Abstract
Background
Major depressive disorder (MDD) is a chronic, life-threatening psychiatric condition characterized by depressed mood, psychomotor alterations, and a markedly diminished interest or pleasure in most activities known as anhedonia. Available pharmacotherapies have limited success and the need for new strategies is clear. Recent studies attribute a major role to the pituitary adenylate cyclase-activating polypeptide (PACAP) system in mediating the response to stress. PACAP knockout mice display profound alterations in depressive-like behaviors, and genetic association studies have demonstrated that genetic variants of the PACAP gene are associated with MDD. However, the effects of PACAP administration on depressive-like behaviors in rodents have not yet been systematically examined.
Objectives
The present study investigated the effects of central administration of PACAP in rats on depressive-like behaviors, using well-established animal models that represent some of the endophenotypes of depression.
Methods
We used intracranial self-stimulation (ICSS) to assess the brain reward function, saccharin preference test to assess anhedonia, social interaction to assess social withdrawal, and forced swim test (FST) to assess behavioral despair.
Results
PACAP raised the current threshold for ICSS, elevation blocked by the PACAP antagonist PACAP(6–38). PACAP reduced the preference for a sweet saccharin solution and reduced the time the rats spent interacting with a novel animal. Interestingly, PACAP administration did not affect immobility in the FST.
Conclusions
Our results demonstrate a role for the central PACAP/PAC1R system in the regulation of depressive-like behaviors and suggest that hyperactivity of the PACAP/PAC1R system may contribute to the pathophysiology of depression, particularly the associated anhedonic symptomatology and social dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is a chronic, life-threatening psychiatric condition which affects 16 % of the population at some point in their lives in the USA (Kessler et al. 2003). MDD is characterized by depressed mood, psychomotor alterations, as well as a markedly diminished interest or pleasure in most activities, also known as anhedonia (American Psychiatric Association 2013). Despite the persistent work on trying to understand the neurobiological substrates of this disorder, there is still much to be discovered regarding the circuitry responsible for the different symptoms of MDD.
Growing evidence attributes a major role to neuropeptide systems in mediating not only the physiological stress response but also depressive and anxiety disorders, making them potential drug targets (Holmes et al. 2003). In particular, there is accumulating evidence that the pituitary adenylate cyclase-activating polypeptide (PACAP) system plays an important role in the behavioral and endocrine responses to stress, as well as in synaptic plasticity and neuroprotection (Hammack and May 2014). PACAP is the most conserved peptide of the growth hormone-releasing hormone (GHRH)/secretin/glucagon/vasoactive intestinal peptides (VIP) superfamily (Vaudry et al. 2009). Two fragments of PACAP exist, PACAP-38 and PACAP-27, the former representing more than 90 % of the total peptide in the brain (Piggins et al. 1996). PACAP functions as a neurohormone and neuromodulator through its G protein-coupled receptor PAC1, which binds PACAP with much greater affinity than VIP (K d = 0.5 vs. 4500 nM) (Harmar et al. 1998). The PACAP/PAC1 system regulates food intake, energy metabolism, body temperature, neuronal survival, and the behavioral response to stress. PACAP and its receptor PAC1 are highly expressed in several nuclei of the hypothalamus as well as in various extra-hypothalamic regions including the amygdala, the hippocampus, and the nucleus accumbens (Joo et al. 2004; Piggins et al. 1996).
Administration of PACAP intracerebroventricularly (i.c.v.), as well as into the paraventricular nucleus of the hypothalamus (PVN), the central nucleus of the amygdala (CeA), and the bed nucleus of the stria terminalis (BNST), has been shown to produce a stress-like response and to activate the hypothalamic–pituitary–adrenal (HPA) axis as well as extrahypothalamic corticotropin-releasing factor (CRF) systems (Agarwal et al. 2005; Dore et al. 2013; Missig et al. 2014; Norrholm et al. 2005). While on one hand PACAP knockout mice have been shown to display attenuations in serum corticosterone levels and depressive-like behaviors after a chronic stress paradigm (Lehmann et al. 2012), other studies have instead described a pro-depressive-like phenotype of PACAP knockout mice, which was, however, not reproduced in later studies (Hashimoto et al. 2009; Hattori et al. 2012). However, the effects of exogenously administered PACAP on depressive-like behaviors have not yet been described.
In this paper, we sought to further characterize the pro-depressive-like effects of PACAP using well-established animal models of anhedonia and behavioral despair, endophenotypes of depression. We used intracranial self-stimulation (ICSS) to assess the brain reward function, forced swim test (FST) to assess behavioral despair, saccharin preference test to assess anhedonia, and social interaction to assess social avoidance, following intracerebroventricular (i.c.v.) administration of PACAP in rats.
Materials and methods
Animals
Male Wistar rats, weighing 300–325 g upon arrival (Charles River, Wilmington, MA), were housed in a 12 h/12 h reverse light cycle (lights off at 11 am), humidity- and temperature-controlled vivarium, with food and water available ad libitum. Animals were allowed a minimum of 2 weeks to habituate to the vivarium; all experiments were done during the rats’ dark cycle. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Principles of Laboratory Animal Care and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee.
