Abstract
Rationale
Environmental factors influence the etiology of many psychiatric disorders. Likewise, environmental factors can alter processes central to motivation. Therefore, motivational deficits present in many disorders may be influenced by early life environmental conditions.
Objective
We examined whether housing animals in different environmental conditions influenced the ability of sensory stimuli to acquire incentive value and whether elevated monoamine activity altered responsing for these stimuli.
Methods
Isolation-housed (IH), pair-housed (PH), and environmentally enriched (EE) male C57BL/6N mice were examined in tests of responding for a conditioned reinforcer (CRf) or an unconditioned sensory reinforcer (USRf). The CRf was previously paired with saccharin delivery through Pavlovian conditioning, while the USRf was not conditioned with a reward. Following baseline tests of responding for the CRf or USRf, the effects of elevated monoamine activity were examined.
Results
At baseline, PH and EE mice responded similarly for the CRf or USRf. IH mice responded more for the CRf but exhibited slower acquisition of responding for the USRf. Administration of citalopram, a serotonin transporter blocker, or atomoxetine, a norepinephrine transporter blocker, decreased responding for the CRf and USRf in all groups. The dopamine transporter blocker GBR 12909 generally increased responding for the CRf and USRf, but further analysis revealed enhanced responding for both reinforcers only in EE mice.
Conclusions
Baseline incentive motivation is strongly influenced by the social component of housing conditions. Furthermore, environmental enrichment increased the sensitivity to elevated dopamine activity, while acute elevations in serotonin and norepinephrine inhibit incentive motivation irrespective of housing condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Motivational impairments are at the core of several psychiatric disorders including depression, schizophrenia, and addiction, and the risk of developing these disorders has been linked in part to environmental conditions experienced early in life (Felitti et al. 1998; Welte et al. 2004; Laviola et al. 2008; Solinas et al. 2010; Akdeniz et al. 2014). Preclinical evidence also suggests that environmental factors directly alter various motivational processes including the valuation of primary rewards (e.g., food or abused drugs) or the attribution of motivational value to sensory stimuli (Fone and Porkess 2008; Simpson and Kelly 2011). These lines of evidence indicate that environmental factors, including conditions in which individuals are raised, may influence psychological and behavioral processes central to motivation.
The impact of environmental factors on neurobiological mechanisms of behavior can be examined experimentally with animals housed in different laboratory conditions (see Nithianantharajah and Hannan 2006 for a review). To determine the influence of positive factors, animals can be housed with environmental enrichment (EE). EE generally involves housing several animals in a large cage that contains a number of enriching factors including a running wheel, multiple enclosures, elevated platforms, and various objects or “toys” to interact with. Conversely, the effects of a negative environment can be examined in socially isolated animals housed with minimal or no enriching factors. Either of these groups should then be compared to a control condition, often socially housed animals living in a standard laboratory environment (Simpson and Kelly 2011).
Incentive motivation, the process by which valuable stimuli elicit appetitive behaviors, is commonly measured by operant responding for primary rewards such as food, sex, or drugs of abuse. In many experimental situations, the availability of primary rewards is signaled by specific sensory stimuli such as lights or tones. Through Pavlovian conditioning, repeated stimulus-reward pairings attribute sensory stimuli with motivational significance based on the stimuli’s ability to predict the reward. Additionally, the conditioning appears to attribute a reward-associated stimulus with incentive properties making it attractive and “wanted” in its own right. This is most clearly demonstrated by the ability of a reward-associated stimulus to support instrumental behavior as a conditioned reinforcer (CRf; Robbins 1978). Interestingly, animals also respond for sensory stimuli even in the absence of an associative relationship with a primary reward (Stewart 1960; Olsen and Winder 2009), which indicates that sensory stimuli themselves can exhibit some incentive properties. A stimulus with the ability to support responding which has not been paired with a primary reward can be described as an “unconditioned sensory reinforcer” (USRf) to emphasize a lack of conditioned value.
A CRf and a USRf are presumably distinct reinforcers; a CRf has learned value due to being paired with a primary reward, while a USRf exhibits some intrinsic value. Notably, if a sensory stimulus presented during Pavlovian conditioning is not paired with primary reward delivery, the stimulus does not support responding (e.g., Beninger and Ranaldi 1992; Mead and Stephens 2003; Browne et al. 2014). These findings underscore the importance of conditioning or habituation in the development of motivational significance. Therefore, whether a sensory stimulus serves as a CRf or a USRf likely depends on separable mechanisms of incentive value attribution. Responding for a CRf may better represent the ability of reward-associated cues to influence motivated behavior, while responding for a USRf may better illustrate the incentive properties of sensory stimuli themselves.
Preclinical studies suggest that housing conditions can alter the reinforcing efficacy of primary rewards and diminish the incentive value of reward-associated stimuli. For example, animals housed with EE respond less for food and self-administer less amphetamine compared to isolated animals (Smith et al. 1997; Bardo et al. 2001). Enriched animals also exhibit diminished cue-induced relapse to sucrose- and drug-seeking behaviors, as well as reduced Pavlovian conditioned approach to a food-associated stimulus (Laviola et al. 2008; Grimm et al. 2008; Solinas et al. 2008; Chauvet et al. 2009; Beckmann and Bardo 2012). Conversely, animals housed in isolation self-administer cocaine at lower doses (Howes et al. 2000), exhibit greater sucrose consumption (Hall et al. 1997), and respond more for a food-associated CRf compared to isolated and socially housed animals (Jones et al. 1990; Smith et al. 1997). These studies suggest that social isolation potentiates reward sensitivity and the attribution of incentive value to stimuli, while enrichment attenuates these processes. However, the effect of housing conditions on the attribution of incentive value to sensory stimuli serving as a CRf or a USRf has not been systematically examined across enriched, socially housed, and isolated groups of animals.
