Abstract
Rationale
Fendiline is a GABAB receptor-positive allosteric modulator and L-type Ca2+ channel blocker that is safe for human use. Based on these pharmacological properties, fendiline may be useful to disrupt associative memories that can drive relapse to drug use in drug-addicted individuals
Objective
The current study evaluated the potential of fendiline to inhibit the maintenance and expression of learned associations between methamphetamine (meth) and an environmental context using conditioned place preference (CPP) in rats, to model for the associative learning that occurs during drug abuse by humans
Methods
Following meth conditioning (1 mg/kg), fendiline (5 mg/kg) was administered at various post-conditioning times to ascertain if there was a temporal window during which fendiline would be effective.
Results
Two once-daily injections of fendiline did not influence the maintenance of CPP regardless of the post-conditioning treatment time while 10 once-daily fendiline treatments inhibited CPP maintenance (p < 0.05). Fendiline administered immediately prior to the CPP test inhibited expression of meth-induced CPP in rats with a fendiline treatment history of 10 once-daily injections (p < 0.05) or those that received two injections that corresponded to the last 2 days of the 10-day treatment (p < 0.05). Fendiline did not produce preference or aversion on its own, nor did it alter motivated motor behavior.
Conclusion
Maintenance and expression of meth CPP is mitigated by repeated fendiline treatments when administered during the days that precede CPP testing. Reduction in the significance of meth-associated cues can reduce relapse; therefore, fendiline may be of value for addiction therapy in abstinent, meth-addicted humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During repeated psychostimulant administration, associations are made between the rewarding effects of the stimulant and the context in which it was administered. After terminating drug treatments, the brain is hyper-responsive to psychostimulant-paired cues (Hotsenpiller et al. 2001; Hotsenpiller and Wolf 2002; Rebec and Sun 2005) which may contribute to cue-induced relapse to drug taking in abstinent addicts (Childress et al. 2008; Ehrman et al. 1992). Aspects of this behavior can be studied using conditioned place preference (CPP), a behavior that can be observed in rodents and humans (Childs and de Wit 2009; Tzschentke 1998). Thus, CPP has been proposed as a means to explore the potential of pharmacotherapies to reduce the significance of drug-associated cues. The current study is focused on methamphetamine (meth), a potent and highly abused psychostimulant, for which no FDA-approved pharmacotherapy is available.
Repeated administration of psychostimulants initiates a cascade of neuronal events that change over the hours and weeks following psychostimulant exposure (Jayaram and Steketee 2005; Zhang et al. 2000, 2001). Time-dependent effects are observed after cessation of drug use by human meth abusers, with different phases being reported during the first 3 to 5 weeks of abstinence (McGregor et al. 2005; Zorick et al. 2010), including time-dependent changes in cognitive function (Simon et al. 2010). On a biochemical level, magnetic resonance spectroscopy analysis of neurotransmitter metabolite levels shows abnormal ratios of choline-N-acetylaspartate in recently abstinent humans vs. those who have abstained for more than 1 year (Nordahl et al. 2005). Temporal instability is also observed in the GABA system as decreased functional coupling of the GABAB receptor (GABABR) to Gi/o proteins is observed at 14 days, but not 1 day, of withdrawal from repeated amphetamine (Zhang et al. 2000). Thus, the dynamic state of the brain after repeated psychostimulant administration may provide “windows of vulnerability” during which the drug memory may be more sensitive to disruption.
The maladapted brain state that occurs after repeated psychostimulant administration is the consequence of numerous factors including adaptations that dampen inhibitory signaling to promote a hyper-excitable brain state. For example, the GABABR system is downregulated (Frankowska et al. 2008; Kushner and Unterwald 2001; Zhang et al. 2000) and high-voltage activated L-type Ca2+ channels are upregulated (Ford et al. 2009; Hu 2007; Nasif et al. 2005). Thus, GABABRs and L-type Ca2+ channels have received attention as possible targets for addiction pharmacotherapy (Brebner et al. 2002; Rose and Grant 2008; Xi and Gardner 2008). The inhibitory actions of GABABRs, activation of inwardly rectifying K+ channels and inhibition of Ca2+ channels (Bowery 1993; Mott and Lewis 1994), should counteract psychostimulant-induced enhancements of neuronal excitability. Indeed, the GABABR agonist baclofen abrogates psychostimulant-induced behaviors, including the maintenance and expression of amphetamine-induced behavioral sensitization (Bartoletti et al. 2004, 2005), expression of meth-induced CPP (Li et al. 2001), nicotine-induced locomotor stimulation (Lobina et al. 2011), nicotine-induced reinstatement of extinguished nicotine seeking (Fattore et al. 2009), as well as the self-administration of meth (Ranaldi and Poeggel 2002), amphetamine (Brebner et al. 2005), and cocaine (Filip et al. 2007; Filip and Frankowska 2007). Baclofen also abrogates meth-induced (Arai et al. 2009; Mizoguchi and Yamada 2011) and cocaine-induced (Porrino et al. 2013) cognitive deficits. Drawbacks to treatment with GABABR agonists include sedation and motor impairment (Cryan et al. 2004; Shoptaw et al. 2003). Positive allosteric modulators (PAMs) of the GABABR selectively augment GABABR-mediated signaling in the presence of receptor-bound endogenous GABA (Gjoni et al. 2006; Urwyler et al. 2005); therefore, GABABR PAMs have fewer negative side effects than direct-acting agonists. Furthermore, receptor desensitization and downregulation that occur with chronic agonist administration are not observed with PAMs (Gjoni and Urwyler 2008). Similar to baclofen, GABABR PAMs (CGP7930 and GS39783) reduce the maintenance of meth-induced CPP (Voigt et al. 2011b), cocaine self-administration (Filip et al. 2007; Halbout et al. 2011; Smith et al. 2004), cocaine- and cue-induced reinstatement of cocaine-seeking (Filip and Frankowska 2007), nicotine self-administration, and counteract nicotine-induced enhancement of brain reward function (Paterson et al. 2008). Attenuation of stimulant-induced behaviors is also observed with L-type Ca2+ channel blockers which inhibit the expression of nicotine-induced (Biala 2003), meth-induced, and cocaine-induced (Suzuki et al. 1992) CPP, cocaine-induced behavioral sensitization (Martin-Iverson and Reimer 1994), the acquisition and consolidation of nicotine-induced cognitive effects (Biala et al. 2013), and attenuate drug-primed reinstatement of nicotine self-administration (Biala and Budzynska 2008). Thus, GABABR PAMs and L-type Ca2+ channel blockers can reduce a variety of stimulant-induce behaviors.