Drugs
Saccharin solution (0.5 % w/v) was prepared using saccharin sodium salt hydrate (Sigma–Aldrich) and tap water. PACAP-38 (agonist) (here called PACAP) and PACAP(6–38) (antagonist) were purchased from the American Peptide Company (Sunnyvale, CA). Both peptides were dissolved in sterile isotonic saline in the presence of 1 % bovine serum albumin (Sigma-Aldrich, St. Louis, MO) and were administered i.c.v. in a volume of 5 μL, either alone or as a cocktail in a single micro-infusion. PACAP doses were chosen based on previous studies showing that similar doses produce anxiogenic and anorectic effects lasting up to 3–6 h (Dore et al. 2013; Mounien et al. 2009; Telegdy and Adamik 2015). PACAP(6–38) doses were chosen also based on previous reports (Burgos et al. 2013; Telegdy and Adamik 2015). Drug pre-treatment time was 30 min in the saccharin preference, social avoidance, and forced swim tests, and 0 min in the ICSS test (since the first two columns of the procedure were not used in the analysis, see below).
Intracranial surgery and micro-infusion procedures
Stereotaxic surgeries were performed as previously described (Iemolo et al. 2012; Sabino et al. 2007). Rats were implanted with a 24-gauge stainless steel cannula (Plastics One, Roanoke, VA) into the lateral ventricle (from bregma = AP -1.00 mm, ML ±1.5 mm, DV −2.3 mm, flat skull) determined using Paxinos and Watson (6th edition). For the ICSS experiment, the i.c.v. coordinates were adjusted to permit both the cannula and the electrode to fit in the same rat (AP, −1.0; ML, −3.2 with 20° vertical tilt; DV, −2.6 from skull; incisor bar set 5.0 mm above the interaural line). After surgery, rats were singly housed and allowed to recover for at least 5 days before behavioral testing began. For micro-infusions, a 31-gauge internal injector with 2.5-mm projection was placed into the guide cannula, connected via PE20 tubing to a Hamilton microsyringe driven by a pump (KD Scientifics, Holliston, MA). Injections were administered at a rate of 2.5 μL/min, and injectors were left in place for an additional minute to prevent backflow.
Intracranial self-stimulation (ICSS) test
Surgery for electrode implantation and ICSS procedure were performed as previously described (Iemolo et al. 2012). Rats were unilaterally implanted with a 0.125-mm-diameter bipolar stainless steel electrode (Plastics One; length ≈ 10.5 mm) into the medial forebrain bundle at the level of the lateral hypothalamus (coordinates from bregma = AP −0.5 mm, ML −1.7 mm, DV −9.7 mm from skull; incisor bar set 5.0 mm above the interaural line).
A rate-independent discrete-trial current intensity procedure originally designed by Dr. Kornetsky was used (Kornetsky and Esposito 1979). Rats were first trained to lever press on a fixed ratio (FR) 1 schedule of reinforcement to obtain 500-ms trains of electrical stimulation. Once stable FR1 operant responding for the electrical stimulus was established, ICSS thresholds were assessed using the following procedure (Iemolo et al. 2012; Valenza et al. 2015). At the beginning of each trial, rats received a non-contingent stimulus (S1), after which they had the opportunity, during a 7.5-s limited period, to lever press, which resulted in the delivery of a contingent stimulus (S2) that was identical to the previous S1. A 7.5–22.5-s (average 15 s) period of time elapsed between S2 delivery and the delivery of the next S1. If no response occurred, this time period began at the end of the 7.5-s period allotted for response. These time periods were randomized so that animals could not “predict” the next S1 delivery. A “trial” consisted of five presentations of S1 at a fixed current intensity (in microamperes). Three or more responses at that intensity were scored as a plus (+) for that trial, whereas two or fewer responses were scored as a minus (−) for that trial. If the animal scored a (+) for the first trial, the second trial began at an intensity of 5 μA lower than the first. The current intensity continued to decrease by the same fixed intensity until the animal scored a (−) for two consecutive trials. When this occurred, the current intensity at the second trial at which a (−) score was obtained was repeated and the current intensities were then ascended by 5 μA for each trial until the animal scored a (+) for two consecutive trials. Each set of ascending or descending current intensities was defined as a “column”, and a total of six alternating descending/ascending columns were performed for each session. The intensity at the midpoint between (+) and (−) was defined as the column threshold. The threshold for each session was calculated as the mean of the last four column thresholds; the first and second column thresholds were, therefore, excluded, as they were the most unstable. To discourage the subject from responding during the inter-trial interval, any response during this period postponed the onset of the S1 for an additional 22.5 s (a length of time that exceeded or was equal to the original random duration of the inter-trial interval).
The reward threshold is defined as the minimal current intensity able to produce a response that maintains the self-stimulation behavior. An increase in the reward threshold indicates that stimulus intensities that were previously perceived as reinforcing are no longer perceived as rewarding, reflecting an overall decrease in reward function (Markou and Koob 1992). The response latency is defined as the mean response latency of all trials within a session during which a positive response occurred. Rats were trained daily until a stable reward threshold was achieved. On drug testing days, animals were injected with PACAP (0, 1, 3 μg/rat) immediately before their ICSS test session, in a within-subject, Latin square design. At least two treatment-free days were allowed between treatment days, and complete return to baseline threshold levels was ascertained before the next treatment was administered.