The behavioral consequences of altered housing conditions may be indicative of changes to underlying neurochemical systems. Incentive motivation is influenced by the monoamine neurotransmitters serotonin (5-hydroxytryptamine; 5-HT), norepinephrine (NE), and dopamine (DA). Elevated 5-HT generally suppresses responding for primary reinforcers and CRfs (Richardson and Roberts 1991; Fletcher 1995; Sanders et al. 2007), and elevated NE has been shown to reduce heroin- and cocaine-seeking on second-order schedules of reinforcement (Economidou et al. 2011). In contrast, elevations in DA can facilitate motivated behaviors; DAergic drugs such as psychostimulants are readily self-administered, and increased DA activity can enhance the incentive salience or value of reward-associated stimuli (Robinson and Berridge 1993; Sutton and Beninger 1999). A number of changes in these monoamine systems have been observed following environmental manipulations. Examples include altered 5-HT release (Brenes et al. 2008; Brenes and Fornaguera 2009) and turnover (Heidbreder et al. 2000), NE release (Galani et al. 2007) and autoreceptor function (Fulford and Marsden 1997), and DA turnover (Hall et al. 1998) and transporter function (Darna et al. 2015). Therefore, the effects of housing condition on incentive motivation may be due to, or interact with, changes in monoamine activity.
The present experiments had three major objectives. The first was to determine whether animals similarly respond for a CRf or a USRf using the same stimulus for both reinforcers. Measuring responding for a CRf and USRf provides two different indices of incentive motivation. Using an identical stimulus for both reinforcers ensures that any differences in response rates are not due to a specific sensory property of the stimulus itself. The second objective was to examine the effects of manipulating housing conditions on responding for a CRf and a USRf. Based on observations that housing condition affects monoamine activity, the third objective was to determine whether elevations of monoamine activity interacted with housing condition to alter incentive motivation. Elevated monoamine activity was achieved by a systemic injection of the 5-HT reuptake blocker citalopram, the NE reuptake blocker atomoxetine, or the DA reuptake blocker GBR 12909.
Methods
Subjects
Responding for a CRf or a USRf was examined in two separate cohorts of male C57BL/6N mice (total N = 71). Mice were purchased from Charles River Laboratories (QC, Canada), arrived in our facility on post-natal day 21, and were immediately separated into the following three housing conditions: pair-housed (PH; two mice per cage), isolation-housed (IH; one mouse per cage), or environmentally enriched (EE; six mice per cage). In Experiment 1, there were 12 mice in each condition; in Experiment 2, there were 10 mice in the PH condition, 13 in the IH condition, and 12 in the EE condition. Mice in the PH and IH groups were housed in cages measuring 30 × 13 × 12 cm with bedding, a cellulose hut, and felt nesting material. In the EE condition, mice were housed in a larger cage measuring 44 × 23 × 15 cm containing a cellulose hut, felt nesting material, and a number of extra enrichment factors. These consisted of multiple water sources, an igloo hut with top-mounted running wheel (Bio-Serv, Flemington, NJ, USA), an enclosure with an elevated platform (Bio-Serv), additional nesting materials, and a random number of small objects. The objects (four to eight per cage) varied in size, texture, and shape and were changed monthly to maintain novelty within the enrichment cage. Behavioral testing began on post-natal day 90.
All mice were housed in the same temperature and humidity controlled room with a 12-h light/dark cycle with lights on at 7:00 A.M. Mice had restricted access to water during behavioral testing, with water available for 2 h each day beginning 1 h after testing. Water restriction was required for training and testing phases of responding for a CRf. Therefore, mice tested for responding for the USRf were also water restricted to ensure that all mice were in a similar physiological state during testing. Food was available ad libitum. This work adhered to the guidelines of the Canadian Council on Animal Care, and protocols were approved by the Center for Addiction and Mental Health Animal Care Committee.
Apparatus
Tests of responding for a CRf and USRf were carried out in 12 mouse operant conditioning chambers measuring 22 × 18 × 13 cm, each enclosed in a sound-attenuating cubicle equipped with a ventilation fan (Med Associates, St Albans, VT). The stainless steel front wall of the chamber contained a horizontally centered reinforcer magazine located 2.5 cm above the floor. The magazine contained an infrared photodetector and a light. A motorized dipper could be raised to deliver 0.02-ml liquid through a hole in the floor of the magazine. The wall also contained two response levers flanking the magazine, a yellow LED stimulus light located above each lever, and a centrally positioned houselight at the top of the wall.
Locomotor activity was assessed using a custom-built system of 16 clear polycarbonate chambers measuring 25 × 45 × 20 cm. Along the long axis of each box was an array of 11 externally mounted infrared photodetectors spaced 4 cm apart and 2 cm above the cage floor. Photocell interruptions were detected and recorded as locomotor activity counts on a DELL desktop computer.
Experiment 1: effect of housing condition and monoamine reuptake blockade on responding for a conditioned reinforcer
Responding for a conditioned reinforcer
The behavioral procedure for responding for a CRf was similar to that described previously (Browne et al. 2014). Testing involved the following three phases: pre-training, Pavlovian conditioning, and operant conditioning.
Pre-training
Mice first received 1 week of acclimatization to water restriction which continued through the entire experiment. To reduce neophobia to saccharin (0.2 % w/v in tap water) used as the primary reinforcer in this experiment, mice were given home-cage access to bottles containing saccharin for the first hour of fluid availability on 3 days of the acclimatization period. Mice were then habituated to operant chambers for three 30-min sessions during which only the houselight was illuminated. Subsequently, to accustom mice to the operation of the dipper, mice received one 30-min session during which they were trained to retrieve saccharin when it was presented in the reinforcer magazine (Browne et al. 2014).
