Abstract
Background
Parkinson’s disease (PD) psychosis is encountered in as many as 50% of patients with advanced disease. Treatment options for PD psychosis are few. In fact, only clozapine and pimavanserin have shown efficacy in randomised controlled trials. Clinicians are often reluctant to prescribe the former, due to the risk of agranulocytosis, while the latter is not widely available yet. Because it is already clinically available and exhibits high affinity for serotonin 2A receptors, a target with which both clozapine and pimavanserin interact, we hypothesised that the anti-depressant mirtazapine might be effective to alleviate PD psychosis.
Methods
Here, we tested the anti-psychotic potential of mirtazapine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned common marmoset. Five MPTP-lesioned marmosets exhibiting psychosis-like behaviours were administered L-3,4-dihydroxyphenylalanine (L-DOPA) in combination with mirtazapine (0.1, 1 and 10 mg/kg) or vehicle. We also tested the effect of mirtazapine on L-DOPA-induced dyskinesia.
Results
The addition of mirtazapine 10 mg/kg to L-DOPA reduced psychosis-like behaviours by 50% (P < 0.05) and dyskinesia by 29% (P < 0.01), when compared to L-DOPA/vehicle. Importantly, the antipsychotic and antidyskinetic effects of mirtazapine were achieved without hindering L-DOPA anti-parkinsonian action.
Conclusions
Our results suggest that mirtazapine may be effective to alleviate PD psychosis and, because the drug is clinically available, clinical trials that would assess its anti-psychotic efficacy in PD could be rapidly undertaken, hopefully leading to a new treatment option for this debilitating condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psychosis, especially visual hallucinations, is encountered in 50% of patients with Parkinson’s disease (PD) 15 years after treatment with L-3,4-dihydroxyphenylalanine (L-DOPA) has begun (Hely et al. 2005). It significantly undermines patients’ quality of life (Friedman 2013).
The treatment of PD psychosis remains an unmet medical need. Thus, clozapine effectively alleviates psychosis (French Clozapine Parkinson Study Group 1999; Parkinson Study Group 1999), but the risk of agranulocytosis (Alvir et al. 1993) prevents this drug from being widely prescribed. Recently, the serotonin 2A/2C (5-HT2A/2C) inverse agonist pimavanserin (Cummings et al. 2014) was approved by the United States (US) Food and Drug Administration (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm498442.htm) and marketed in the US as Nuplazid™ “for the treatment of hallucinations and delusions associated with PD psychosis” (http://www.acadia-pharm.com/product/), but it is not widely available yet, being restricted to the US market.
Mirtazapine is an atypical anti-depressant that harbours affinity for a breadth of receptors, including 5-HT2A receptors (de Boer 1996), suggesting that it may be useful to alleviate PD psychosis. The fact that it has low affinity for dopamine D2 receptors (Anttila and Leinonen 2001) renders it less susceptible to interfere with L-DOPA anti-parkinsonian action and makes it an attractive drug to administer to PD patients. Case reports have suggested that mirtazapine may effectively alleviate hallucinations, both auditory and visual, experienced by PD patients (Godschalx-Dekker and Siegers 2014; Nagata et al. 2012, 2013; Tagai et al. 2013). However, there is also a case report where mirtazapine triggered psychiatric features in a PD patient (Normann et al. 1997).
As such, whereas mirtazapine may appear, based on its pharmacological profile, as an interesting candidate to alleviate PD psychosis, the evidence are anecdotal and conflicting. Here, we sought to assess the anti-psychotic effect of mirtazapine on psychosis-like behaviours in the gold-standard animal model of PD, the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate. We concomitantly determined the effects of mirtazapine on L-DOPA-induced dyskinesia and parkinsonian disability.