Saccharin preference test
On day 1 of the saccharin preference test, rats were presented with two water bottles for 4 h in their home cages at dark cycle onset. On day 2, rats received one bottle containing water and another containing a 0.5 % saccharin solution for 2 h. The saccharin bottle was then exchanged for a water bottle for two additional hours. On day 3, rats were allowed access to both saccharin and water bottles for 4 h. Rats were water deprived for 20 h on day 1, 2, and 3 to encourage drinking in a short period of time. On day 4, the test day, the drug was administered 30 min before bottle presentation; water and saccharin consumption was measured by weighing the bottles before and at the end of the 4-h test period.
Social avoidance test
The social avoidance paradigm in rats was based on the social approach-avoidance test previously described in both rats and mice (Lukas et al. 2011), with slight modifications. The test took place during the rats’ active phase, under red lights. Rats were placed in a novel arena (100 × 100 × 40 cm). After 30 s of habituation, an empty wire-mesh cage (64 × 32 × 25 cm) was placed at one side wall of the arena for 4 min, during which the experimental rat could gain familiarity with the arena and the novel empty cage. An unknown male Wistar conspecific (social stimulus) was then inserted into the cage for an additional 4 min. The arena was cleaned with quatricide between rats. The test was recorded with a camera and the time the experimental rat spent interacting with the conspecific was analyzed by an experimenter blind to the treatments.
Forced swim test (FST)
A 1-day protocol was used. Thirty minutes after drug administration, rats were placed into a clear acrylic cylinder (25 cm diameter) filled with water (24 °C; 42 cm deep) for 15 min. The test was videotaped and later scored for the time rats spent climbing, swimming, and immobile by an experimenter blind to the treatments. Latency to first becoming immobile was also recorded. Behaviors were scored as described in Cryan et al. (2005). Animals were considered immobile when performing the minimum movements required to keep their head above water, in the absence of other behaviors. Climbing was defined as upward-directed movement of the forepaws aimed toward the sides of the cylinder. Swimming was defined as horizontal movement throughout the cylinder. At the end of the session, rats were removed from the cylinder, dried, and placed into a polycarbonate cage located on a heating pad for 15 min, and then returned to their home cage.
Statistical analysis
ICSS dose-response data were analyzed using a repeated measure analysis of variance (ANOVA) with dose as a within-subject factor. ICSS threshold blockade data were analyzed using a two-way repeated measure ANOVA with PACAP and antagonist as within-subject factors. Data from the social avoidance, sucrose intake, and FST studies were analyzed using one-way ANOVAs. Pairwise post hoc comparisons were made using Student-Newman-Keuls test; Student’s t test was used when comparing two groups. Statistical significance was set at p <0.05. The software/graphic packages used were SigmaPlot 11.0 and Statistica 7.0.
Results
Central administration of PACAP elevates ICSS threshold
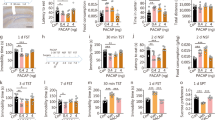
As shown in Fig. 1a, PACAP (0–3 μg/rat, i.c.v.) significantly affected the ICSS threshold (F(2,12) = 29.75, p < 0.001; significant linear trend, p < 0.001). Post hoc analysis showed that the highest dose of PACAP (3 μg) elevated the ICSS reward threshold. PACAP did not affect latency to respond, as shown in Fig. 1b (F(2,12) = 1.14, n.s.; not significant linear trend).
The PAC1R antagonist PACAP(6–38) blocks PACAP-induced elevation of ICSS threshold
Administration of the selective PAC1R antagonist PACAP(6–38) (0–7.5 μg/rat, i.c.v.) did not affect ICSS threshold per se (F(2,18) = 0.93, n.s.; not significant linear trend), as shown in Fig. 2a. However, when co-administered with PACAP (3 μg/rat, i.c.v.), PACAP(6–38) (2.5 μg/rat) blocked PACAP-induced elevation in ICSS threshold (PACAP, F(1,6) = 4.68, n.s.; antagonist, F(1,6) = 0.87, n.s.; PACAP × antagonist, F(1,6) = 11.88, p = 0.014), as shown in Fig. 2b. Neither PACAP nor PACAP(6–38) affected latency to respond (data not shown).