Pavlovian conditioning
At the beginning of each 40-min session, the houselight was turned on. Throughout the session, a conditioned stimulus (CS) was presented immediately prior to saccharin delivery 30 times according to a random time 60-s schedule. The CS consisted of a 5-s period of houselight off, illumination of both yellow stimulus lights, and the sound of the dipper mechanism at the end of the 5 s. Stimulus lights remained on and the houselight remained off for eight additional seconds, during which the dipper remained elevated to give animals time to collect the saccharin reward. The dipper then descended, the stimulus lights were turned off, and the houselight was re-illuminated. The total number of head entries into the reinforcer magazine during the 5-s CS period (prior to saccharin delivery) and the number of entries during a 5-s period immediately prior to each CS (pre-CS period) were recorded. Mice were tested once daily for 14 sessions.
Operant conditioning
Mice were placed in the chambers with two response levers present: an active lever and an inactive lever. Responses on the active lever produced the CS (now referred to as a CRf) on a random ratio (RR)2 schedule of reinforcement. The CRf consisted of a shortened version of the CS presented during the Pavlovian phase: 5-s period of houselight off, stimulus lights on, and elevation of the dipper (without saccharin) during the last 2 s. Responses on the inactive lever were recorded but had no consequence. Mice first received a pre-test session that lasted until 10 responses were recorded on the active lever or until 40 min had elapsed. This pre-test served to minimize potential confounding novelty of the response levers on subsequent test days (Kelley and Delfs 1991; Fletcher 1995). Following this session, all tests of responding for conditioned reinforcement were carried out with the same parameters in 40-min sessions. Mice were tested once daily for five sessions.
Following baseline testing, the effects of acute elevations of monoamine activity through serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT) blockade on responding for a CRf were examined across housing conditions. For SERT blockade, mice were treated with vehicle, 10 or 20 mg/kg citalopram prior to testing. For NET blockade, mice were treated with vehicle, 0.3, 1, or 3 mg/kg atomoxetine prior to testing. For DAT blockade, mice were treated with vehicle, 2.5, 5, or 10 mg/kg GBR 12909 prior to testing. Test days were separated by 72 h, and doses were administered according to a Latin square design. Drugs were tested in a fixed order for all mice: citalopram, atomoxetine, and then GBR 12909.
Locomotor activity
Following completion of conditioned reinforcement testing, mice were provided free access to water and locomotor activity was then measured for 1 h over three daily sessions.
Experiment 2: effect of housing condition and monoamine reuptake blockade on responding for an unconditioned sensory reinforcer
Locomotor activity
As in Experiment 1, locomotor activity was measured for 1 h over three daily sessions in mice that were not water restricted. Testing was completed prior to responding for a USRf.
Responding for an unconditioned sensory reinforcer
All mice were acclimatized to the water restriction schedule for 1 week on which they were maintained for the duration of the study. The behavioral procedure for responding for a USRf was adapted from Olsen and Winder (2009). Mice were first habituated to the operant conditioning chambers for three daily 30-min sessions, during which only the houselight was illuminated and no levers were available. In subsequent sessions, two response levers were presented: an active lever and an inactive lever. Responses on the active lever delivered the USRf, while responses on the inactive lever were recorded but had no programmed consequence. The USRf was identical to the stimulus used as the CRf in Experiment 1, a 5-s period of houselight off and stimulus lights on and elevation of the empty dipper during the last 2 s. Each daily session lasted 60 min. The longer session length relative to Experiment 1 was used because pilot studies showed that this was needed to ensure comparable levels of responding between experiments.
Mice first acquired responding on a fixed ratio 1 (FR1) schedule of reinforcement for 7 days, in which every active lever response was reinforced. Mice were then shifted to an RR2 schedule for seven additional baseline sessions. The RR2 schedule of reinforcement was used to promote higher levels of responding than on the FR1 schedule and ensure that testing was completed on the same schedule as mice responding for a CRf. The effects of citalopram, atomoxetine, and GBR 12909 on responding for a USRf were then examined on the RR2 schedule. The drug treatment protocol was identical to that presented in Experiment 1.
Drugs
Citalopram hydrobromide (Toronto Research Chemicals, Toronto, Canada), atomoxetine hydrochloride (Tocris, Bristol, UK), and GBR 12909 dihydrochloride (Tocris, Bristol, UK) were all dissolved in 0.9 % saline and administered intraperitoneally in a volume of 10 ml/kg 20 min prior to behavioral testing. All doses are expressed in terms of the free base. Animals received two acclimatization injections of saline in their homecage prior to drug testing phases. Drug treatments were in the order of citalopram (10 and 20 mg/kg), atomoxetine (0.1, 0.3, and 1 mg/kg), and GBR 12909 (2.5, 5, and 10 mg/kg). As we predicted that citalopram and atomoxetine treatment would suppress responding, these drugs were administered first. Therefore, any potential reductions in response rates due to repeated testing would have less of an impact on citalopram or atomoxetine’s effects. Also, GBR 12909 was administered last to avoid any potential sensitizing effects of elevated DA activity on subsequent tests of responding for either reinforcer. The doses of citalopram used were based on previous work conducted in our lab using tests of responding for CRf (Browne et al. in preparation), and doses of atomoxetine and GBR 12909 were based on published findings (Baarendse and Vanderschuren 2012; Young et al. 2010).