Behavioral consequences of abused drugs are often categorized into distinct phases: (1) development (when the behavior develops, also termed induction), (2) maintenance, and (3) expression (when the behavior is expressed in response to drug-associated cues). The latter two phases are relevant targets for addiction therapy. The capacity of GABABRs and L-type Ca2+ channels to modify the maintenance of meth-induced behaviors is unknown, and thus is the focus of the current study. The dual pharmacological actions of fendiline, a GABABR PAM and L-type Ca2+ channel blocker, and its safety profile of fendiline in humans (Bayer and Mannhold 1987), compelled us to ascertain the ability of fendiline to mitigate maintenance and expression of meth-induced CPP. This study is an important first step to determine if fendiline may be an effective therapy to mitigate meth-induced relapse in humans.
Materials and methods
Animals
Male Sprague–Dawley rats (n = 118, Harlan, Indianapolis, IN) weighing 225–250 g were acclimated to the vivarium for at least 5 days prior to experimentation. Cage mates received identical treatments and were housed in a climate-controlled environment on a 12-h light/dark cycle (lights on 7 am / lights off 7 pm) with ad libitum access to food and water. The Rush University Medical Center housing facilities are accredited through the Association for Assessment and Accreditation of Laboratory Animal Care, and all experiments were carried out in accordance with the conditions set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with the approval of the Rush University Medical Center Institutional Animal Care and Use Committee.
Drugs
(+)Methamphetamine HCl (Sigma, St. Louis, MO) was dissolved in 0.9 % saline and administrated as 1 mg/ml/kg (as the base). Fendiline [N-(3,3-diphenylpropyl)-α-methylbenzylamine] (Sigma, St. Louis, MO) was dissolved in a 25 % ethanol/water solution and administered as 5 mg/ml/kg. (+/−)Baclofen, (Sigma, St. Louis, MO) was dissolved in 0.9 % saline and administered as 4 mg/ml/kg. Pentobarbital (Ovation Pharmaceuticals, Inc., Deerfield, IL) was administered as 10 mg/kg. Treatments were administered intraperitoneally (i.p.) and doses were selected based on the following: Our prior dose–response evaluations determined that 1 mg/kg i.p. meth produces persistent CPP (Herrold et al. 2013; Voigt et al. 2011a; b). The fendiline dose was guided by “Human Equivalent Doses” (HED) conversions (Bayer and Mannhold 1987; Chappell and Mordenti 1991) and from reports on doses tested in rats. Fendiline doses of 20–37.5 mg/kg alter blood pressure and heart rate in rats (Kozlovskii et al. 1996; Kozlovskii 1997; Maksimenko et al. 1997). The HED for 30 mg/kg fendiline is 336 mg/70 kg human, which would not be well-tolerated as a chronic therapy (Bayer and Mannhold 1987). To better align with HEDs and to avoid autonomic consequences, we opted to test lower doses. Our pilot studies with 10 mg/kg fendiline impaired spontaneous motor activity, while the selected dose of 5 mg/kg was devoid of motor effects (Fig. 5). The baclofen (4 mg/kg) dose was within the range that attenuates meth-induced CPP (1.25–5 mg/kg, i.p.) (Li et al. 2001; Voigt et al. 2011a) and amphetamine self-administration (1.8–5.6 mg/kg, i.p.) (Brebner et al. 2005) in rats. The 4 mg/kg baclofen dose in rats equates to approximately 24 mg in humans (US Department of Health and Human Services 2005), which is within the dosage range that reduce aspects of alcohol (Ameisen 2005) and nicotine (Cousins et al. 2001) dependence, and cocaine self-administration (Haney et al. 2006) in humans. Pentobarbital (a GABAA receptor PAM) was used as a positive control for motoric assessments at a dose of 10 mg/kg, which our pilot study verified is sufficient to induce motor slowing without a loss of righting reflex.
Conditioned place preference
The test room was dimly lit (54–108 lux) with white noise (San Diego Instruments, San Diego, CA) continuously present. The apparatus (AccuScan Instruments, Inc., Columbus, OH) (63 cm × 30 cm × 30 cm) consisted of three chambers divided by Plexiglas sliding doors; two large conditioning chambers (25 cm × 30 cm × 30 cm) separated by a small center chamber (13 cm × 30 cm × 30 cm). Each chamber had distinct visual and tactile cues (chamber A, vertical lines on walls and an overturned paint dish glued to the center of a randomly patterned floor; chamber B, horizontal lines on walls and a square patterned floor; center chamber, no stripes on walls and a smooth, slightly raised platform floor). Time spent in each chamber and motor activity was monitored via two sets of photobeams (24 in the horizontal plane and 12 in the vertical plane). All studies were conducted during the light cycle.