a Effect of selective PAC1 antagonist PACAP(6–38) (0, 2.5, 7.5 μg/rat, i.c.v.) on reward threshold (n = 10). b Effects of PACAP(6–38) (0, 2.5 μg/rat, i.c.v.) and PACAP (0, 3 μg/rat) on reward threshold (n = 7). Data represent mean ± SEM. *p < 0.05 versus vehicle-treated group; #p < 0.05 versus PACAP-treated group (Student-Newman Keuls test)
Central administration of PACAP reduces saccharin intake and preference
As shown in Fig. 3a, PACAP (0–3 μg/rat, i.c.v.) significantly affected the intake of a saccharin solution (dose, F(2,20) = 7.26, p < 0.01; significant linear trend, p = 0.001). Post hoc analysis showed that both doses of PACAP (1 and 3 μg) significantly decreased 4-h saccharin intake (Fig. 3a, left panel). PACAP did not affect concurrent water intake (F(2,20) = 1.11, n.s.; not significant linear trend), although a not significant trend to increase was noticed (Fig. 3a, middle panel). Importantly, total fluid intake was not affected by the PACAP treatment (F(2,20) = 2.00, n.s.; not significant linear trend) (Fig. 3a, right panel). PACAP treatment also affected saccharin preference (F(2,20) = 5.77, p = 0.01; significant linear trend, p < 0.01), with the highest dose of PACAP (3 μg) significantly decreasing 4-h saccharin preference, as shown in Fig. 3b.
Central administration of PACAP induces social avoidance
As shown in Fig. 4, PACAP (0–3 μg/rat, i.c.v.) significantly increased social avoidance in a social interaction test (F(2,23) = 7.40, p < 0.01; significant linear trend, p = 0.001). Post hoc analysis showed that both doses of PACAP (1 and 3 μg) significantly reduced the time the rats spent approaching an unfamiliar rat during the 4-min test.
Central administration of PACAP does not affect immobility time in the FST
As shown in Fig. 5a, PACAP did not significantly affect immobility time in the FST (PACAP, F(2,23) = 0.81, n.s.; time, F(2,46) = 116.33, p < 0.001; PACAP × time, F(4,46) = 0.06, n.s.). Interestingly, PACAP tended to increase the latency to first become immobile, as shown in Fig. 5b (F(2,23) = 2.96, p = 0.07; significant linear trend p < 0.05). As shown in Fig. 5c, PACAP did not significantly alter time spent swimming (PACAP, F(2,23) = 0.43, n.s.; time, F(2,46) = 14.44, p < 0.001; PACAP × time, F(4,46) = 0.98, n.s.) or climbing (PACAP, F(2,23) = 1.32, n.s.; time, F(2,46) = 189.35, p < 0.001; PACAP × time, F(4,46) = 1.19, n.s.). When the entire 15-min sessions were analyzed together, PACAP had still no effect on immobility, swimming, or climbing time (see insets of Fig. 5a, c, d).
Discussion
Growing evidence attributes a major role to neuropeptide systems in the mediation of the stress response, making them potential drug targets for the treatment of affective disorders (for a review, see Holmes et al. 2003). The PACAP/PAC1R system has been proposed to play a major role in mediating the behavioral and endocrine responses to stress (Hammack and May 2014). Although human and animal studies have suggested the possible involvement of the PACAP system in the pathophysiology of anxiety and depressive disorders, the effects of exogenously administered PACAP on depressive-like behaviors in rodents have not yet been systematically examined. These series of studies comprehensively characterize the effects of PACAP on depressive-like behaviors in the rat, using well-established animal models of anhedonia, behavioral despair, and social withdrawal. We demonstrate that central administration of PACAP induces a pro-depressant phenotype in rats. PACAP administered i.c.v. raised the current threshold for ICSS, and this elevation was blocked by co-administration of the PAC1R antagonist PACAP(6–38). PACAP reduced the consumption and preference for a sweet saccharin solution and reduced the time the rats spent interacting with a novel conspecific animal. On the other hand, PACAP administration did not increase immobility in the FST. The results collectively suggest a role for the central PACAP/PAC1R system in the regulation of depressive-like behaviors.
Depression, as in other mental illnesses, consists of endophenotypes which can be reproduced independently and evaluated in animals with the goal of identifying novel therapies for depression (Hasler et al. 2004). The depressive phenotype can be dissected into separate key components that are biologically and clinically meaningful and can be assessed quantitatively, called psychopathological endophenotypes (e.g., depressed mood, impaired reward function or anhedonia, impaired learning and memory, psychomotor alterations, increased stress sensitivity, etc.) (Hasler et al. 2004). In the present investigation, we utilized reward-based (ICSS, consumption of sweet solutions), behavioral despair (FST), and social withdrawal models to explore the role of the PACAP/PAC1R system.
PACAP-treated rats showed a dose-dependent increase in ICSS threshold, defined as the current intensity that supports operant behavior in the discrete-trial current-intensity intracranial self-stimulation procedure, confirming previous results obtained with higher doses of the peptide (Dore et al. 2013). An increase in ICSS threshold is indicative of a decrease of the rewarding properties of the electrical stimulations (i.e., higher currents are needed to feel the same pleasure) (Markou and Koob 1992). A number of conditions known to trigger depressive states in humans also cause deficits in the brain reward system function of animals as measured by ICSS; this is the case of chronic stress, such as chronic variable stress or chronic social defeat (Der-Avakian et al. 2014; Moreau et al. 1995), as well as withdrawal from abused drugs, such as ethanol and cocaine and nicotine (Markou and Koob 1991; Schulteis et al. 1995). For this reason, increases in ICSS thresholds, reflecting deficits in brain reward circuitries, are widely recognized as a sign of “anhedonia”, i.e., diminished interest or pleasure in rewarding activities and a core symptom of major depression and other affective disorders (American Psychiatric Association 2013). In line with this notion is the observation that chronic treatment with antidepressant drugs is able to attenuate increases in ICSS threshold (Markou et al. 1992).