Materials and methods
Induction of parkinsonism and dyskinesia in the common marmoset
Five common marmosets (Callithrix jacchus; WorldWide Primates, USA; 2 males and 3 females), weighing 350–400 g, were housed in groups of 2–3 under conditions of controlled temperature (24 ± 1 °C), humidity (50%) and a 12 h light/dark cycle (07:15 lights on). Animals were cared for in accordance with a protocol approved by McGill University Animal Care Committee in accordance with the regulations defined by the Canadian Council on Animal Care. They had unlimited access to food, fresh fruits and water. Home cages were enriched with primate toys and perches. Prior to the start of studies, animals were acclimatised to handling, administration of subcutaneous (s.c.) treatments, as well as transfer to observation cages for behavioural assessment.
Parkinsonism was induced by injections of MPTP hydrochloride (2 mg/kg s.c. daily or every other day, for 5 days, tailored to the animals’ reaction to MPTP; Sigma-Aldrich, Canada). The MPTP administration phase was followed by a 6-week recovery period to allow parkinsonian symptoms to develop and stabilise. Treatment-related complications, including dyskinesia and psychosis-like behaviours, were elicited by treatment with oral L-DOPA/benserazide (henceforth referred to as L-DOPA, 15/3.75 mg/kg once daily; Sigma-Aldrich, Canada) for a minimum of 30 days. This treatment regimen has been previously demonstrated to produce a stable model of L-DOPA-induced dyskinesia and psychosis-like behaviours (Huot et al. 2012, 2014).
Administration of mirtazapine, in combination with L-DOPA, to parkinsonian marmosets
On days of behavioural assessment, at 08:00, marmosets were injected with a therapeutic dose of L-DOPA 15/3.75 mg/kg s.c. (Sigma-Aldrich, Canada) in combination with either vehicle (0.9% NaCl) or mirtazapine, (0.1, 1 and 10 mg/kg s.c.; Cedarlane Laboratories, Canada). Drug administration schedule was randomised according to a Latin square design in which all animals received all treatments, in a random order. After administration of a given treatment, each marmoset was placed individually into an observation cage (36 × 33 × 22 in. containing food, water and a wooden perch, and left undisturbed for the 6 h duration of the experiment. Behaviour was recorded via webcam and analysed post hoc by a movement disorders neurologist blinded to the treatment given. At least 48 h were left between each treatment in any animal.
Motor activity
A quantitative assessment of marmoset activity was made using the webcam-based motion detection software Zone Trigger (Omega Unfold Inc., Montreal, Canada). Activity counts were detected every minute during the 6 h duration of the experiment, logged as a .txt document and exported into a Microsoft Office Excel (Microsoft, Redmond, USA) format for processing.
Behavioural analysis
The scales used for assessment of behaviour were described in detail previously (Fox et al. 2010; Huot et al. 2011). Parkinsonian disability was rated for 5 min every 10 min using a parkinsonian disability scale combining measures of range of movement, bradykinesia, posture and attention/alertness. Range of movement was rated on a 0 to 9 scale: 0 = running, jumping between roof, walls, perch, using limbs through a wide range of activity; 9 = no movement. Bradykinesia was rated from 0 to 3: 0 = normal initiation and speed of movement; 3 = prolonged freezing, akinesia, inability to move. Postural abnormalities were rated 0 or 1: 0 = normal balance, upright posture, head held up; 1 = impaired balance, crouched posture, head down. Attention/alertness was rated 0 or 1; 0 = normal head checking movements, movement of neck in variable directions, smooth, small movements; 1 = reduced or absent head checking, head in one position for more than 50% of observation period. The score attributed to each of the behaviours assessed was the most representative of the 5 min observation period. A global parkinsonian disability score was calculated as a combination of the behaviours mentioned above, equally weighted, according to the following formula: (range of movement × 1) + (bradykinesia × 3) + (posture × 9) + (alertness × 9). The maximal parkinsonian disability score per 5 min observation period was 36.