Experiments 1–3: effects of fendiline on methamphetamine-induced CPP
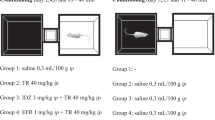
A 30-min pre-test was conducted 72 h prior to conditioning to determine unconditioned preference. As a group (n = 108), rats spent approximately equal time in each chamber (45 ± 2 % in chamber A and 47 ± 2 % in chamber B, p = 0.56); however, individual rats tended to spend more time in one chamber. To avoid a ceiling effect that may occur when rats are paired with a rewarding stimulus in the initially preferred chamber, for conditioning, meth was administered in the chamber in which the rats spent the least amount of time during the pre-test, as has been previously employed (Calcagnetti and Schechter 1994; Cunningham et al. 2003; Kurokawa et al. 2012; Nomikos and Spyraki 1988; Yu et al. 2013). As a control, we verified that the meth dose and treatment protocol did not alter anxiety (elevated plus maze; data not shown) which is sometimes a concern with the biased CPP design. Conditioning occurred over 6 days (Fig. 1). As CPP outcomes are the same whether the meth- or the saline-conditioning session occurs first (Voigt et al. 2011b), meth-conditioned rats were given meth on days 1, 3, and 5 and saline on days 2, 4, and 6. During conditioning, rats were placed into the appropriate chamber of the CPP box immediately after the injection for 45 min. A 30-min, drug-free CPP test was conducted 3 days after the last conditioning session. Rats that did not increase time spent in the meth-paired chamber by 10 % (180 s) during the CPP test compared to the same chamber during the pre-test were excluded from the study (26/108 rats failed to meet this criterion). Culling rats based on the strength of learning has been used previously (Brenhouse and Andersen 2008; Guo et al. 2008; Paolone et al. 2009) to assure that only those rats that clearly acquired the task (i.e., made the association between drug and context) were used to determine the potential of various ligands to subsequently disrupt CPP. Rats were assigned to a treatment group such that the magnitude of preference expressed during CPP test 1 was approximately equal for all groups. Home cage fendiline or vehicle injections were administered according to specific protocols outlined below. Three days after the final home cage injection, CPP was tested again. The 3-day period between the last home cage injection and the preference test was imposed to allow for fendiline to be cleared from the system. While no information is available on the in vivo clearance of fendiline in rats, human elimination half-life is reported to be 20 h (400 mg/day for 19 days; p.o.) (Weyhenmeyer et al. 1987) and 20–35 h (3 mg, i.v. or 50–75 mg, p.o.) (Kukovetz et al. 1982). As 4.5 half lives is considered sufficient for complete elimination, the 72-h washout period selected for rats was considered below that necessary to influence CPP outcomes. Experiment 1 (Fig. 1a) was designed to ascertain if early post-conditioning treatments of fendiline disrupted the short-term maintenance of meth-induced CPP. Meth-conditioned rats were injected with fendiline or vehicle in the home cage, once daily for 2 days (days 10 and 11; Fig. 1a). Experiment 2 (Fig. 1b) was designed to determine the effect of fendiline on the long-term maintenance of meth-induced CPP. Meth-conditioned rats received either: (1) 10 days of fendiline vehicle, (2) 10 days of fendiline, (3) 2 days of fendiline followed by 8 days of vehicle, or (4) eight injections of vehicle followed by 2 days of fendiline. These rats were then used to evaluate the effects of fendiline on the expression of CPP when tested immediately after a fendiline injection in Experiment 3 (Fig. 1b). Seven to 10 days after CPP test 2, rats were “re-conditioned” (R1–R4) to re-establish preference in all groups and prevent extinction of CPP that might occur after repeated testing. Three days after re-conditioning, rats were given a drug-free CPP test (CPP test 3) to verify place preference, and the 10 % criterion for task acquisition was re-applied. Three days later, fendiline or vehicle was administered immediately prior to CPP test 4. Behavioral effects of fendiline were apparent within the first 5 min after fendiline administration during CPP test 4 (vehicle history with vehicle challenge, 1,135 ± 60s (n = 9); 2-day-early fendiline history with fendiline challenge, 693 ± 96 (n = 12); 2-day-late fendiline history with fendiline challenge, 453 ± 88 (n = 14); 10-day fendiline history with fendiline challenge, 506 ± 120 (n = 12), one-way ANOVA p = 0.0001); thus, the fendiline administration protocol was appropriate to detect fendiline-induced effects on CPP.
Treatment protocols. a Experiment 1 evaluated the effects of home cage fendiline on the short-term maintenance of meth-induced CPP. b Experiment 2 evaluated the effects of fendiline on the long-term maintenance of meth-induced CPP and experiment 3 evaluated the effects of an acute fendiline injection on the expression of meth-induced CPP. Experiment 4 (not illustrated) assessed fendiline-induced effects on motor behavior on a rotarod. c Experiment 5 determined if fendiline was rewarding or aversive. Ø, no drug; V or Veh, vehicle (1 ml/kg); F or Fend, fendiline; M, meth; S, saline; R, reconditioning protocol
Experiment 4: effects of fendiline on motivated motor function
The rotarod was used to ascertain the effects of fendiline on motivated motor behavior. The rotarod (San Diego Instruments, San Diego, CA) consisted of four 11-cm-wide lanes positioned to achieve a 46-cm fall height. The rotating drum (7 cm diameter) was accelerated 5–40 rpm over 5 min. A subset of meth-conditioned rats given vehicle home cage treatments were used (vehicle group in experiment 1). Rats were trained on the rotarod apparatus until they remained on the rotating drum for 3 min. On the test day, fendiline, baclofen, pentobarbital, or vehicle was administered and latency to fall from the apparatus was measured at time 0 (i.e., prior to injection), and 20, 40 min, and 1, 3, 6, and 24 h after the injection. A repeated measures design was used wherein all rats were evaluated with each ligand. Rats were “re-trained” on the apparatus 24 h prior to each test to ensure that the 3-min minimum criterion was maintained across multiple tests.
Experiment 5: assessment of fendiline in CPP/conditioned place aversion
This experiment (Fig. 1c) was designed to determine if fendiline was rewarding or aversive. Rats were pre-tested and as a group time spent in each chamber was not significantly different (38 ± 6 % in chamber A and 53 ± 6 % in chamber B, p = 0.24, n = 12). Any rat that spent more than 75 % of the pre-test in one chamber was excluded from further analysis (i.e., 2 of 12). A counterbalanced design was used (half received fendiline in the initially preferred chamber and half in the initially non-preferred chamber) because it was unknown if fendiline would produce a preference or an aversion. Conditioning occurred over 10 days; with two 45-min conditioning sessions each day. Rats were given fendiline vehicle and immediately placed in one CPP chamber in the morning and 4 h later the other chamber was paired with fendiline. This fendiline dosing paradigm emulated the most robust fendiline treatment paradigm used (i.e., 10 once-daily injections). Three days after the final conditioning session, rats were given a 30-min, drug-free CPP test and motor activity was monitored.
Statistical analysis
Conditioned preference or aversion was defined as spending significantly more time in the drug- or vehicle-treated chamber, respectively. Two-way repeated measures ANOVA (chamber × CPP test) followed by a post hoc Newman–Keuls test was used to determine differences between time spent in each chamber (time spent in the center not included in the analysis). Motor activity during CPP test 4 was assessed using a one-way ANOVA followed by a post hoc Newman–Keuls. Rotarod assessments of motor function were conducted using a repeated measures ANOVA (treatment × time) followed by a post hoc Newman–Keuls. Statistical outliers (n = 14 out of 118 rats), identified as greater than two standard deviations above or below the group average (CPP or motor data), were removed from the analysis. p < 0.05 was considered significant. All data are shown as mean ± SEM.
Results
Experiment 1: effects of fendiline administered during the early post-conditioning phase on short-term maintenance of meth-induced CPP (Fig. 1a)
Following conditioning, preference for the meth-paired chamber was observed before and after two once-daily treatments of vehicle or fendiline. Figure 2a shows vehicle treatment ((n = 8); chamber, F (1,14) = 77.807, p < 0.001; test and interaction, not significant). Figure 2b shows fendiline treatment ((n = 9); chamber, F (1,16) = 14.746, p = 0.001; test and interaction, not significant). Post hoc analysis demonstrated significant preference for the meth-paired chamber during both CPP tests for vehicle-treated (p < 0.01) and fendiline-treated (p < 0.01 for CPP test 1 and p < 0.05 for CPP test 2) rats. While the time spent in the meth-paired chamber was reduced by fendiline, the preference for the meth-paired chamber was still significant; thus, 2 days of fendiline administered during the early post-conditioning phase did not disrupt the short-term maintenance of meth-induced CPP.