We also found that co-infusion of the PAC1R antagonist PACAP(6–38) blocked the PACAP-induced elevations of the ICSS thresholds. It is important to note that although the well-characterized PACAP(6–38) is used as a PAC1R antagonist in many studies, it also exhibits high potency at VPAC2 receptors (Dickinson et al. 1997). The finding that PAC1R knockout mice behave as PACAP knockout in the context of stress-related behaviors (Mustafa et al. 2015), together with the observation that VIP appears to have an anxiolytic-like profile (Ivanova et al. 2014) and promote social contact and pair bonding (Kingsbury and Goodson 2014), strongly suggests the exclusive involvement of PAC1R in the regulation of mood and social interaction. However, future studies will need to test more specific PACAP receptor antagonists as become available in order to evaluate the specific role of the individual receptors in the observed effects. Interestingly, PACAP(6–38) had no per se effect on ICSS threshold, suggesting the lack of a tonically active PACAP/PAC1 system in baseline, non-aversive condition. Our results are in line with previous results showing a lack of effect of PACAP(6–38) in unstressed animals as compared to animals exposed to chronic variable stress (Roman et al. 2014), and are reminding of the “normalizing” profile of CRF receptor antagonists on brain stimulation reward as well as anxiety-like behavior, which have been shown to affect these behaviors exclusively in conditions of high stress or arousal (Heinrichs et al. 1992; Macey et al. 2000). An alternative explanation for the lack of effect of PACAP(6–38) in ICSS can be related to the fact that the behavioral tests in this study were performed during the dark cycle, when the PACAP signaling may be less active as compared to the light cycle.
Importantly, neither PACAP nor PACAP(6–38) treatment affected the latency to start lever pressing, which is considered a measure of operant performance and therefore general motor activity in the ICSS procedure (Markou and Koob 1992). This finding excludes potential locomotor suppressive effects of the peptides, in line with a lack of increased immobility in the FST as well as previous reports showing no locomotor impairment following administration of PACAP in rodents (Dore et al. 2013; Iemolo et al. 2015; Kocho-Schellenberg et al. 2014; Mounien et al. 2009; Resch et al. 2011). We believe that the demonstration of the attenuation in the brain reward system function following administration of PACAP is a very significant one, as ICSS can be considered a direct read-out of intact reward functioning in the brain.
Even though in this study we have not directly measured the effect of PACAP on HPA activation, several previous reports have shown that similar doses of PACAP do activate the HPA axis (Agarwal et al. 2005; Dore et al. 2013; Norrholm et al. 2005). However, considering the rapid effects of PACAP, our data suggest instead an effect of PACAP on hedonic behaviors, independently of HPA axis activation. This hypothesis is also supported by the observation that acute administration of steroids has been found to either not affect or even increase brain stimulation reward (Barr et al. 2000; Goodwin et al. 1992; Slusher 1965). Moreover, bilateral adrenalectomy has been shown to fail to alter self-stimulation thresholds acutely (Abrahamsen and Carr 1997; Abrahamsen et al. 1997). Consequently, it appears that acute activation of the HPA stress system, through the release of ACTH and corticosterone, either does not affect or even stimulate rather than inhibit self-stimulation, and therefore it is unlikely that the HPA axis stress system may mediate the effects of PACAP on ICSS observed in the present study.
We found that rats treated with PACAP showed a dose-dependent decrease in intake of a palatable saccharin solution, which was reduced 49.9 % by the highest dose, compared to vehicle. Interestingly, a statistically non-significant trend to increased concurrent water intake (53.3 % by the highest dose, compared to vehicle) was observed, which speaks against the alternative hypothesis of general malaise. As a consequence, total fluid intake was not significantly affected by the treatment. Chronic stress is known to reduce the intake and preference of sweet solutions (as well as water) and chronic treatment with antidepressant drugs has been shown to increase sucrose preference as well as attenuate the reduction in sucrose consumption by chronic stress (Sampson et al. 1991; Willner et al. 1987). Sucrose/saccharin preference shares a common theoretical basis with ICSS. Reduced intake and preference for sweet solutions represents the loss of interest, fatigue, and loss of energy common during depressive episodes. Like ICSS, preference for sweet solutions can be a measure of the affective state and motivation of rodents, and thus is considered another measure of anhedonia (D’Souza and Markou 2010). Therefore, we demonstrate that central administration of PACAP causes a generalized decrease in sensitivity to rewards. It should be noted, however, that an alternative explanation for the reduction of saccharin intake may involve a potential reduction of place preference by PACAP, rather than a pure attenuation of the hedonic response; future studies investigating the effects of PACAP on place conditioning may help shed light on this question.