L-DOPA-induced psychosis-like behaviours and dyskinesia were also assessed. Both psychosis-like behaviours and dyskinesia were rated from 0 to 4. The following psychosis-like behaviours were assessed: hyperkinesia, response to non-apparent stimuli (hallucinatory-like behaviour), repetitive grooming and stereotypies. Each of these was rated from 0 to 4, where 0 = absent; 4 = severe, at times interfering with normal activity, present more than 30% of the observation period. For dyskinesia, 0 = absent, whereas 4 = severe, continuous, replacing normal activity, present more than 70% of the observation period. For any 5 min period of assessment, the psychosis-like behaviours score attributed was the most disabling of any of the four sub-scores observed. In any 5 min period of assessment, choreiform and dystonic dyskinesias were graded separately and the dyskinesia score given reflected the most disabling dyskinesia observed. Several articles assessing psychosis-like behaviours in the MPTP-lesioned marmoset have been published (Fox et al. 2010, 2006; Huot et al. 2012, 2011; Visanji et al. 2006) and the scale used here to rate behaviours was detailed and validated in (Fox et al. 2010).
Parkinsonian disability, dyskinesia and psychosis-like behaviours scores were cumulated for each hour across the entire 6 h of observation. Duration of anti-parkinsonian benefit, on-time, was defined as the number of minutes for which bradykinesia was absent (score 0).
Statistical analysis
Continuous motor activity scores are presented as the mean and were analysed by two-way repeated measures analysis of variance (RM ANOVA) followed by Tukey’s post hoc tests. Categorical, discontinuous scores for parkinsonian disability, dyskinesia and psychosis-like behaviours severity are presented as the median with individual values and were analysed using non-parametric Friedman followed by Dunn’s post hoc tests. On-time data are presented as the mean ± standard error (SEM) and were analysed by one-way RM ANOVA followed by Tukey’s post hoc tests. Time course data for parkinsonian disability were ranked by marmoset across each of the four treatments and analysed by a two-way ANOVA followed by Tukey’s post hoc tests. Statistical significance was assigned when P < 0.05. Statistical analyses were computed using GraphPad Prism 6.0 h (GraphPad Software Inc., La Jolla, USA).
Results
Mirtazapine was well tolerated by animals at all doses administered. We did not observe any adverse events, including sedation.
Mirtazapine reduces the intensity of L-DOPA-induced motor activity
As shown in Fig. 1a, the addition of mirtazapine (each of 0.1, 1 and 10 mg/kg) to L-DOPA produced a significant reduction of global motor activity (≈70%) compared to L-DOPA alone (F time(5,24) = 1.743, P > 0.05; F treatment(3,72) = 6.723, P < 0.001; F interaction(15,72) = 0.136, P > 0.05; two-way RM ANOVA). However, mirtazapine did not significantly reduce motor activity at any particular time point in the post hoc test. This important reduction of motor activity was driven by one marmoset who appeared to be markedly hyperactive. As shown in Fig. 1b, following exclusion of this animal, mirtazapine no longer produced a significant reduction of motor activity (F time(5,18) = 10.32, P < 0.001; F treatment(3,54) = 1.184, P > 0.05; F interaction(15,54) = 0.460, P > 0.05; two-way RM ANOVA).
a Time course of motor activity in MPTP-lesioned marmosets treated with L-DOPA in combination with mirtazapine (0.1, 1 and 10 mg/kg) or vehicle. Although mirtazapine significantly reduced global motor activity, it did not reduce it significantly at any specific time point. b Time course of motor activity in MPTP-lesioned marmosets treated with L-DOPA in combination with mirtazapine (0.1, 1 and 10 mg/kg) or vehicle following exclusion of an animal that displayed particularly high motor activity
Mirtazapine reduces the severity of L-DOPA-induced psychosis-like behaviours
As illustrated in Fig. 2a, adding mirtazapine to L-DOPA resulted in a significant reduction of psychosis-like behaviours severity (Friedman statistic [FS] = 11.94, P < 0.01). Thus, when mirtazapine 10 mg/kg was added to L-DOPA, psychosis-like behaviours were reduced by ≈50% (P < 0.05, Dunn’s post hoc test), when compared to L-DOPA/vehicle. The addition of mirtazapine 0.1 or 1 mg/kg to L-DOPA did not reduce psychosis-like behaviours, when compared to L-DOPA alone.