The short-term maintenance of meth-induced CPP was not inhibited by fendiline (timeline in Fig. 1a). Preference for the meth-paired chamber determined in CPP test 1 was not statistically altered by two once-daily injections of vehicle (a), or fendiline (b). Newman–Keuls test; ** p < 0.01; * p < 0.05. Filled diamond, time spent in meth-paired chamber; open square, time spent in saline-paired chamber; filled triangle, time spent in center chamber
Experiment 2: effects of fendiline administration on the long-term maintenance of meth-induced CPP (Fig. 1b)
To ascertain if increasing the number of fendiline treatments could disrupt the long-term maintenance of meth-induced CPP, 10 home-cage treatments were administered. As shown in Fig. 3a, meth-induced CPP was not disrupted by 10 injections of fendiline vehicle (n = 11; chamber, F (1,20) = 63.355, p < 0.001; test and interaction, not significant). Thus, preference exhibited during CPP test 1 was not diminished by repeated testing or by home cage vehicle treatments. As shown in Fig. 3b, 10 once-daily treatments of fendiline disrupted meth-induced CPP (n = 12; chamber, F (1,22) = 11.129, p = 0.003; test, not significant; interaction, F (1,22) = 4.800, p = 0.039), as the preference exhibited during CPP test 1 (p < 0.01) was no longer evident in CPP test 2 (post hoc Newman–Keuls). To determine if the ability of fendiline to antagonize maintenance of meth-induced place preference reflected processes that occurred only at the beginning or at the end of the 10-day fendiline treatment period fendiline injections were given on protocol days 10 and 11 (2-day-early fendiline) or 18 and 19 (2-day-late fendiline) (Fig. 1b). Neither early nor late fendiline treatments disrupted CPP. Figure 3c shows 2-day-early fendiline (n = 14; chamber, F (1,26) = 47.050, p < 0.001; test, not significant; interaction, F (1,26) = 11.056, p = 0.003). Figure 3d shows 2-day-late fendiline (n = 16; chamber, F (1,30) = 44.607, p < 0.001; test and interaction, not significant). While the 2-day-early and 2-day-late fendiline treatments slightly reduced preference for the meth-paired chamber, CPP was retained with sufficient magnitude to preserve significance (Newman–Keuls; p < 0.01). Thus, treatment duration, and not the post-conditioning phase in which the fendiline was administered, was critical for inhibiting the maintenance of meth-induced CPP.
The long-term maintenance of meth-induced CPP was inhibited by 10, but not two, injections of fendiline (timeline in Fig. 1B, Experiment 2). All groups showed preference for the meth-paired chamber during CPP test 1. a Rats administered 10 once-daily injections of vehicle expressed CPP during CPP test 2. b Rats administered 10 once-daily injections of fendiline did not express CPP during CPP test 2. c and d Rats administered two once-daily injections of fendiline, which corresponded to the first 2 days (c), or the last 2 days (d) of the 10-day treatment, retained preference for the meth-paired chamber during CPP test 2. Newman–Keuls test; ** p < 0.01. Filled diamond, time spent in meth-paired chamber; open square, time spent in saline-paired chamber; filled triangle, time spent in center chamber
Spontaneous motor activity was monitored during CPP test 2 to determine if fendiline treatment history impacted motor activity during testing for preference. No significant changes in motor activity were observed. Horizontal activity outcomes were representative of all horizontal and vertical motor activity. Horizontal activity: 10-day vehicle (n = 11) 3,649 ± 246, 10-day fendiline (n = 12) 3,373 ± 362, 2-day-early fendiline (n = 14) 4,280 ± 232, and 2-day-late fendiline (n = 16) 3,695 ± 209. These results demonstrate that spontaneous motor behavior during the CPP test was not altered by fendiline treatment history.
Experiment 3: effects of fendiline on the expression of meth-induced CPP
Experiment 3 was designed to ascertain if fendiline, administered immediately prior to the CPP test could disrupt the expression of meth-induced CPP. Rats used in this experiment were “re-conditioned” (Fig. 1b) to ensure that preference during CPP test 3 was similar in all groups, independent of treatment history (nine rats failed to meet the 10 % criterion for CPP and were excluded from further study: three in the vehicle, two in 10-day, one in 2-day-early, and three in 2-day-late fendiline groups). The reconditioning procedure revealed that a history of 10 fendiline injections (sufficient to disrupt CPP during test 2) did not impede the re-acquisition of meth-induced CPP. Meth-conditioned rats with a vehicle treatment history and a vehicle challenge maintained preference during both CPP tests 3 and 4 (Fig. 4a; n = 9; chamber, F (1,16) = 18.780, p = 0.001; test and interaction, not significant; post hoc Newman–Keul’s, p < 0.01 for both tests). Thus, vehicle injection immediately prior to CPP test 4 did not disrupt the ability of the rats to express a preference. Rats in the 2-day-early fendiline treatment group also expressed a preference for the meth-paired chamber during both the drug-free CPP test 3 and the fendiline-challenged CPP test 4 (Fig. 4c; n = 12; chamber, F (1,22) = 14.315, p = 0.001; test and interaction, not significant; post hoc Newman–Keuls, p < 0.01 for both tests). In contrast, CPP was nullified by the fendiline challenge for rats in the 10-day fendiline and 2-day-late treatment history groups. The 10-day fendiline history is shown in Fig. 4b (n = 12; chamber and test, not significant; interaction, F (1,22) = 14.483, p = 0.001; post hoc Newman–Keul’s, p < 0.01 for CPP test 3). The 2-day-late Fendiline History is shown in Fig. 4d (n = 12; chamber, F (1,26) = 7.105, p = 0.013; test, not significant; interaction, F (1,36) = 8.574, p = 0.007; post hoc Newman–Keul’s, p < 0.01 for CPP test 3). These findings indicate that fendiline treatment history is critical in determining the ability of a fendiline challenge to disrupt the expression of CPP.