Based on the effects on ICSS and saccharin preference, a pro-depressant effect of PACAP can be concluded. PACAP is well known for inducing anorexia (Hawke et al. 2009; Morley et al. 1992; Mounien et al. 2009), and this raises the possibility that the observed effects could be the result of this. However, saccharin is a non-caloric reinforcer (unlike sucrose, reason for which it was chosen in this study) and food deprivation is known to potentiate the brain reward function as assessed by ICSS (Carr et al. 2000; Goodall and Carey 1975). These observations suggest that the anti-rewarding effect of PACAP is unlikely to be a result of decreased food intake.
In this series of studies, we also found that central PACAP administration caused a dose-dependent decrease in social interaction, i.e., it produced social withdrawal. The social interaction/avoidance test is an ethologically relevant model of neophobia, depression, and anxiety that uses the natural form of social behavior as a dependent variable (File and Seth 2003). Although decreases in social interaction are most common in anxiety states, it is important to note that social dysfunction represents one of the core symptoms of depression-related diseases (Merikangas and Angst 1995). Other “stress” neuropeptides, such as corticotropin-releasing factor and cholecystokinin, have been shown to reduce social interaction (Dunn and File 1987; To and Bagdy 1999), and chronic stress can also dramatically impact social interaction (Becker et al. 2008; Berton et al. 2006; Krishnan et al. 2007). A previous report that mice with a deletion of the PACAP gene show increased social interaction and an attenuation of social defeat-induced social withdrawal is in line with our results (Hattori et al. 2012; Lehmann et al. 2012).
Finally, we assessed whether central PACAP administration altered behavior in the forced swim test. The FST is based on the observation that animals develop an immobile posture in an inescapable cylinder filled with water, and in this test, immobility in the FST is interpreted as an incapacity or reluctance to maintain effort, rather than a generalized hypoactivity (Willner 1990), and this reluctance correlates with the psychomotor impairment shown by depressed patients in tests requiring sustained effort (Weingartner and Silberman 1982). Immobility is, therefore, interpreted as a passive stress-coping strategy (behavioral despair) (Detke and Lucki 1996; Porsolt et al. 1978). We found that PACAP at the doses used in this study did not reliably affect immobility, swimming, or climbing in the FST. Noteworthy, PACAP treatment slightly increased the latency of the animals to first become immobile, which would essentially suggest an improved coping with stressful stimuli. Several considerations should be made regarding the FST. Originally developed to screen antidepressant medications, the forced swim test has more recently been used as a putative assessment of depressive-like behavior. However, its validity and reproducibility as a model of “depression” is debated and conflicting findings have been reported following chronic stressors (Der-Avakian et al. 2014); therefore, caution should be exercised to avoid over-extrapolation of the behavioral outcome of the FST. It is conceivable that the central PACAP/PAC1R system is not involved in the mechanism that allows for the development of passive behavior (immobility), which disengages the animal from active forms of coping with stressful stimuli. Interestingly, PACAP knockout mice have been reported to show decreased immobility in the FST (Hashimoto et al. 2009), an effect which may however have been confounded by increased motor activity of the mutants.
As immobility time is typically lowest during the first day of FST and increases on day 2 when a 2-day protocol is performed, we chose to test the effects of PACAP on FST using a 1-day protocol to avoid a possible floor effect and therefore maximize the chances of observing an increase in immobility following administration of the drug. However, we cannot exclude the possibility that in a 2-day paradigm PACAP could have had an effect, even though the increase in latency to become immobile observed following PACAP administration would suggest otherwise.
One limitation of this study is that the PACAP antagonist blockade was performed exclusively in the ICSS experiment and not in the social interaction or saccharin preference tests, due to the fact that while the ICSS test used a within-subject design (therefore minimizing the number of experimental subjects), the remainder of the tests used instead a between-subject design. Future studies will need to directly ascertain that the effects of PACAP on social withdrawal and saccharin preference are indeed also mediated by PAC1R.
Although the tests used in this study all assess some aspects of depression, they measure different behavioral outcomes: ICSS measures the sensitivity of the brain reward system (the median forebrain bundle), saccharin preference test measures the motivation for natural rewards, social interaction measures social behavior and recognition, and FST measures the coping with a potential life-threatening situation. Given the marked heterogeneity of the paradigms used, it is likely that the four tests rely on different neurobiological substrates and neurotransmitters. Furthermore, since i.c.v., rather than brain site-specific, infusions were used in this study, additional studies will be necessary to determine the site of action of PACAP in the context of depressive-like behaviors.
Many other stress-responsive systems have been implicated in the etiology and pathophysiology of mood disorders, including the kappa opioid receptor (KOR) and the corticotropin releasing factor (CRF) systems. KORs are indeed involved in stress and depressive-like behaviors (Knoll and Carlezon 2010); stress promotes the synthesis and release of dynorphin (Chartoff et al. 2009) and KOR activation produces dysphoria, decreased brain reward function, and pro-depressive-like behaviors both in humans and in rodents (Bals-Kubik et al. 1993; Pfeiffer et al. 1986; Todtenkopf et al. 2004). Interestingly, PACAP has been shown also to increase CRF transcription (Agarwal et al. 2005); since a CRF receptor antagonist was shown to be able to prevent the anti-rewarding effects of PACAP (Dore et al. 2013) and the CRF system is thought to be upstream of KOR (Land et al. 2008), we may speculate that PACAP’s effects may be mediated by the initial activation of CRF followed by the activation of the dynorphin/KOR system.
