liang L, Hubert R, Mamoun M, Farahi J, Mandelkern MA (2014) Radiation dosimetry and biodistribution of the translocator protein radiotracer [(11)C]DAA1106 determined with PET/CT in healthy human volunteers. Nucl Med Biol 41:871–875
Brody AL, Hubert R, Mamoun MS, Enoki R, Garcia LY, Abraham P, Young P, Mandelkern MA (2016) Nicotinic acetylcholine receptor availability in cigarette smokers: effect of heavy caffeine or marijuana use. Psychopharmacology 233:3249–3257
Brody AL, Hubert R, Enoki R, Garcia LY, Mamoun MS, Okita K, London ED, Nurmi EL, Seaman LC, Mandelkern MA (2017) Effect of cigarette smoking on a marker for neuroinflammation: a [(11)C]DAA1106 positron emission tomography study. Neuropsychopharmacology 42:1630–1639
Chaki S, Funakoshi T, Yoshikawa R, Okuyama S, Okubo T, Nakazato A, Nagamine M, Tomisawa K (1999) Binding characteristics of [3H]DAA1106, a novel and selective ligand for peripheral benzodiazepine receptors. Eur J Pharmacol 371:197–204
Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B (2008) Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging 35:2304–2319
Colasanti A, Guo Q, Giannetti P, Wall MB, Newbould RD, Bishop C, Onega M, Nicholas R, Ciccarelli O, Muraro PA, Malik O, Owen DR, Young AH, Gunn RN, Piccini P, Matthews PM, Rabiner EA (2016) Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry 80:62–72
Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O'Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK (2009) beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry 66:666–676
Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, Roosa HV, Gage KL, Stathis M, Rais R, Rojas C, McGlothan JL, Watkins CC, Sacktor N, Guilarte TR, Zhou Y, Sawa A, Slusher BS, Caffo B, Kassiou M, Endres CJ, Pomper MG (2014) Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neuro-Oncol 20:219–232
De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L (2005) Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation 2:4
Esterlis I, Cosgrove KP, Batis JC, Bois F, Stiklus SM, Perkins E, Seibyl JP, Carson RE, Staley JK (2010) Quantification of smoking-induced occupancy of {beta}2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. J Nucl Med 51:1226–1233
Fagerstrom KO (1978) Measuring the degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3:235–241
Foucault-Fruchard L, Antier D (2017) Therapeutic potential of alpha7 nicotinic receptor agonists to regulate neuroinflammation in neurodegenerative diseases. Neural Regen Res 12:1418–1421
Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM (2009) Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract 63:360–367
Gao Z, Nissen JC, Ji K, Tsirka SE (2014) The experimental autoimmune encephalomyelitis disease course is modulated by nicotine and other cigarette smoke components. PLoS One 9:e107979
Goncalves RB, Coletta RD, Silverio KG, Benevides L, Casati MZ, da Silva JS, Nociti FH Jr (2011) Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res 60:409–424
Gonzalez H, Elgueta D, Montoya A, Pacheco R (2014) Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol 274:1–13
Haarman BC, Burger H, Doorduin J, Renken RJ, Sibeijn-Kuiper AJ, Marsman JB, de Vries EF, de Groot JC, Drexhage HA, Mendes R, Nolen WA, Riemersma-Van der Lek RF (2016) Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder—a combined magnetic resonance imaging and positron emission tomography study. Brain Behav Immun 56:21–33
Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP, Suridjan I, Wilson AA, Meyer JH, Remington G, Houle S, Rusjan PM, Mizrahi R (2016) Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F]FEPPA. Am J Psychiatry. https://doi.org/10.1176/appi.ajp.2016.16020171
Hamelin L, Lagarde J, Dorothee G, Leroy C, Labit M, Comley RA, de Souza LC, Corne H, Dauphinot L, Bertoux M, Dubois B, Gervais P, Colliot O, Potier MC, Bottlaender M, Sarazin M, Clinical It (2016) Early and protective microglial activation in Alzheimer's disease: a prospective study using 18F-DPA-714 PET imaging. Brain 139:1252–1264
Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, Pittman B, Lee JY, O'Connor KC, Pelletier D, Carson RE (2013) The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain Behav Immun 33:131–138
Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict 86:1119–1127
Heck JD (2009) Smokers of menthol and nonmenthol cigarettes exhibit similar levels of biomarkers of smoke exposure. Cancer Epidemiol Biomark Prev 18:622–629
Hukkanen J, Jacob P III, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 57:79–115
Ikoma Y, Yasuno F, Ito H, Suhara T, Ota M, Toyama H, Fujimura Y, Takano A, Maeda J, Zhang MR, Nakao R, Suzuki K (2007) Quantitative analysis for estimating binding potential of the peripheral benzodiazepine receptor with [(11)C]DAA1106. J Cereb Blood Flow Metab 27:173–184