The expression of meth-induced CPP was inhibited by fendiline only in rats with a treatment history of 10 fendiline injections, or two injections corresponding to the last two treatments of the 10-day treatment protocol (timeline in Fig. 1b, Experiment 3). All “re-conditioned” rats demonstrated preference for the meth-paired chamber during CPP test 3. In contrast, fendiline given immediately prior to CPP test 4, had a treatment history-dependent effect on the preference expression during this test. a Rats with a treatment history of 10 fendiline vehicle injections expressed CPP following the vehicle challenge (CPP test 4). c CPP was also retained when the fendiline challenge was given to rats with a treatment history of two fendiline injections during the first two treatment protocol days (early fendiline history). b and d Fendiline challenge immediately prior to CPP test 4 blocked preference in rats with a treatment history of 10 once daily fendiline injections (b) or two fendiline injections on the last 2 days of the 10-day protocol (d). Newman–Keuls test; ** p < 0.01. Filled diamond, time spent in meth-paired chamber; open square, time spent in saline-paired chamber; filled triangle, time spent in center chamber
As the results with CPP indicated that the brain may have adapted to 10 fendiline injections, we monitored motor activity during CPP test 4 to determine if motor behavior also showed changes indicative of neuronal adaptations. Horizontal activity was reduced in all fendiline-challenged groups (10-day vehicle history with vehicle challenge 3,259 ± 268 (n = 9), 10-day fendiline history with fendiline challenge 1,611 ± 270 (n = 12), 2-day-early fendiline history with fendiline challenge 1,999 ± 290 (n = 12); 2-day-late fendiline history with fendiline challenge 1,478 ± 142 9 n = 14); ANOVA p < 0.001; post hoc Newman–Keul’s p < 0.001 for all fendiline-challenged groups vs. the 10-day vehicle history with vehicle challenge). Similar outcomes were obtained for the other horizontal and vertical motor activity (data not shown). While activity was significantly decreased by fendiline independent of treatment history, it did not impede the capacity of rats to express meth-induced CPP as rats with the 2-day-early fendiline treatment history demonstrated CPP despite having significantly reduced motor activity (Fig. 4c).
Experiment 4: effect of fendiline on rotarod performance
To investigate the effects of fendiline on motor behavior, rats that were meth-conditioned and given home cage vehicle treatments in experiment 1 were tested on the rotarod to determine if fendiline-induced deficits in motivated motor function. Saline served as a negative control and baclofen and pentobarbital as positive controls. As shown in Fig. 5, baclofen (p < 0.05) and pentobarbital (p < 0.01) impaired motivated motor function at 20- and 40-min post-injection. No impairments were observed with fendiline or its vehicle (treatment, not significant; time, F (6,270) = 3.357, p = 0.003; interaction, F (24,270) = 3.587, p < 0.0001). Thus, fendiline did not alter motivated motor function, an important consideration for interpreting fendiline-induced effects on meth-induced CPP.
Motivated motor behavior assessed on the rotarod was not inhibited by fendiline (experiment 4). Baclofen and pentobarbital, significantly impaired performance on the rotarod compared to saline-treated rats at 20- and 40-min post-injection; an effect that was not observed for fendiline or fendiline vehicle. Newman–Keuls test; ** p < 0.01; * p < 0.05. Open square, baclofen vehicle; open triangle, fendiline vehicle; filled square, baclofen; filled triangle, fendiline; filled circle, pentobarbital
Experiment 5: assessments of fendiline in CPP / conditioned place aversion
To best interpret the effects of fendiline, we evaluated the capacity of fendiline to produce preference or aversion. A conditioning paradigm for fendiline was employed that mimicked the most robust fendiline protocol used to disrupt meth-induced CPP (10 once daily injections). Ten days of fendiline conditioning did not induce a chamber bias (n = 8; pre-test (843 ± 103 vs. 760 ± 100 s), CPP/CPA test (777 ± 113 vs. 839 ± 105 s)); chamber, test, and interaction: not significant); therefore, fendiline was neither rewarding nor aversive. Furthermore, motor activity revealed that behavioral response to fendiline remained unchanged throughout 10-day protocol indicating that neither sensitization nor tolerance occurred as a result of the 10 once daily treatments (horizontal activity: day 1 fendiline, 1,009 ± 92 vs. day 10 fendiline, 1,263 ± 106 photobeam breaks, p = 0.09).
Discussion
The current study revealed that meth conditioning induced a preference for the meth-paired chamber that was not diminished by home cage injections nor repeated testing; however, CPP was disrupted by fendiline administration in a duration-dependent manner. To the best of our knowledge, this is the first evaluation of the ability of fendiline to alter behavioral effects of a psychostimulant. Consequently, it was prudent to demonstrate that the fendiline dose employed was not rewarding or aversive. While acute fendiline administration reduced general motor activity (effects were equivalent for all groups independent of treatment history, i.e., the 10-day fendiline group did not exhibit greater motor deficits than the 2-day-early fendiline group), this effect did not interfere with motivated motor behavior assessed with the rotarod, nor did it impair the ability of rats to demonstrate a preference for the meth-paired chamber (e.g., the 2-day-early Fendiline group had decreased motor activity but still expressed CPP). Together these data indicate that the CPP outcomes of fendiline administration were independent of effects on motor function. Last, the duration of the fendiline treatment proved to be more relevant for attenuating meth-induced CPP, than the post-conditioning time during which fendiline was administered. The findings showed that sustained interruptions of mechanisms critical for the maintenance of meth-induced CPP occurred only when more than two fendiline injections were administered and suggest that chronic treatment renders fendiline most effective.
Maintenance of place preference
The maintenance of associative memories involves numerous neurotransmitter receptors, ion channels, and downstream mediators (Alberini et al. 2006; Bailey et al. 2004; Wang et al. 2006). Fendiline is a GABABR PAM (Kerr et al. 2002; Ong et al. 2005) and an L-type Ca2+ channel blocker (Nawrath et al. 1998; Tripathi et al. 1993), and each of these may alter substrates critical for memory maintenance. While it is clear that fendiline potentiates GABABR signaling in vitro (Chen et al. 2005; Ong and Kerr 2005), there is controversy regarding the ability of fendiline to function as a GABABR PAM in vivo (Urwyler et al. 2004); therefore, the respective contribution of fendiline acting as a GABABR PAM or as an L-type Ca2+ channel blocker in the current study awaits further studies with selective ligands. Nonetheless, the outcomes obtained with fendiline are exciting, especially in terms of addition therapy, and fendiline warrants further investigation for its clinical utility in this capacity.