It is important to note that the anhedonic effects of acute drug administrations cannot obviously be regarded as the same as those of chronic administration of stressors. However, since chronic exposure to stress ultimately results in similar behavioral endpoints as acute PACAP administration, we hypothesize that chronic stress may lead to a recruitment of the PACAP system, which would in turn produce anhedonic behaviors. A limitation of the present study is that it involved the exogenous administration of the peptide; subsequent studies will need to assess the role of the endogenous PACAP system using animal models of depression and pharmacological antagonists or viral knockdown of PACAP and/or PAC1.
In summary, while during acute stress PACAP/PAC1R signaling may increase the motivation to escape threat (by producing aversion) and thus facilitate behavioral responses, prolonged PACAP/PAC1R system activation signaling in response to chronic stress may instead lead to the long-lasting changes seen in depression. Together, our results suggest that the hyperactivity of the PACAP/PAC1R system may contribute to the pathophysiology of depression, particularly the anhedonic symptomatology and social dysfunction associated with the disease.
References
Abrahamsen GC, Carr KD (1997) Effect of adrenalectomy on cocaine facilitation of lateral hypothalamic self-stimulation. Brain Res 755:156–161
Abrahamsen GC, Kandawire MJ, Carr KD (1997) Aminoglutethimide, a corticosteroid synthesis inhibitor, facilitates brain stimulation reward in food-restricted rats: an investigation of underlying mechanisms. Psychopharmacology (Berlin) 133:405–412
Agarwal A, Halvorson LM, Legradi G (2005) Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res 138:45–57
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, DSM-5. American Psychiatric Association, Washington, DC, etc., pp Online resource (XLIV, 947 p.)
Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993) Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264:489–495
Barr AM, Brotto LA, Phillips AG (2000) Chronic corticosterone enhances the rewarding effect of hypothalamic self-stimulation in rats. Brain Res 875:196–201
Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ (2008) Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry 13:1079–1092
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868
Burgos JR, Iresjo BM, Smedh U (2013) Pituitary adenylate cyclase-activating polypeptide 6-38 blocks cocaine- and amphetamine-regulated transcript peptide-induced hypophagia in rats. PLoS One 8:e72347
Carr KD, Kim G, Cabeza de Vaca S (2000) Hypoinsulinemia may mediate the lowering of self-stimulation thresholds by food restriction and streptozotocin-induced diabetes. Brain Res 863:160–168
Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA Jr (2009) Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol 75:704–712
Cryan JF, Valentino RJ, Lucki I (2005) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569
Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A (2014) Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry 76:542–549
Detke MJ, Lucki I (1996) Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res 73:43–46
Dickinson T, Fleetwood-Walker SM, Mitchell R, Lutz EM (1997) Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides 31:175–185
Dore R, Iemolo A, Smith KL, Wang X, Cottone P, Sabino V (2013) CRF mediates the anxiogenic and anti-rewarding, but not the anorectic effects of PACAP. Neuropsychopharmacology 38:2160–2169
D’Souza MS, Markou A (2010) Neural substrates of psychostimulant withdrawal-induced anhedonia. Curr Top Behav Neurosci 3:119–178
Dunn AJ, File SE (1987) Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Behav 21:193–202
File SE, Seth P (2003) A review of 25 years of the social interaction test. Eur J Pharmacol 463:35–53
Goodall EB, Carey RJ (1975) Effects of d- versus l-amphetamine, food deprivation, and current intensity on self-stimulation of the lateral hypothalamus, substantia nigra, and medial frontal cortex of the rat. J Comp Physiol Psychol 89:1029–1045
Goodwin GM, Muir WJ, Seckl JR, Bennie J, Carroll S, Dick H, Fink G (1992) The effects of cortisol infusion upon hormone secretion from the anterior pituitary and subjective mood in depressive illness and in controls. J Affect Disord 26:73–83
Hammack SE, May V (2014) Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol Psychiatry
Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA (1998) International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev 50:265–270
Hashimoto H, Hashimoto R, Shintani N, Tanaka K, Yamamoto A, Hatanaka M, Guo X, Morita Y, Tanida M, Nagai K, Takeda M, Baba A (2009) Depression-like behavior in the forced swimming test in PACAP-deficient mice: amelioration by the atypical antipsychotic risperidone. J Neurochem 110:595–602
Hasler G, Drevets WC, Manji HK, Charney DS (2004) Discovering endophenotypes for major depression. Neuropsychopharmacology 29:1765–1781
Hattori S, Takao K, Tanda K, Toyama K, Shintani N, Baba A, Hashimoto H, Miyakawa T (2012) Comprehensive behavioral analysis of pituitary adenylate cyclase-activating polypeptide (PACAP) knockout mice. Front Behav Neurosci 6:58
Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM (2009) PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci : Off J Soc Neurosci 29:14828–14835
Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF (1992) Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res 581:190–197
Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G (2003) Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci 24:580–588
Iemolo A, Valenza M, Tozier L, Knapp CM, Kornetsky C, Steardo L, Sabino V, Cottone P (2012) Withdrawal from chronic, intermittent access to a highly palatable food induces depressive-like behavior in compulsive eating rats. Behav Pharmacol 23:593–602