Koshimori Y, Ko JH, Mizrahi R, Rusjan P, Mabrouk R, Jacobs MF, Christopher L, Hamani C, Lang AE, Wilson AA, Houle S, Strafella AP (2015) Imaging striatal microglial activation in patients with Parkinson’s disease. PLoS One 10:e0138721
Kreisl WC, Lyoo CH, Liow JS, Wei M, Snow J, Page E, Jenko KJ, Morse CL, Zoghbi SS, Pike VW, Turner RS, Innis RB (2016) (11)C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol Aging 44:53–61
Li H, Shi Q (2015) Drugs and diseases interacting with cigarette smoking in US prescription drug labelling. Clin Pharmacokinet 54:493–501
Li X, Han X, Bao J, Liu Y, Ye A, Thakur M, Liu H (2016) Nicotine increases eclampsia-like seizure threshold and attenuates microglial activity in rat hippocampus through the alpha7 nicotinic acetylcholine receptor. Brain Res 1642:487–496
Low D, Ginhoux F (2018) Recent advances in the understanding of microglial development and homeostasis. Cell Immunol 330:68–78
Lyoo CH, Ikawa M, Liow JS, Zoghbi SS, Morse CL, Pike VW, Fujita M, Innis RB, Kreisl WC (2015) Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect Neuroinflammation measured with PET Radioligand binding to translocator protein. J Nucl Med 56:701–706
Maeda J, Suhara T, Zhang MR, Okauchi T, Yasuno F, Ikoma Y, Inaji M, Nagai Y, Takano A, Obayashi S, Suzuki K (2004) Novel peripheral benzodiazepine receptor ligand [11C]DAA1106 for PET: an imaging tool for glial cells in the brain. Synapse 52:283–291
Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I, De Luca V, Wilson AA, Houle S (2012) Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab 32:968–972
Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie JP Jr (2009) Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiol Biomark Prev 18:35–41
Narendran R, Lopresti BJ, Mason NS, Deuitch L, Paris J, Himes ML, Kodavali CV, Nimgaonkar VL (2014) Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J Neurosci 34:9945–9950
Noda M, Kobayashi AI (2017) Nicotine inhibits activation of microglial proton currents via interactions with alpha7 acetylcholine receptors. J Physiol Sci 67:235–245
Okubo T, Yoshikawa R, Chaki S, Okuyama S, Nakazato A (2004) Design, synthesis and structure-affinity relationships of aryloxyanilide derivatives as novel peripheral benzodiazepine receptor ligands. Bioorg Med Chem 12:423–438
Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS (2007) Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 102:1979–1986
Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, Innis RB, Pike VW, Reynolds R, Matthews PM, Parker CA (2011) Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med 52:24–32
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP (2012) An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32:1–5
Park HJ, Lee PH, Ahn YW, Choi YJ, Lee G, Lee DY, Chung ES, Jin BK (2007) Neuroprotective effect of nicotine on dopaminergic neurons by anti-inflammatory action. Eur J Neurosci 26:79–89
Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S (2006) Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the coronary artery risk development in young adults (CARDIA) study. Arch Intern Med 166:1915–1922
Pluvy I, Garrido I, Pauchot J, Saboye J, Chavoin JP, Tropet Y, Grolleau JL, Chaput B (2015) Smoking and plastic surgery, part I. pathophysiological aspects: update and proposed recommendations. Ann Chir Plast Esthet 60:e3–e13
Quik M (2004) Smoking, nicotine and Parkinson's disease. Trends Neurosci 27:561–568
Quik M, Zhang D, McGregor M, Bordia T (2015) Alpha7 nicotinic receptors as therapeutic targets for Parkinson's disease. Biochem Pharmacol 97:399–407
Rinker B (2013) The evils of nicotine: an evidence-based guide to smoking and plastic surgery. Ann Plast Surg 70:599–605
Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A (2014) Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data. J Cereb Blood Flow Metab 34:1060–1069
Rom O, Avezov K, Aizenbud D, Reznick AZ (2013) Cigarette smoking and inflammation revisited. Respir Physiol Neurobiol 187:5–10
SAMHSA (2009) Substance abuse and mental health services administration. Office of applied studies. The NSDUH Report: Use of Menthol Cigarettes
Schetters STT, Gomez-Nicola D, Garcia-Vallejo JJ, Van Kooyk Y (2017) Neuroinflammation: microglia and T cells get ready to tango. Front Immunol 8:1905
Schuh KJ, Stitzer ML (1995) Desire to smoke during spaced smoking intervals. Psychopharmacology 120:289–295
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, Meyer JH (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–275
Shi FD, Piao WH, Kuo YP, Campagnolo DI, Vollmer TL, Lukas RJ (2009) Nicotinic attenuation of central nervous system inflammation and autoimmunity. J Immunol 182:1730–1739