Following repeated psychostimulant exposure, brain adaptations are brain region and withdrawal time specific. For example, the activated form of CREB (pCREB) is increased in the frontal cortex at three, but not at 14 days of withdrawal from repeated meth (McDaid et al. 2006). CREB is a mediator not only of psychostimulant-induced adaptations but also of learning and memory (Berke and Hyman 2000; Kelley 2004; Nestler 2001); therefore, modifying CREB may be a viable mechanism for disrupting processes necessary to maintain meth-induced CPP. Extracellular GABA concentrations also are differentially regulated in the medial prefrontal cortex following repeated stimulant exposure whereby cocaine-sensitized rats have elevated GABA levels at one and 7 days after the last cocaine injection, but not at 28 days (Jayaram and Steketee 2005). Although these time-dependent effects are reported for GABA and CREB, we did not see different effects when two fendiline injections were administered during the “early” or “late” withdrawal periods in experiment 2. However, it might be that at different withdrawal times, fendiline may have been more effective.
The protocols employed in the current study identified treatment duration-dependent effects of fendiline. We showed that 10 injections of fendiline, but not two, inhibited the maintenance of meth-induced CPP, suggesting that relatively sustained fendiline-induced changes in neuronal signaling were necessary to disrupt memory maintenance. The nature of these neuroadaptations likely do not involve upregulation of GABABRs as more selective GABABR PAMs (GS39783 and CGP7930) do not influence GABABR expression (Gjoni and Urwyler 2008). However, additional assessments that focus on time-dependent changes in GABABRs and downstream mediators, including L-type Ca2+ channels may provide important mechanistic insights about how fendiline disrupted CPP maintenance.
Expression of place preference
Treatment history influenced the ability of fendiline to inhibit the expression of meth-induced CPP. Fendiline challenge immediately prior to CPP testing blocked CPP expression only for rats with treatment histories during the later phases of withdrawal with two (2-day-late) or 10 fendiline injections, but not in rats administered fendiline during early withdrawal (2-day-early). These outcomes may reflect different time periods of “vulnerability” to fendiline and/or a critical length of time between terminating fendiline treatment and testing for CPP expression. Our findings also determined that tolerance or sensitization do not occur in response to repeated fendiline treatments, as the CPP-inhibiting effect of two fendiline injections (2-day-late) was also observed following 10 injections. These outcomes are clinically relevant as long-term treatments will likely be necessary for anti-addiction therapy.
Re-exposure to cues associated with abused substances increases neuronal activity in a region specific manner in humans (Childress et al. 1999, 2008; Kilts et al. 2004) and rodents (Brown et al. 1992; Rhodes et al. 2005; Zombeck et al. 2008). Augmenting inhibitory neurotransmission inhibits mnemonic processes (Castellano et al. 1989; Swartzwelder et al. 1987; Zarrindast et al. 2004). Accordingly, augmenting GABA receptor signaling via systemic administration of gamma vinyl-GABA, to prevent GABA degradation, inhibits the reinstatement of CPP (DeMarco et al. 2009). Thus, it is plausible that fendiline-induced inhibition of neuronal activity may be involved in the ability of fendiline to inhibit the expression of meth-induced CPP.
Study considerations and summary
The goal of this study was to identify withdrawal times and treatment duration necessary for fendiline to disrupt the maintenance and expression of meth-induced CPP. A caveat that deserves consideration is the potential influence of the fendiline vehicle (25 % ethanol) on experimental outcomes. This ethanol concentration (0.2 g/kg) is subthreshold to that which induces behavioral sensitization (de Macedo et al. 2013; Masur and dos Santos 1988) and CPP (Rezayof et al. 2012). Moreover, we verified that the 25 % ethanol vehicle did not induce motor impairment on the rotarod (Steiner et al. 2011). Thus, while alcohol can represent an active drug treatment, the dose administered in the current study was below that necessary to influence motor function. Another caveat deserving discussion is the choice of the fendiline dose. As detailed in the “Methods”, the dose was based on literature for its use in rats and dosing equivalency for safe use in humans. Given that the focus of the current study was on withdrawal time and treatment duration, the experimental design did not accommodate a dose–response analysis. Nonetheless, future studies with multiple doses of fendiline will be informative as to efficacy, tolerability, and toxicity of fendiline, especially during chronic use.
In summary, our results reveal that fendiline treatment successfully disrupted the maintenance of drug–context associations in specific fendiline administration protocols. Ten days of fendiline treatment produced the most robust inhibitory effects on CPP expression; however, the late withdrawal period appeared to be important as two injections of fendiline during this time promoted the ability of a fendiline challenge to mitigate meth-induced preference. These observations suggest that there may be post-conditioning “windows of opportunity” for fendiline to be particularly effective in blocking the maintenance of CPP. As cue-induced relapse is highly problematic in human meth-addicts, by reducing the impact of drug-associated cues, fendiline or other GABAB or calcium channel ligands may provide much needed assistance to maintain abstinence.
References
Alberini CM, Milekic MH, Tronel S (2006) Mechanisms of memory stabilization and de-stabilization. Cell Mol Life Sci 63:999–1008
Ameisen O (2005) Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol 40:147–150
Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Kamei H, Kim HC, Yamada K (2009) GABAB receptor agonist baclofen improves methamphetamine-induced cognitive deficit in mice. Eur J Pharmacol 602:101–104
Bailey CH, Kandel ER, Si K (2004) The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron 44:49–57
Bartoletti M, Gubellini C, Ricci F, Gaiardi M (2004) The GABAB agonist baclofen blocks the expression of sensitisation to the locomotor stimulant effect of amphetamine. Behav Pharmacol 15:397–401
Bartoletti M, Gubellini C, Ricci F, Gaiardi M (2005) Baclofen blocks the development of sensitization to the locomotor stimulant effect of amphetamine. Behav Pharmacol 16:553–558
Bayer R, Mannhold R (1987) Fendiline: a review of its basic pharmacological and clinical properties. Pharmatherapeutica 5:103–136
Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532
Biala G (2003) Calcium channel antagonists suppress nicotine-induced place preference and locomotor sensitization in rodents. Pol J Pharmacol 55:327–335