Iemolo A, Ferragud A, Cottone P, Sabino V (2015) Pituitary adenylate cyclase-activating peptide in the central amygdala causes anorexia and body weight loss via the melanocortin and the TrkB systems. Neuropsychopharmacology
Ivanova M, Belcheva S, Belcheva I, Stoyanov Z, Tashev R (2014) Modulatory effect of VIP injected into hippocampal CA1 area on anxiety in olfactory bulbectomized rats. Acta Neurobiol Exp 74:317–327
Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI (2004) Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol 476:388–413
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey R (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105
Kingsbury MA, Goodson JL (2014) Pair bond formation is impaired by VPAC receptor antagonism in the socially monogamous zebra finch. Behav Brain Res 272:264–268
Knoll AT, Carlezon WA Jr (2010) Dynorphin, stress, and depression. Brain Res 1314:56–73
Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, Edwards S, Wasserman E, Toufexis DJ, Braas KM, May V, Hammack SE (2014) PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology 39:1614–1623
Kornetsky C, Esposito RU (1979) Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc 38:2473–2476
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404
Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C (2008) The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci : Off J Soc Neurosci 28:407–414
Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE (2012) PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology
Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011) The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36:2159–2168
Macey DJ, Koob GF, Markou A (2000) CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res 866:82–91
Markou A, Koob GF (1991) Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4:17–26
Markou A, Koob GF (1992) Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav 51:111–119
Markou A, Hauger RL, Koob GF (1992) Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology (Berlin) 109:305–314
Merikangas KR, Angst J (1995) Comorbidity and social phobia: evidence from clinical, epidemiologic, and genetic studies. Eur Arch Psychiatry Clin Neurosci 244:297–303
Missig G, Roman CW, Vizzard MA, Braas KM, Hammack SE, May V (2014) Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology 86:38–48
Moreau JL, Scherschlicht R, Jenck F, Martin JR (1995) Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behav Pharmacol 6:682–687
Morley JE, Horowitz M, Morley PM, Flood JF (1992) Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides 13:1133–1135
Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jegou S (2009) Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology 34:424–435
Mustafa T, Jiang SZ, Eiden AM, Weihe E, Thistlethwaite I, Eiden LE (2015) Impact of PACAP and PAC1 receptor deficiency on the neurochemical and behavioral effects of acute and chronic restraint stress in male C57BL/6 mice. Stress: 1-11
Norrholm SD, Das M, Legradi G (2005) Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN). Regul Pept 128:33–41
Paxinos G, Watson C (6th edition) The rat brain in stereotaxic coordinates. Academic Press, Sydney ; Orlando, pp xxvi, 237 p. of plates
Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776
Piggins HD, Stamp JA, Burns J, Rusak B, Semba K (1996) Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol 376:278–294
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391
Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S (2011) Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul, Integr Comp Physiol 301:R1625–R1634
Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, Hammack SE, May V (2014) PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 47:151–165
Sabino V, Cottone P, Steardo L, Schmidhammer H, Zorrilla EP (2007) 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berlin) 192:537–546
Sampson D, Willner P, Muscat R (1991) Reversal of antidepressant action by dopamine antagonists in an animal model of depression. Psychopharmacology (Berlin) 104:491–495
Schulteis G, Markou A, Cole M, Koob GF (1995) Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci U S A 92:5880–5884
Slusher MA (1965) Influence of adrenal steroids on self-stimulation rates in rats. Proc Soc Exp Biol Med Soc Exp Biol Med 120:617–620
Telegdy G, Adamik A (2015) Neurotransmitter-mediated anxiogenic action of PACAP-38 in rats. Behav Brain Res 281:333–338
To CT, Bagdy G (1999) Anxiogenic effect of central CCK administration is attenuated by chronic fluoxetine or ipsapirone treatment. Neuropharmacology 38:279–282
Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berlin) 172:463–470
Valenza M, Steardo L, Cottone P, Sabino V (2015) Diet-induced obesity and diet-resistant rats: differences in the rewarding and anorectic effects of D-amphetamine. Psychopharmacology (Berlin)
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357
Weingartner H, Silberman E (1982) Models of cognitive impairment: cognitive changes in depression. Psychopharmacol Bull 18:27–42
Willner P (1990) Animal models of depression: an overview. Pharmacol Ther 45:425–455
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berlin) 93:358–364
Acknowledgments
We thank Stephen St. Cyr for technical assistance and Andrew Kim for editorial assistance. This publication was made possible by grants # MH093650, MH091945, DA030425, and GM008541 from the National Institute of Health, by the Peter McManus Charitable Trust, and the Peter Paul Career Development Professorship (P.C.).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mariel P. Seiglie and Karen L. Smith contributed equally to this work.
Rights and permissions
About this article
Cite this article
Seiglie, M.P., Smith, K.L., Blasio, A. et al. Pituitary adenylate cyclase-activating polypeptide induces a depressive-like phenotype in rats. Psychopharmacology 232, 3821–3831 (2015). https://doi.org/10.1007/s00213-015-4045-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4045-4