a Peak dose psychosis-like behaviours in MPTP-lesioned marmosets treated with L-DOPA in combination with mirtazapine (0.1, 1 and 10 mg/kg) or vehicle. b On-time with disabling psychosis-like behaviours in MPTP-lesioned marmosets treated with L-DOPA in combination with mirtazapine (0.1, 1 and 10 mg/kg) or vehicle. *P < 0.05; **P < 0.01; ***P < 0.001
As shown in Fig. 2b, mirtazapine significantly decreased the duration of on-time with disabling psychosis-like behaviours (F(3,12) = 14.07, P < 0.001, one-way RM ANOVA). Thus, after administration of L-DOPA/vehicle, duration of on-time with disabling psychosis-like behaviours was ≈154 min, while it was ≈76 min after L-DOPA/mirtazapine 1 mg/kg (≈50% reduction, P < 0.01, Tukey’s post hoc test), and ≈56 min after L-DOPA/mirtazapine 10 mg/kg (≈64% reduction, P < 0.001, Tukey’s post hoc test). On-time with disabling psychosis-like behaviours was also significantly shorter when L-DOPA/mirtazapine 10 mg/kg was compared to L-DOPA/mirtazapine 0.1 and 1 mg/kg (P < 0.01 and P < 0.05, respectively, Tukey’s post hoc test).
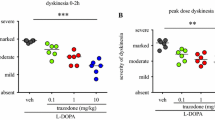
Mirtazapine reduces the severity of L-DOPA-induced dyskinesia
As illustrated in Fig. 3a, adding mirtazapine to L-DOPA resulted in a significant reduction of dyskinesia severity (FS = 12.67, P < 0.001). Thus, when mirtazapine 10 mg/kg was added to L-DOPA, dyskinesia was reduced by ≈29% (P < 0.01, Dunn’s post hoc test), when compared to L-DOPA/vehicle. The addition of mirtazapine 0.1 or 1 mg/kg to L-DOPA did not reduce dyskinesia severity, when compared to L-DOPA alone.
a Peak dose dyskinesia in MPTP-lesioned marmosets treated with L-DOPA in combination with mirtazapine (0.1, 1 and 10 mg/kg) or vehicle. b On-time with disabling dyskinesia in MPTP-lesioned marmosets treated with L-DOPA in combination with mirtazapine (0.1, 1 and 10 mg/kg) or vehicle. *P < 0.05; **P < 0.01; ***P < 0.001
As shown in Fig. 3b, mirtazapine significantly decreased the duration of on-time with disabling dyskinesia (F(3,12) = 23.29, P < 0.001, one-way RM ANOVA). Thus, after administration of L-DOPA/vehicle, duration of on-time with disabling dyskinesia was ≈138 min, while it was ≈98 min after L-DOPA/mirtazapine 0.1 mg/kg (≈29% reduction, P < 0.05, Tukey’s post hoc test), ≈60 min after L-DOPA/mirtazapine 1 mg/kg (≈57% reduction, P < 0.001, Tukey’s post hoc test) and ≈40 min after L-DOPA/mirtazapine 10 mg/kg (≈71% reduction, P < 0.001, Tukey’s post hoc test). On-time with disabling dyskinesia was also significantly shorter when L-DOPA/mirtazapine 10 mg/kg was compared to L-DOPA/mirtazapine 0.1 and 1 mg/kg (P < 0.01 and P < 0.05, respectively, Tukey’s post hoc test).
Mirtazapine does not hinder L-DOPA anti-parkinsonian action
As illustrated in Fig. 4, mirtazapine did not alter the anti-parkinsonian action of L-DOPA. Thus, parkinsonian disability was similar across all treatments (Fig. 4a), as was duration of on-time, which averaged 3 h in all treatments (Fig. 4b).