Biala G, Budzynska B (2008) Calcium-dependent mechanisms of the reinstatement of nicotine-conditioned place preference by drug priming in rats. Pharmacol Biochem Behav 89:116–125
Biala G, Kruk-Slomka M, Jozwiak K (2013) Influence of acute or chronic calcium channel antagonists on the acquisition and consolidation of memory and nicotine-induced cognitive effects in mice. Naunyn Schmiedebergs Arch Pharmacol 386:651–664
Bowery NG (1993) GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol 33:109–147
Brebner K, Childress AR, Roberts DC (2002) A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol 37:478–484
Brebner K, Ahn S, Phillips AG (2005) Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology (Berlin) 177:409–417
Brenhouse HC, Andersen SL (2008) Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci 122:460–465
Brown EE, Robertson GS, Fibiger HC (1992) Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci 12:4112–4121
Calcagnetti DJ, Schechter MD (1994) Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry 18:925–933
Castellano C, Brioni JD, Nagahara AH, McGaugh JL (1989) Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol 52:170–179
Chappell WR, Mordenti J (1991) Extrapolation of toxicological and pharmacological data from animals to humans. In: Testa B (ed) Drug Research. Academic Press, New York, pp 1–116
Chen Y, Phillips K, Minton G, Sher E (2005) GABA(B) receptor modulators potentiate baclofen-induced depression of dopamine neuron activity in the rat ventral tegmental area. Br J Pharmacol 144:926–932
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP (1999) Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18
Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP (2008) Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One 3:e1506
Childs E, de Wit H (2009) Amphetamine-induced place preference in humans. Biol Psychiatry 65:900–904
Cousins MS, Stamat HM, de Wit H (2001) Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob Res 3:123–129
Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 310:952–963
Cunningham CL, Ferree NK, Howard MA (2003) Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 170:409–422
de Macedo GC, Kawakami SE, Vignoli T, Sinigaglia-Coimbra R, Suchecki D (2013) The influence of orexins on ethanol-induced behavioral sensitization in male mice. Neurosci Lett. doi:10.1016/j.neulet.2013.07.010:
DeMarco A, Dalal RM, Pai J, Aquilina SD, Mullapudi U, Hammel C, Kothari SK, Kahanda M, Liebling CN, Patel V, Schiffer WK, Brodie JD, Dewey SL (2009) Racemic gamma vinyl-GABA (R, S-GVG) blocks methamphetamine-triggered reinstatement of conditioned place preference. Synapse 63:87–94
Ehrman RN, Robbins SJ, Childress AR, O’Brien CP (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 107:523–529
Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P (2009) Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. Eur Neuropsychopharmacol 19:487–498
Filip M, Frankowska M (2007) Effects of GABA(B) receptor agents on cocaine priming, discrete contextual cue and food induced relapses. Eur J Pharmacol 571:166–173
Filip M, Frankowska M, Przegalinski E (2007) Effects of GABA(B) receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol 574:148–157
Ford KA, Wolf ME, Hu XT (2009) Plasticity of L-type Ca2+ channels after cocaine withdrawal. Synapse 63:690–697
Frankowska M, Wydra K, Faron-Gorecka A, Zaniewska M, Kusmider M, Dziedzicka-Wasylewska M, Filip M (2008) Neuroadaptive changes in the rat brain GABA(B) receptors after withdrawal from cocaine self-administration. Eur J Pharmacol 599:58–64
Gjoni T, Urwyler S (2008) Receptor activation involving positive allosteric modulation, unlike full agonism, does not result in GABAB receptor desensitization. Neuropharmacology 55:1293–1299
Gjoni T, Desrayaud S, Imobersteg S, Urwyler S (2006) The positive allosteric modulator GS39783 enhances GABA(B) receptor-mediated inhibition of cyclic AMP formation in rat striatum in vivo. J Neurochem 96:1416–1422
Guo N, Garcia MM, Harlan RE (2008) A morphine-paired environment alters c-Fos expression in the forebrain of rats displaying conditioned place preference or aversion. Behav Neurosci 122:1078–1086
Halbout B, Quarta D, Valerio E, Heidbreder CA, Hutcheson DM (2011) The GABA-B positive modulator GS39783 decreases psychostimulant conditioned-reinforcement and conditioned-reward. Addict Biol 16:416–427
Haney M, Hart CL, Foltin RW (2006) Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology 31:1814–1821
Herrold AA, Voigt RM, Napier TC (2013) mGluR5 is necessary for maintenance of methamphetamine-induced associative learning. Eur Neuropsychopharmacol 23:691–696
Hotsenpiller G, Wolf ME (2002) Extracellular glutamate levels in prefrontal cortex during the expression of associative responses to cocaine related stimuli. Neuropharmacology 43:1218–1229
Hotsenpiller G, Giorgetti M, Wolf ME (2001) Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci 14:1843–1855
Hu XT (2007) Cocaine withdrawal and neuro-adaptations in ion channel function. Mol Neurobiol 35:95–112
Jayaram P, Steketee JD (2005) Effects of cocaine-induced behavioural sensitization on GABA transmission within rat medial prefrontal cortex. Eur J Neurosci 21:2035–2039
Kelley AE (2004) Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44:161–179
Kerr DI, Ong J, Puspawati NM, Prager RH (2002) Arylalkylamines are a novel class of positive allosteric modulators at GABA(B) receptors in rat neocortex. Eur J Pharmacol 451:69–77
Kilts CD, Gross RE, Ely TD, Drexler KP (2004) The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry 161:233–241
Kozlovskii VL (1997) The relationship between anticonvulsant and anti-anxiety effects of calcium channel blockers. Eksp Klin Farmakol 60:19–22
Kozlovskii VL, Mosin AE, Ivakina LV (1996) The effect of the subchronic administration of calcium-channel blockers on CNS excitability. Eksp Klin Farmakol 59:14–16
Kukovetz WR, Brunner F, Beubler E, Weyhenmeyer R, Lohaus R, Grob M, Mayer D (1982) Single dose pharmacokinetics of fendiline in humans. Eur J Drug Metab Pharmacokinet 7:105–110
Kurokawa K, Mizuno K, Ohkuma S (2012) Possible involvement of type 1 inositol 1,4,5-trisphosphate receptors up-regulated by dopamine D1 and D2 receptors in mouse nucleus accumbens neurons in the development of methamphetamine-induced place preference. Neuroscience 227:22–29
Kushner SA, Unterwald EM (2001) Chronic cocaine administration decreases the functional coupling of GABA(B) receptors in the rat ventral tegmental area as measured by baclofen-stimulated 35S-GTPgammaS binding. Life Sci 69:1093–1102
Li SM, Yin LL, Ren YH, Pan LS, Zheng JW (2001) GABA(B) receptor agonist baclofen attenuates the development and expression of d-methamphetamine-induced place preference in rats. Life Sci 70:349–356
Lobina C, Carai MA, Froestl W, Mugnaini C, Pasquini S, Corelli F, Gessa GL, Colombo G (2011) Activation of the GABA(B) receptor prevents nicotine-induced locomotor stimulation in mice. Front Psychiatry 2:76