Statistical analysis
Analyses were performed using Statistica (v7). For all analyses, significance was set at p ≤ 0.05. In Experiment 1, the number of magazine entries during Pavlovian conditioning sessions was analyzed using a three-way repeated measures ANOVA with CS period and day of training as within-subjects factors, and housing condition (group) as a between-subjects factor. For operant testing (i.e., responding for a CRf or USRf), the number of responses was analyzed with a three-way repeated measures ANOVA with lever (active and inactive) and session as within-subjects factors and group as a between-subjects factor. To determine whether administration of each transporter blocker altered responding, data were analyzed individually for each drug using three-way repeated measures ANOVAs with group as a between-subject factor, and lever and dose as within-subjects factors. Where noted, two-way ANOVAs including lever and dose as within-subjects factors were also examined for each group. Locomotor activity data were analyzed using two-way repeated measures ANOVAs comparing the activity between groups across each session. Post hoc pairwise comparisons were performed using the Tukey’s honest significant difference test. When a main effect of drug was observed, post hoc analyses were conducted to compare responding on the active lever under vehicle conditions with each dose of the drug. To confirm that baseline responding was stable during drug testing, correlations for reliability of testing were conducted across vehicle treatments. Correlation coefficients ranged from 0.42 to 0.84 (all p < 0.012), demonstrating that behavior did not significantly vary across vehicle conditions. Additionally, post hoc comparisons of active lever responding during vehicle conditions did not differ between housing groups in either experiment (all p > 0.05).
Results
Experiment 1: effect of housing condition and monoamine reuptake blockade on responding for a conditioned reinforcer
Training and baseline testing
Over the course of Pavlovian conditioning, PH, IH, and EE mice all learned to approach the reward magazine upon CS presentation at similar rates (Fig. 1a). Magazine entries were higher during CS periods relative to pre-CS periods overall (F (1, 33) = 74.82, p < 0.001). This difference emerged over time (day × CS period F (13, 429) = 35.98, p < 0.001) equally for all three groups (group F (2, 33) = 0.77, ns). During the operant conditioning phase, responses on the active lever that delivered the CRf were higher than responses on the inactive lever for all groups (lever F (1, 33) = 31.10, p < 0.001; Fig. 1b). However, mice in the IH group responded more for the CRf compared to PH and EE mice (group F (2, 33) = 3.74, p < 0.05; lever × group F (2, 33) = 3.68, p < 0.05).
Effects of housing conditions on Pavlovian conditioned approach and responding for a conditioned reinforcer. a The mean (±SEM) numbers of reward magazine entries during 5-s CS presentations prior to saccharin delivery (CS; filled symbols) and during a 5-s period just before CS onset (pre-CS; open symbols) were similar across pair-housed (PH; n = 12), isolation-housed (IH; n = 12), and enriched (EE; n = 12) mice. b The mean (±SEM) number of responses on the lever delivering the CS as a conditioned reinforcer (active lever; filled symbols) and the inactive lever (open symbols) for PH, IH, and EE mice. IH mice made significantly more responses for the conditioned reinforcer compared to both PH and EE mice, which exhibited similar levels of responding
Effect of citalopram on responding for a CRf
Citalopram decreased active lever responding similarly in all three groups (dose F (2, 66) = 34.60, p < 0.001; dose × lever F (2, 66) = 23.70, p < 0.001; and dose × lever × group F (4, 66) = 1.43, ns; Fig. 2a–c). Post hoc Tukey’s HSD tests confirmed that, relative to vehicle, 10 mg/kg citalopram reduced responding for the CRf in IH and EE mice and 20 mg/kg decreased responding in all groups (all p < 0.05).
Effects of monoamine reuptake blockade on responding for a conditioned reinforcer across pair-housed (PH; left panels; n = 12), isolation-housed (IH; middle panels; n = 12), and enriched (EE; right panels; n = 12) mice. Bars represent the mean (±SEM) number of responses on the lever producing the conditioned reinforcer (active lever; filled bars) and on the inactive lever (open bars) following citalopram (a–c), atomoxetine (d–f), or GBR 12909 (g–i) challenge. *p < 0.05 compared to vehicle
Effect of atomoxetine on responding for a CRf
Atomoxetine decreased responding on the active lever in all three groups (dose F (3, 99) = 17.21, p < 0.001; dose × lever F (3, 99) = 10.80, p < 0.001; and dose × lever × group F (6, 99) = 1.86, ns; Fig. 2d–f). Although the overall group × dose interaction was not significant, post hoc tests confirmed that, relative to vehicle, 1 mg/kg atomoxetine significantly reduced responding for the CRf in IH mice and the 3 mg/kg dose reduced active lever responding in all groups (all p < 0.05).
Effect of GBR 12909 on responding for a CRf
GBR 12909 significantly increased responding on the active lever (dose F (3, 99) = 4.97, p < 0.01; dose × lever F (3, 99) = 3.39, p < 0.05; Fig. 2g–i). Although the dose × group interaction was not significant (F (6, 99) = 1.33, ns), visual inspection of the data in Fig. 2g–i suggested that GBR 12909 enhanced responding only in the EE group. Thus, we performed separate two-way repeated measure ANOVAs for each housing group. These analyses revealed that GBR 12909 did not significantly affect responding in PH (dose F (3, 33) = 1.42, ns; dose × lever F (3, 33) = 1.00, ns) or IH mice (dose F (3, 33) = 2.54, ns; dose × lever F (3, 33) = 1.68, ns). However, GBR 12909 significantly enhanced responding for the CRf in EE mice (dose F (3, 33) = 3.44, p < 0.05; dose × lever F (3, 33) = 3.41, p < 0.05). Post hoc tests confirmed that, relative to the vehicle injection, animals increased responding on the active lever at the 10-mg/kg dose (p < 0.05) and moderately at the 5-mg/kg dose (p = 0.09).