Discussion
In this study, we have demonstrated that mirtazapine effectively reduces both psychosis-like behaviours and dyskinesia, in the MPTP-lesioned marmoset. Importantly, it did so without hindering L-DOPA anti-parkinsonian action and was well tolerated by animals.
Methodological considerations
In our study, mirtazapine produced a significant decrease in motor activity which reached statistical significance at the ANOVA, but not the post hoc test level. This decrease in motor activity was largely driven by one animal who presented marked hyperactive behaviour following administration of L-DOPA. Upon exclusion of this animal, mirtazapine no longer significantly reduced global motor activity counts. It has previously been shown that such hyperactive behaviour is a potential correlate of impulse-control disorders and dopamine dysregulation syndrome (Johnston et al. 2011). Although we refrain to make strong conclusions based on a single animal, this suggests that mirtazapine might have some usefulness in the treatment of impulse control disorders and dopamine dysregulation syndrome. Importantly, upon exclusion of the hyperactive animal from other statistical analyses, the anti-psychotic and anti-dyskinetic effects of mirtazapine remained significant and mirtazapine did not hinder the anti-parkinsonian action of L-DOPA (data not shown).
The MPTP-lesioned common marmoset has been used to assess the effect of drugs on PD psychosis since 2006 (Visanji et al. 2006). Although PD psychosis is not a merely pharmacological phenomenon, dopaminergic agents may play a role in its pathophysiology (Goetz et al. 1998). Importantly, clozapine significantly alleviated PLBs in the parkinsonian marmoset (Visanji et al. 2006), which is not unlike the situation in clinical settings (Seppi et al. 2011).
Because marmosets body weight is similar to rats, we have selected our doses here based upon a pharmacokinetic study conducted in rats (Rouini et al. 2014) that demonstrated that doses akin to the ones we used lead to plasma levels comparable to those achieved in human after administration of mirtazapine 15–45 mg daily (Delbressine et al. 1998; Jaquenoud Sirot et al. 2012).
Clinical implications
It is noteworthy that mirtazapine had already demonstrated a modest anti-dyskinetic effect in an open-label study (Meco et al. 2003) and, as such, the most important finding of our study is the effect of mirtazapine on psychotic behaviours. Of note, mirtazapine could also improve parkinsonian tremor (Gordon et al. 2002), which suggests that the drug may be used to treat several manifestations and treatment-related complications of PD.
Because mirtazapine is a non-selective drug that interacts with several pharmacological targets (Anttila and Leinonen 2001), we can only speculate on the mechanism(s) that underlies its therapeutic efficacy. Amongst all of the targets for which mirtazapine exhibits high affinity, including alpha-adrenoceptors, 5-HT1A, 5-HT2A and 5-HT2C receptors, only interaction with 5-HT2A/2C receptors has been validated, in a randomised-controlled clinical trial, as an effective anti-psychotic strategy, although several clinical trials have validated alpha-adrenoceptor blockade (Lewitt et al. 2012; Rascol et al. 2001) and 5-HT1A stimulation (Kannari et al. 2002) as effective anti-dyskinetic approaches, sometimes at the expense of L-DOPA anti-parkinsonian benefit. It is noteworthy that 5-HT2A blockade was also shown to alleviate L-DOPA-induced dyskinesia, at the pre-clinical (Huot et al. 2011; Vanover et al. 2008) and clinical (Maertens de Noordhout and Delwaide 1986; Meco et al. 1988) levels. Given mirtazapine affinity for 5-HT2A receptors, and inasmuch as 5-HT2A receptor blockade is a validated anti-psychotic and anti-dyskinetic strategy, it certainly underlies, at least in part, the therapeutic benefits conferred by mirtazapine in our study.