Maksimenko EI, Zaitsev AA, Ailamazian EK, Ignatov I, Mikhailov AA (1997) Comparison of the tocolytic and hemodynamic effects of calcium channel blockers in pregnant rats. Eksp Klin Farmakol 60:25–27
Martin-Iverson MT, Reimer AR (1994) Effects of nimodipine and/or haloperidol on the expression of conditioned locomotion and sensitization to cocaine in rats. Psychopharmacology (Berl) 114:315–320
Masur J, dos Santos HM (1988) Response variability of ethanol-induced locomotor activation in mice. Psychopharmacology (Berl) 96:547–550
McDaid J, Graham MP, Napier TC (2006) Methamphetamine-induced sensitization differentially alters pCREB and DeltaFosB throughout the limbic circuit of the mammalian brain. Mol Pharmacol 70:2064–2074
McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM (2005) The nature, time course and severity of methamphetamine withdrawal. Addiction 100:1320–1329
Mizoguchi H, Yamada K (2011) Pharmacologic treatment with GABA(B) receptor agonist of methamphetamine-induced cognitive impairment in mice. Curr Neuropharmacol 9:109–112
Mott DD, Lewis DV (1994) The pharmacology and function of central GABAB receptors. Int Rev Neurobiol 36:97–223
Nasif FJ, Hu XT, White FJ (2005) Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci 25:3674–3679
Nawrath H, Klein G, Rupp J, Wegener JW, Shainberg A (1998) Open state block by fendiline of L-type Ca++ channels in ventricular myocytes from rat heart. J Pharmacol Exp Ther 285:546–552
Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128
Nomikos GG, Spyraki C (1988) Cocaine-induced place conditioning: importance of route of administration and other procedural variables. Psychopharmacology (Berlin) 94:119–125
Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH (2005) Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 62:444–452
Ong J, Kerr DI (2005) Clinical potential of GABAB receptor modulators. CNS Drug Rev 11:317–334
Ong J, Parker DA, Marino V, Kerr DI, Puspawati NM, Prager RH (2005) 3-Chloro,4-methoxyfendiline is a potent GABA(B) receptor potentiator in rat neocortical slices. Eur J Pharmacol 507:35–42
Paolone G, Botreau F, Stewart J (2009) The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berlin) 202:403–409
Paterson NE, Vlachou S, Guery S, Kaupmann K, Froestl W, Markou A (2008) Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats. J Pharmacol Exp Ther 326:306–314
Porrino LJ, Hampson RE, Opris I, Deadwyler SA (2013) Acute cocaine induced deficits in cognitive performance in rhesus macaque monkeys treated with baclofen. Psychopharmacology (Berlin) 225:105–114
Ranaldi R, Poeggel K (2002) Baclofen decreases methamphetamine self-administration in rats. Neuroreport 13:1107–1110
Rebec GV, Sun W (2005) Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav 84:653–666
Rezayof A, Ghandipour M, Nazari-Serenjeh F (2012) Effect of co-injection of arachydonilcyclopropylamide and ethanol on conditioned place preference in rats. Physiol Behav 107:301–308
Rhodes JS, Ryabinin AE, Crabbe JC (2005) Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci 119:759–771
Rose ME, Grant JE (2008) Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the theoretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry 20:145–155
Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W (2003) Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry 64:1440–1448
Simon SL, Dean AC, Cordova X, Monterosso JR, London ED (2010) Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs 71:335–344
Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DC (2004) Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology (Berlin) 173:105–111
Steiner MA, Lecourt H, Strasser DS, Brisbare-Roch C, Jenck F (2011) Differential effects of the dual orexin receptor antagonist almorexant and the GABA(A)-alpha1 receptor modulator zolpidem, alone or combined with ethanol, on motor performance in the rat. Neuropsychopharmacology 36:848–856
Suzuki T, Shiozaki Y, Masukawa Y, Misawa M (1992) Effects of calcium antagonists on the cocaine- and methamphetamine-induced conditioned place preference. Arukoru Kenkyuto Yakubutsu Ison 27:81–90
Swartzwelder HS, Tilson HA, McLamb RL, Wilson WA (1987) Baclofen disrupts passive avoidance retention in rats. Psychopharmacology (Berlin) 92:398–401
Tripathi O, Schreibmayer W, Tritthart HA (1993) Fendiline inhibits L-type calcium channels in guinea-pig ventricular myocytes: a whole-cell patch-clamp study. Br J Pharmacol 108:865–869
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Urwyler S, Gjoni T, Kaupmann K, Pozza MF, Mosbacher J (2004) Selected amino acids, dipeptides and arylalkylamine derivatives do not act as allosteric modulators at GABAB receptors. Eur J Pharmacol 483:147–153
Urwyler S, Gjoni T, Koljatic J, Dupuis DS (2005) Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology 48:343–353
US Department of Health and Human Services (2005) Guidance for industry on estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. p 42346
Voigt RM, Herrold AA, Napier TC (2011a) Baclofen facilitates the extinction of methamphetamine-induced conditioned place preference in rats. Behav Neurosci 125:261–267
Voigt RM, Herrold AA, Riddle JL, Napier TC (2011b) Administration of GABA(B) receptor positive allosteric modulators inhibit the expression of previously established methamphetamine-induced conditioned place preference. Behav Brain Res 216:419–423
Wang H, Hu Y, Tsien JZ (2006) Molecular and systems mechanisms of memory consolidation and storage. Prog Neurobiol 79:123–135
Weyhenmeyer R, Fenzl E, Apecechea M, Rehm KD, Dyde CJ, Johnson KJ, Friedel R (1987) Tolerance and pharmacokinetics of oral fendiline. Arzneimittelforschung 37:58–62
Xi ZX, Gardner EL (2008) Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev 1:303–327
Yu YJ, Chang CH, Gean PW (2013) AMPA receptor endocytosis in the amygdala is involved in the disrupted reconsolidation of methamphetamine-associated contextual memory. Neurobiol Learn Mem 103:72–81
Zarrindast MR, Shamsi T, Azarmina P, Rostami P, Shafaghi B (2004) GABAergic system and imipramine-induced impairment of memory retention in rats. Eur Neuropsychopharmacol 14:59–64
Zhang K, Tarazi FI, Campbell A, Baldessarini RJ (2000) GABA(B) receptors: altered coupling to G-proteins in rats sensitized to amphetamine. Neuroscience 101:5–10
Zhang Y, Loonam TM, Noailles PA, Angulo JA (2001) Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci 937:93–120
Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS (2008) Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav 93:637–650
Zorick T, Nestor L, Miotto K, Sugar C, Hellemann G, Scanlon G, Rawson R, London ED (2010) Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction 105:1809–1818
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voigt, R.M., Riddle, J.L. & Napier, T.C. Effect of fendiline on the maintenance and expression of methamphetamine-induced conditioned place preference in Sprague–Dawley rats. Psychopharmacology 231, 2019–2029 (2014). https://doi.org/10.1007/s00213-013-3347-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3347-7