Experiment 2: effect of housing condition and monoamine reuptake blockade on responding for an unconditioned sensory reinforcer
Acquisition and baseline testing
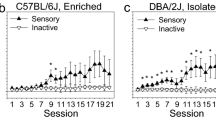
Over the course of testing, all groups of mice responded more on the active lever delivering the USRf compared to the inactive lever (Fig. 3). On the FR1 schedule of reinforcement, IH mice made significantly fewer responses on the active lever compared to both PH and EE mice as demonstrated in Fig. 3a (lever × day F (6, 192) = 4.88, p < 0.05; group F (2, 32) = 4.27, p < 0.05). However, all groups of mice eventually showed similar levels of responding on the RR2 schedule of reinforcement (group F (2, 32) = 0.30, ns; lever × day F (6, 192) = 0.47, ns); responding on the active lever was higher than on the inactive lever in all groups on the RR2 schedule (lever F (1, 32) = 29.57, p < 0.001).
Effects of housing conditions on responding for an unconditioned sensory reinforcer (USRf) on fixed ratio 1 (FR1) and random ratio 2 (RR2) schedules of reinforcement. For clarity, responding on the active and inactive levers is presented on separate panels. a The mean (±SEM) number of responses on the lever delivering the USRf (active lever) and b the inactive lever across pair-housed (PH; n = 10), isolation-housed (IH; n = 13), and enriched (EE; n = 12) mice. IH mice made significantly fewer responses for the USRf than PH and EE mice on the FR1 schedule of reinforcement but reached similar levels during testing on the RR2 schedule
Effect of citalopram on responding for a USRf
Citalopram significantly decreased active lever responding (dose F (2, 64) = 23.34, p < 0.001; dose × lever F (2, 64) = 9.32, p < 0.001; and dose × lever × group F (4, 64) = 0.45, ns; Fig. 4a–c). Post hoc tests confirmed that, relative to vehicle, both 10 and 20 mg/kg citalopram significantly reduced active lever responses in all three groups of mice (all p < 0.05).
Effects of monoamine reuptake blockade on responding for an unconditioned sensory reinforcer (USRf) across pair-housed (PH; left panels; n = 10), isolation-housed (IH; middle panels; n = 13), and enriched (EE; right panels; n = 12) mice. Bars represent the mean (±SEM) number of responses on the lever producing the USRf (active lever; filled bars) and on the inactive lever (open bars) following citalopram (a–c), atomoxetine (d–f), or GBR 12909 (g–i) challenge. *p < 0.05 compared to vehicle
Effect of atomoxetine on responding for a USRf
Atomoxetine decreased active lever responding (dose F (3, 96) = 19.12, p < 0.001; dose × lever F (3, 96) = 5.52, p < 0.01; Fig. 4d–f). However, a dose × group interaction was also observed (F (6, 96) = 2.67, p < 0.05), indicating that the effect of atomoxetine differed across housing conditions. Therefore, we performed two-way repeated measure ANOVA separately for each group and found that atomoxetine generally decreased responding in all three groups (dose all Fs > 6.4 and all ps < 0.01). However, a dose × lever interaction was observed in PH (F (3, 27) = 3.37, p < 0.05) and IH mice (F (3, 36) = 3.42, p < 0.05) but not EE mice (F (3,33) = 1.58, ns). Nonetheless, post hoc tests confirmed that atomoxetine reduced responding on the active lever at both 1 and 3 mg/kg in PH and EE mice and 3 mg/kg decreased responding in IH mice (all p < 0.05).
Effect of GBR 12909 on responding for a USRf
Treatment with GBR 12909 significantly increased responding on the active lever (dose F (3, 96) = 2.97, p < 0.05; dose × lever F (3, 96) = 3.02, p < 0.05; Fig. 4g–i). While the effect of GBR 12909 appeared similar across groups (dose × group F (6, 96) = 0.66, ns), like the effects of GBR 12909 on responding for a CRf, post hoc tests revealed that only EE mice showed significantly increased responding for the USRf following the 5-mg/kg dose (p < 0.05).
Locomotor activity
Locomotor activity was examined across three sessions after completion of tests of operant responding in Experiment 1 (Fig. 5a) and before beginning operant responding tests in Experiment 2 (Fig. 5b). In both experiments, locomotor activity decreased across sessions (Experiment 2, day F (2, 66) = 8.47, p < 0.001; Experiment 1, day F (2, 62) = 3.29, p < 0.05) but did not differ between groups (Experiment 2, day × group F (4, 62) = 1.99, ns; Experiment 1, day × group F (2, 66) = 1.20, ns).
Effects of housing conditions on locomotor activity. a The mean (±SEM) number of activity counts across three test sessions in pair-housed (PH; n = 12), isolation-housed (IH; n = 12), and enriched (EE; n = 12) mice following all behavioral manipulations in Experiment 1. b The mean (±SEM) numbers of activity counts across three test sessions in PH (n = 10), IH (n = 13), and EE (n = 12) mice prior to any behavioral manipulations in Experiment 2
Discussion
These experiments found that mice responded for an identical stimulus serving as a CRf or a USRf and that responding was influenced by post-weaning housing condition. Whereas EE and PH mice showed similar response rates for both reinforcers, IH mice exhibited higher responding for a CRf but slower acquisition of responding for a USRf. The differences observed between the socially housed animals and IH mice suggest that changes specifically in the social component of the housing condition had the most significant impact on responding for these two reinforcers. These results also demonstrated that elevating monoamine activity altered responding for a CRf and USRf. Treatment with citalopram or atomoxetine, which, respectively, blocks the 5-HT and NE transporters, decreased responding for both a CRf and USRf across all housing conditions. Conversely, while DA transporter blockade with GBR 12909 enhanced responding in general, further analyses comparing active lever presses between each dose and vehicle found a significant increase in responding for both reinforcers only in EE mice.