Shiffman SM, Jarvik ME (1976) Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology 50:35–39
Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J (2004) Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem 89:337–343
Song N, Wang J, Jiang H, Xie J (2018) Astroglial and microglial contributions to iron metabolism disturbance in Parkinson’s disease. Biochim Biophys Acta 1864:967–973
Spielberger C (1983) Manual for the state-trait anxiety inventory consulting psychologists press, Palo Alto, CA
Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O'Malley S, van Dyck CH (2006) Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci 26:8707–8714
Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, Okubo Y, Suhara T (2010) Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 13:943–950
Towler J (2000) Cigarette smoking and its effects on wound healing. J Wound Care 9:100–104
Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A, Veronese M (2015) The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans 43:586–592
Varrone A, Oikonen V, Forsberg A, Joutsa J, Takano A, Solin O, Haaparanta-Solin M, Nag S, Nakao R, Al-Tawil N, Wells LA, Rabiner EA, Valencia R, Schultze-Mosgau M, Thiele A, Vollmer S, Dyrks T, Lehmann L, Heinrich T, Hoffmann A, Nordberg A, Halldin C, Rinne JO (2015) Positron emission tomography imaging of the 18-kDa translocator protein (TSPO) with [18F]FEMPA in Alzheimer's disease patients and control subjects. Eur J Nucl Med Mol Imaging 42:438–446
Venneti S, Lopresti BJ, Wang G, Slagel SL, Mason NS, Mathis CA, Fischer ML, Larsen NJ, Mortimer AD, Hastings TG, Smith AD, Zigmond MJ, Suhara T, Higuchi M, Wiley CA (2007) A comparison of the high-affinity peripheral benzodiazepine receptor ligands DAA1106 and (R)-PK11195 in rat models of neuroinflammation: implications for PET imaging of microglial activation. J Neurochem 102:2118–2131
Venneti S, Wang G, Nguyen J, Wiley CA (2008) The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol 67:1001–1010
Waisman A, Ginhoux F, Greter M, Bruttger J (2015) Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol 36:625–636
Wang M, Gao M, Zheng QH (2012) Fully automated synthesis of PET TSPO radioligands [11C]DAA1106 and [18F]FEDAA1106. Appl Radiat Isot 70:965–973
Ward MM, Swan GE, Jack LM (2001) Self-reported abstinence effects in the first month after smoking cessation. Addict Behav 26:311–327
Werley MS, Coggins CR, Lee PN (2007) Possible effects on smokers of cigarette mentholation: a review of the evidence relating to key research questions. Regul Toxicol Pharmacol 47:189–203
Williams JM, Gandhi KK, Steinberg ML, Foulds J, Ziedonis DM, Benowitz NL (2007) Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob Res 9:873–881
Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK, Nozaki S, Fujimura Y, Koeda M, Asada T, Suhara T (2008) Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry 64:835–841
Yasuno F, Kosaka J, Ota M, Higuchi M, Ito H, Fujimura Y, Nozaki S, Takahashi S, Mizukami K, Asada T, Suhara T (2012) Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [(11)C]DAA1106. Psychiatry Res 203:67–74
Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ (2013) Influence of TSPO genotype on 11C-PBR28 standardized uptake values. J Nucl Med 54:1320–1322
Zhang MR, Kida T, Noguchi J, Furutsuka K, Maeda J, Suhara T, Suzuki K (2003) [(11)C]DAA1106: radiosynthesis and in vivo binding to peripheral benzodiazepine receptors in mouse brain. Nucl Med Biol 30:513–519
Zurcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE, Akeju O, Catana C, Rosen BR, Cudkowicz ME, Hooker JM, Atassi N (2015) Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. Neuroimage Clin 7:409–414
Acknowledgements
The authors thank Josephine Ribe and Christopher Davis for performing positron emission tomography scanning for the study.
Funding
This study was supported by the Tobacco-Related Disease Research Program (A.L.B. grant #588000), the National Institute on Drug Abuse (A.L.B. (R01 DA044909)), and the Department of Veterans Affairs, Office of Research and Development (CSR&D Merit Review Award I01 CX000412 (A.L.B.)). This research was also supported, in part, by the DOMONKAI fund from the Department of Psychiatry, Graduate School of Medicine, at Chiba University (K.O.), endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies (E.D.L.) and Marjorie M. Green Trust (E.D.L.), and the National Institutes of Health (T32 DA024635 (L.S.)). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Brody, A.L., Gehlbach, D., Garcia, L.Y. et al. Effect of overnight smoking abstinence on a marker for microglial activation: a [11C]DAA1106 positron emission tomography study. Psychopharmacology 235, 3525–3534 (2018). https://doi.org/10.1007/s00213-018-5077-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5077-3