To date, the only clinically available drug that was demonstrated to be effective at reducing both PD psychosis and dyskinesia without impairing the therapeutic benefit conferred by L-DOPA in controlled trials is clozapine. Thus, clozapine effectively reduced psychosis (French Clozapine ParkinsonStudy Group 1999; Parkinson Study Group 1999) and dyskinesia (Durif et al. 2004) but, as mentioned above, its propensity to cause agranulocytosis makes clinicians less inclined to prescribe it. Our results here suggest that mirtazapine could alleviate both L-DOPA-induced psychosis and dyskinesia in PD. Because mirtazapine is a clinically available molecule, small clinical trials that would look at its anti-psychotic potential could be undertaken, hopefully leading to a repositioning of the drug for the treatment of PD psychosis.
References
Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA (1993) Clozapine-induced agranulocytosis. Incidence and risk factors in the United States N Engl J Med 329:162–167. doi:10.1056/NEJM199307153290303
Anttila SA, Leinonen EV (2001) A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev 7:249–264
Cummings J et al (2014) Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383:533–540. doi:10.1016/S0140-6736(13)62106-6
de Boer T (1996) The pharmacologic profile of mirtazapine. J Clin Psychiatry 57(Suppl 4):19–25
Delbressine LP et al (1998) Pharmacokinetics and biotransformation of mirtazapine in human volunteers. Clinical drug investigation 15:45–55
Durif F et al (2004) Clozapine improves dyskinesias in Parkinson disease: a double-blind, placebo-controlled study. Neurology 62:381–388
Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM (2010) Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson’s disease. Can J Neurol Sci 37:86–95
Fox SH, Visanji NP, Johnston TH, Gomez-Ramirez J, Voon V, Brotchie JM (2006) Dopamine receptor agonists and levodopa and inducing psychosis-like behavior in the MPTP primate model of Parkinson disease. Arch Neurol 63:1343–1344. doi:10.1001/archneur.63.9.1343
French Clozapine Parkinson Study Group (1999) Clozapine in drug-induced psychosis in Parkinson’s disease. The French Clozapine Parkinson Study Group. Lancet 353:2041–2042
Friedman JH (2013) Parkinson disease psychosis: update. Behav Neurol 27:469–477. doi:10.3233/BEN-129016
Godschalx-Dekker JA, Siegers HP (2014) Reduction of parkinsonism and psychosis with mirtazapine. A Case Report Pharmacopsychiatry. doi:10.1055/s-0034-1367014
Goetz CG, Pappert EJ, Blasucci LM, Stebbins GT, Ling ZD, Nora MV, Carvey PM (1998) Intravenous levodopa in hallucinating Parkinson’s disease patients: high-dose challenge does not precipitate hallucinations. Neurology 50:515–517
Gordon PH, Pullman SL, Louis ED, Frucht SJ, Fahn S (2002) Mirtazapine in Parkinsonian tremor. Parkinsonism Relat Disord 9:125–126
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney multicenter study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 20:190–199. doi:10.1002/mds.20324
Huot P, Johnston TH, Gandy MN, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2012) The monoamine re-uptake inhibitor UWA-101 improves motor fluctuations in the MPTP-lesioned common marmoset. PLoS One 7:e45587. doi:10.1371/journal.pone.0045587
Huot P et al (2011) Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of on-time. J Neurosci 31:7190–7198. doi:10.1523/JNEUROSCI.1171-11.2011
Huot P et al (2014) UWA-121, a mixed dopamine and serotonin re-uptake inhibitor, enhances L-DOPA anti-parkinsonian action without worsening dyskinesia or psychosis-like behaviours in the MPTP-lesioned common marmoset. Neuropharmacology 82:76–87. doi:10.1016/j.neuropharm.2014.01.012
Jaquenoud Sirot E et al (2012) Multicenter study on the clinical effectiveness, pharmacokinetics, and pharmacogenetics of mirtazapine in depression. J Clin Psychopharmacol 32:622–629. doi:10.1097/JCP.0b013e3182664d98