An important aspect of the present experiments was that the stimulus used as a CRf and a USRf was identical. The only difference between the CRf and USRf arose from the animal’s experience with either stimulus. The CRf stimulus acquired value due to a direct pairing with a primary reward through Pavlovian conditioning. In contrast, the ability of a USRf to support responding without conditioning suggests that the value of a USRf is likely based on intrinsic incentive properties of the stimulus itself. Therefore, the psychological mechanisms by which a CRf and USRf acquire salient properties are potentially different. This notion is supported in the present experiments by the distinct pattern of responding for a CRf and USRf exhibited by IH mice. Future studies are necessary to determine whether different brain regions are involved in responding for these reinforcers.
In the Pavlovian conditioning phase of Experiment 1, all three groups of mice learned to approach the reward magazine upon CS presentation at similar rates, indicating that simple stimulus-reward learning was not affected by housing conditions. However, housing condition clearly altered subsequent responding for the reward-associated stimulus serving as a CRf. While PH and EE mice showed similar levels of responding, IH mice made significantly more responses than these groups across all baseline sessions. These results are similar to a previous study in which isolated rats exhibited higher responding for a CRf compared to socially housed controls (Jones et al. 1990). In the present experiments, there was no difference in locomotor activity between the different housing groups, indicating that enhanced responding for a CRf in IH mice is not a reflection of general activity levels. Together, these results demonstrate that while housing condition did not alter the ability of animals to learn the predictive value of a reward-associated stimulus, the stimulus may have acquired greater incentive value in IH mice.
The EE and PH mice share an element of social enrichment, but they differ in terms of physical enrichment. Given that EE and PH mice did not exhibit different patterns of responding for a CRf, the physical enrichment or additional cagemates in the EE condition seemingly did not produce a sufficient change in the incentive value of a reward-associated stimulus. This appears to contrast with previous studies using rats showing that animals housed with EE exhibit blunted cue-induced reinstatement for food or drug seeking (Grimm et al. 2008; Chauvet et al. 2009; Beckmann and Bardo 2012). However, these reinstatement procedures assess whether a reward-associated stimulus can invigorate a previously extinguished behavior; this reinstatement procedure does not demonstrate whether the stimulus itself supports responding based on its incentive value. Furthermore, many studies that examine the effects of environmental manipulations compare EE to IH animals (e.g., Grimm et al. 2008; Beckmann and Bardo 2012). The lack of an appropriate pair-housed control group makes it difficult to discern whether enrichment or isolation altered behavior (see Simpson and Kelly 2011).
Results from Experiment 2 showed that the rate at which mice acquired responding for a USRf was also influenced by post-weaning housing condition. While socially housed mice demonstrated similar levels of responding, IH mice initially made very few responses for the USRf and took longer to reach the same level of responding as the EE and PH groups. Contrary to these results, a previous study found that enriched rats made fewer responses for a visual stimulus compared to both IH and PH rats (Cain et al. 2006). However, procedural differences could account for this discrepancy. In the study by Cain et al. (2006), rats were either habituated to the levers or had performed lever pressing behavior prior to tests of responding for a visual stimulus. This procedure resulted in responding for the stimulus which steadily decreased over repeated testing, likely reflecting a response pattern initially supported by novelty. In contrast, animals in the present experiments were not habituated to response levers, and lever pressing was only reinforced by the USRf. This procedure produced selective responding for the USRf that was stable over repeated testing. Our procedures and results are consistent with previous studies (Stewart 1960; Olsen and Winder 2009, 2012; Shin et al. 2010; Keller et al. 2014) and persuasively show that the USRf supported responding due to its intrinsic value as opposed to novelty.
One potential contributing factor to the slow acquisition of responding for the USRf in IH mice in Experiment 2 may be an initial neophobic reaction to the sensory stimulus itself. Generalized neophobia is a distinctive behavioral phenotype of rodents reared in isolation (Gentsch et al. 1982; Fone et al. 1996; Fone and Porkess 2008). Greater neophobia in IH mice could have an inhibitory effect on responding for the USRf early on in Experiment 2 due to the unfamiliar stimulus resulting from lever pressing. This effect contrasts with Experiment 1, wherein 14 sessions of Pavlovian conditioning familiarized animals with the stimulus, thus preventing neophobic response suppression in the subsequent operant phase. In fact, IH mice demonstrated the greatest level of responding for the same stimulus when it served as a CRf. However, no differences in overall locomotor activity on the first day of testing (when the context was novel) were observed in the present study between any housing conditions. Therefore, if neophobia is a contributing factor in acquisition of responding for a USRf, it could be specific to the response-contingent stimulus itself as opposed to reflecting a generalized novelty-induced behavioral suppression.
Manipulations that elevate extracellular 5-HT consistently reduce responding for primary reinforcers such as food, intracranial self-stimulation, and drugs (Kornblith and Hoebel 1976; Katz and Carroll 1977; Richardson and Roberts 1991; Lee and Kornetsky 1998; Sanders et al. 2007). Additionally, the 5-HT releaser dexfenfluramine has been shown to decrease responding for a CRf, while lesions of 5-HT neurons may facilitate responding (Fletcher 1995; Fletcher et al. 1999). In the present experiments, acute SERT blockade with citalopram produced a clear reduction in responding across groups for both a CRf and a USRf, which supports the notion that 5-HT exerts a broad, inhibitory control over incentive motivation.
Changes in NE activity may also alter the incentive value of a reward-associated stimulus. These experiments demonstrated that atomoxetine significantly reduced responding for a CRf and a USRf. Consistent with this finding, atomoxetine has been shown to suppress cue-induced reinstatement of cocaine- and heroin-seeking behavior (Economidou et al. 2011). Treatment with amphetamine, which has a higher affinity for NET over DAT in mice (Han and Gu 2006), has also reduced responding for a CRf in C57BL/6 mice (Browne et al. 2014). However, elevated NE reduces performance of other behavioral tasks that do not directly measure incentive motivation. For example, atomoxetine and other NET blockers—but not citalopram or GBR 12909—increased the number of trials omitted on the five-choice serial reaction time task, a test of attention, and motor impulsivity (Baarendse and Vanderschuren 2012; Navarra et al. 2008). Therefore, reduced responding for a CRf or USRf following elevated NE activity may not be fully explained by decreased incentive motivation.
Elevated DA activity tends to enhance motivation. In rats, treatment with psychostimulants such as amphetamine or cocaine increases responding for a CRf (Robbins 1978; Robbins et al. 1983; and see Sutton and Beninger 1999 for review), an effect attributed to increased mesolimbic DA activity (Taylor and Robbins 1984; Cador et al. 1991). Interestingly, enriched rats have been shown to exhibit a heightened locomotor activity response to acute challenges with amphetamine (Bowling et al. 1993) or the DA agonist apomorphine (Hoffmann et al. 2009). In the present experiments, the selective DAT blocker GBR 12909 increased responding for both reinforcers only in EE mice. Therefore, EE appeared to enhance the sensitivity to acute elevations of DA. The enhanced sensitivity to DA may be influenced by the increased number and complexity of social interactions in the EE group (caused by the greater number of animals in the enrichment cage compared to PH or IH groups), additional exercise from the presence of a running wheel, or the number of enriching factors. Additional experiments that control for these variables would provide insight into how EE enhances the effects of acute DAergic manipulations.
In conclusion, these experiments show that post-weaning housing conditions can influence basal incentive motivation in mice determined by differential responding for a CRf or for a USRf. The main contributing factor to this effect was the social component of the housing condition. These results also suggest that augmented 5-HT or NE activity broadly inhibits incentive motivation. Conversely, increased DA activity enhanced incentive motivation specifically in animals housed with enrichment, indicative of an altered sensitivity of underlying DA systems compared to animals housed without enrichment.
References
Akdeniz C, Tost H, Meyer-Lindenberg A (2014) The neurobiology of social environmental risk for schizophrenia: an evolving research field. Soc Psychiatry Psychiatr Epidemiol 49:507–517. doi:10.1007/s00127-014-0858-4
Baarendse PJJ, Vanderschuren LJMJ (2012) Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology (Berlin) 219:313–326. doi:10.1007/s00213-011-2576-x
Bardo MT, Klebaur JE, Valone JM, Deaton C (2001) Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berlin) 155:278–284
Beckmann JS, Bardo MT (2012) Environmental enrichment reduces attribution of incentive salience to a food-associated stimulus. Behav Brain Res 226:331–334. doi:10.1016/j.bbr.2011.09.021
Beninger RJ, Ranaldi R (1992) The effects of amphetamine, apomorphine, SKF 38393, quinpirole and bromocriptine on responding for conditioned reward in rats. Behav Pharmacol 3:155–163
Bowling SL, Rowlett JK, Bardo MT (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32:885–893
Brenes JC, Fornaguera J (2009) The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav Brain Res 198:199–205. doi:10.1016/j.bbr.2008.10.036
Brenes JC, Rodríguez O, Fornaguera J (2008) Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol Biochem Behav 89:85–93. doi:10.1016/j.pbb.2007.11.004
Browne JDC, Soko AD, Fletcher PJ (2014) Responding for conditioned reinforcement in C57BL/6 and CD-1 mice, and Sprague–Dawley rats: effects of methylphenidate and amphetamine. Psychopharmacology (Berlin) 231:4503–4516. doi:10.1007/s00213-014-3602-6
Cador M, Taylor JR, Robbins TW (1991) Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology (Berlin) 104:377–385
Cain ME, Green TA, Bardo MT (2006) Environmental enrichment decreases responding for visual novelty. Behav Processes 73:360–366. doi:10.1016/j.beproc.2006.08.007
Chauvet C, Lardeux V, Goldberg SR et al (2009) Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology 34:2767–2778. doi:10.1038/npp.2009.127
Darna M, Beckmann JS, Gipson CD et al (2015) Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res 1599:115–125. doi:10.1016/j.brainres.2014.12.034
Economidou D, Dalley JW, Everitt BJ (2011) Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry 69:266–274. doi:10.1016/j.biopsych.2010.09.040
Felitti VJ, Anda RF, Nordenberg D et al (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–258
Fletcher PJ (1995) Effects of d-fenfluramine and metergoline on responding for conditioned reward and the response potentiating effect of nucleus accumbens d-amphetamine. Psychopharmacology (Berlin) 118:155–163
Fletcher PJ, Korth KM, Chambers JW (1999) Selective destruction of brain serotonin neurons by 5,7-dihydroxytryptamine increases responding for a conditioned reward. Psychopharmacology (Berlin) 147:291–299
Fone KCF, Porkess MV (2008) Behavioural and neurochemical effects of post-weaning social isolation in rodents—relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32:1087–1102. doi:10.1016/j.neubiorev.2008.03.003
Fone KC, Shalders K, Fox ZD et al (1996) Increased 5-HT2C receptor responsiveness occurs on rearing rats in social isolation. Psychopharmacology (Berlin) 123:346–352
Fulford AJ, Marsden CA (1997) Social isolation in the rat enhances alpha 2-autoreceptor function in the hippocampus in vivo. Neuroscience 77:57–64