Johnston TH et al (2011) Fatty acid amide hydrolase (FAAH) inhibition reduces L-3,4-dihydroxyphenylalanine-induced hyperactivity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned non-human primate model of Parkinson’s disease. J Pharmacol Exp Ther 336:423–430. doi:10.1124/jpet.110.169532
Kannari K et al (2002) Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson’s disease. No To Shinkei 54:133–137
Lewitt PA et al (2012) Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study). Neurology 79:163–169. doi:10.1212/WNL.0b013e31825f0451
Maertens de Noordhout A, Delwaide PJ (1986) Open pilot trial of ritanserin in parkinsonism. Clin Neuropharmacol 9:480–484
Meco G, Fabrizio E, Di Rezze S, Alessandri A, Pratesi L (2003) Mirtazapine in L-dopa-induced dyskinesias. Clin Neuropharmacol 26:179–181
Meco G, Marini S, Linfante I, Modarelli F, Agnoli A (1988) Controlled single-blind crossover study of ritanserin and placebo in L-dopa-induced dyskinesias in Parkinson’s disease. Curr Ther Res 43:262–270
Nagata T, Shinagawa S, Tagai K, Nakayama K (2012) A case in which mirtazapine reduced auditory hallucinations in a patient with Parkinson disease Int Psychogeriatr:1–3 doi:10.1017/S1041610212002037
Nagata T, Shinagawa S, Tagai K, Nakayama K (2013) A case in which mirtazapine reduced auditory hallucinations in a patient with Parkinson disease. Int Psychogeriatr 25:1199–1201. doi:10.1017/S1041610212002037
Normann C, Hesslinger B, Frauenknecht S, Berger M, Walden J (1997) Psychosis during chronic levodopa therapy triggered by the new antidepressive drug mirtazapine. Pharmacopsychiatry 30:263–265
Parkinson Study Group (1999) Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. The Parkinson Study Group. N Engl J Med 340:757–763
Rascol O et al (2001) Idazoxan, an alpha-2 antagonist, and L-DOPA-induced dyskinesias in patients with Parkinson’s disease. Mov Disord 16:708–713
Rouini MR, Lavasani H, Sheikholeslami B, Owen H, Giorgi M (2014) Pharmacokinetics of mirtazapine and its main metabolites after single intravenous and oral administrations in rats at two dose rates. Daru 22:13. doi:10.1186/2008-2231-22-13
Seppi K et al (2011) The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord 26(Suppl 3):S42–S80. doi:10.1002/mds.23884
Tagai K, Nagata T, Shinagawa S, Tsuno N, Ozone M, Nakayama K (2013) Mirtazapine improves visual hallucinations in Parkinson’s disease: a case report. Psychogeriatrics : the official journal of the Japanese Psychogeriatric Society 13:103–107. doi:10.1111/j.1479-8301.2012.00432.x
Vanover KE et al (2008) A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav 90:540–544. doi:10.1016/j.pbb.2008.04.010
Visanji NP, Gomez-Ramirez J, Johnston TH, Pires D, Voon V, Brotchie JM, Fox SH (2006) Pharmacological characterization of psychosis-like behavior in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 21:1879–1891. doi:10.1002/mds.21073
Acknowledgements
This work was supported by Université de Montréal, Centre Hospitalier de l’Université de Montréal, Parkinson Society Canada, Fonds de Recherche Québec – Santé and the Weston Brain Institute.We would like to thank Drs Jonathan Brotchie and Tom Johnston for their advice during MPTP administration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animals were cared for in accordance with a protocol approved by McGill University Animal Care Committee in accordance with the regulations defined by the Canadian Council on Animal Care.
Conflict of Interest
PH has received payments from Philippe Huot MD Inc. The other authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hamadjida, A., Nuara, S.G., Veyres, N. et al. The effect of mirtazapine on dopaminergic psychosis and dyskinesia in the parkinsonian marmoset. Psychopharmacology 234, 905–911 (2017). https://doi.org/10.1007/s00213-017-4530-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4530-z