Galani R, Berthel M-C, Lazarus C et al (2007) The behavioral effects of enriched housing are not altered by serotonin depletion but enrichment alters hippocampal neurochemistry. Neurobiol Learn Mem 88:1–10. doi:10.1016/j.nlm.2007.03.009
Gentsch C, Lichtsteiner M, Kraeuchi K, Feer H (1982) Different reaction patterns in individually and socially reared rats during exposures to novel environments. Behav Brain Res 4:45–54
Grimm JW, Osincup D, Wells B et al (2008) Environmental enrichment attenuates cue-induced reinstatement of sucrose seeking in rats. Behav Pharmacol 19:777–785. doi:10.1097/FBP.0b013e32831c3b18
Hall FS, Humby T, Wilkinson LS, Robbins TW (1997) The effects of isolation-rearing on sucrose consumption in rats. Physiol Behav 62:291–297
Hall FS, Wilkinson LS, Humby T et al (1998) Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav 59:859–872
Han DD, Gu HH (2006) Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6:6. doi:10.1186/1471-2210-6-6
Heidbreder CA, Weiss IC, Domeney AM et al (2000) Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100:749–768
Hoffmann LC, Schütte SRM, Koch M, Schwabe K (2009) Effect of “enriched environment” during development on adult rat behavior and response to the dopamine receptor agonist apomorphine. Neuroscience 158:1589–1598. doi:10.1016/j.neuroscience.2008.11.035
Howes SR, Dalley JW, Morrison CH et al (2000) Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berlin) 151:55–63
Jones GH, Marsden CA, Robbins TW (1990) Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms of the nucleus accumbens. Psychopharmacology (Berlin) 102:364–372
Katz RJ, Carroll BJ (1977) Intracranial reward after Lilly 110140 (fluoxetine HCl): evidence for an inhibitory role for serotonin. Psychopharmacology (Berlin) 51:189–193
Keller KL, Vollrath-Smith FR, Jafari M, Ikemoto S (2014) Synergistic interaction between caloric restriction and amphetamine in food-unrelated approach behavior of rats. Psychopharmacology (Berlin) 231:825–840. doi:10.1007/s00213-013-3300-9
Kelley AE, Delfs JM (1991) Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology 103:187–196
Kornblith CL, Hoebel BG (1976) A dose–response study of anorectic drug effects on food intake, self-stimulation, and stimulation-escape. Pharmacol Biochem Behav 5:215–218
Laviola G, Hannan AJ, Macrì S et al (2008) Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis 31:159–168. doi:10.1016/j.nbd.2008.05.001
Lee K, Kornetsky C (1998) Acute and chronic fluoxetine treatment decreases the sensitivity of rats to rewarding brain stimulation. Pharmacol Biochem Behav 60:539–544
Mead AN, Stephens DN (2003) Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J Neurosci 23:9500–9507
Navarra R, Graf R, Huang Y et al (2008) Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry 32:34–41. doi:10.1016/j.pnpbp.2007.06.017
Nithianantharajah J, Hannan AJ (2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7:697–709. doi:10.1038/nrn1970
Olsen CM, Winder DG (2009) Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology 34:1685–1694. doi:10.1038/npp.2008.226
Olsen CM, Winder DG (2012) Stimulus dynamics increase the self-administration of compound visual and auditory stimuli. Neurosci Lett 511:8–11. doi:10.1016/j.neulet.2011.12.068
Richardson NR, Roberts DC (1991) Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci 49:833–840
Robbins TW (1978) The acquisition of responding with conditioned reinforcement: effects of pipradrol, methylphenidate, d-amphetamine, and nomifensine. Psychopharmacology (Berlin) 58:79–87
Robbins TW, Watson BA, Gaskin M, Ennis C (1983) Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology (Berlin) 80:113–119
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Sanders AC, Hussain AJ, Hen R, Zhuang X (2007) Chronic blockade or constitutive deletion of the serotonin transporter reduces operant responding for food reward. Neuropsychopharmacology 32:2321–2329. doi:10.1038/sj.npp.1301368
Shin R, Cao J, Webb SM, Ikemoto S (2010) Amphetamine administration into the ventral striatum facilitates behavioral interaction with unconditioned visual signals in rats. PLoS One 5:e8741. doi:10.1371/journal.pone.0008741
Simpson J, Kelly JP (2011) The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behav Brain Res 222:246–264. doi:10.1016/j.bbr.2011.04.002
Smith JK, Neill JC, Costall B (1997) Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology (Berlin) 131:23–33
Solinas M, Chauvet C, Thiriet N et al (2008) Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci U S A 105:17145–17150. doi:10.1073/pnas.0806889105
Solinas M, Thiriet N, Chauvet C, Jaber M (2010) Prevention and treatment of drug addiction by environmental enrichment. Prog Neurobiol 92:572–592. doi:10.1016/j.pneurobio.2010.08.002
Stewart J (1960) Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol 53:187–193
Sutton MA, Beninger RJ (1999) Psychopharmacology of conditioned reward: evidence for a rewarding signal at D1-like dopamine receptors. Psychopharmacology 144:95–110
Taylor JR, Robbins TW (1984) Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology 84:405–412
Welte JW, Barnes GM, Wieczorek WF et al (2004) Risk factors for pathological gambling. Addict Behav 29:323–335
Young JW, Goey AKL, Minassian A, et al (2010) GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berlin) 208:443–54. doi:10.1007/s00213-009-1744-8
Acknowledgments
This research was supported by a Doctoral award from the Natural Sciences and Engineering Research Council to CJB and a Canadian Institutes of Health Research operating grant to PJF. FDZ receives salary support through a Canadian Institutes of Health Research Postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Browne, C.J., Fletcher, P.J. & Zeeb, F.D. Responding for a conditioned reinforcer or unconditioned sensory reinforcer in mice: interactions with environmental enrichment, social isolation, and monoamine reuptake inhibitors. Psychopharmacology 233, 983–993 (2016). https://doi.org/10.1007/s00213-015-4178-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4178-5