Abstract
Rationale
Electronic cigarettes are becoming increasingly popular among smokers worldwide. Commonly reported reasons for use include the following: to quit smoking, to avoid relapse, to reduce urge to smoke, or as a perceived lower-risk alternative to smoking. Few studies, however, have explored whether electronic cigarettes (e-cigarettes) deliver measurable levels of nicotine to the blood.
Objective
This study aims to explore in experienced users the effect of using an 18-mg/ml nicotine first-generation e-cigarette on blood nicotine, tobacco withdrawal symptoms, and urge to smoke.
Methods
Fourteen regular e-cigarette users (three females), who are abstinent from smoking and e-cigarette use for 12 h, each completed a 2.5 h testing session. Blood was sampled, and questionnaires were completed (tobacco-related withdrawal symptoms, urge to smoke, positive and negative subjective effects) at four stages: baseline, 10 puffs, 60 min of ad lib use and a 60-min rest period.
Results
Complete sets of blood were obtained from seven participants. Plasma nicotine concentration rose significantly from a mean of 0.74 ng/ml at baseline to 6.77 ng/ml 10 min after 10 puffs, reaching a mean maximum of 13.91 ng/ml by the end of the ad lib puffing period. Tobacco-related withdrawal symptoms and urge to smoke were significantly reduced; direct positive effects were strongly endorsed, and there was very low reporting of adverse effects.
Conclusions
These findings demonstrate reliable blood nicotine delivery after the acute use of this brand/model of e-cigarette in a sample of regular users. Future studies might usefully quantify nicotine delivery in relation to inhalation technique and the relationship with successful smoking cessation/harm reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco smoking constitutes a major public health crisis causing estimated 81,700 deaths per year in England alone (The NHS Information Centre 2011), yet 21 % of the population continue to smoke (The NHS Information Centre 2011). Whilst nicotine is highly addictive, at levels commonly ingested by smokers, it is relatively non-toxic; the combustion products tar and carbon monoxide (CO), by contrast, are responsible for the major health risks (Royal College of Physicians 2000). This recognition led to the development of nicotine replacement therapy (NRT)—pharmaceutical grade nicotine delivered in the form of patch, gum, lozenge, sub-lingual tablet, inhalator and both mouth and nasal spray used to quit smoking or as a longer-term less harmful alternative. Even with the use of NRT, 93 % of quit attempts ultimately end in failure (Etter and Stapleton 2006), suggesting that these non-cigarette pharmaceutical products have limitations.
Electronic cigarettes (e-cigarettes) are battery-operated devices that deliver nicotine via inhaled vapour. In the UK, they are currently regulated as a consumer product under the General Product Safety Directive, although the Medicines and Healthcare Products Regulatory Agency (2013) recently announced plans to regulate these as medicines from 2016. Since their introduction into the Chinese market in 2004, e-cigarettes have gained popularity worldwide with sales increasing year on year. E-cigarette liquid contains glycerol and/or propylene glycol, flavourings and nicotine (0–24 mg/ml). This liquid is vaporised by an atomiser which is activated by ‘drawing’ on the device or pressing a button. ‘Smoking’; an e-cigarette, therefore, mimics the act of smoking and is commonly referred to as ‘vaping’. There are over 100 different e-cigarette brands commonly divided into first- and second-generation types. First-generation e-cigarettes are simple two-piece devices which tend to resemble tobacco cigarettes in size and shape. These devices use a ‘cartomiser’ system in which the atomiser and e-cigarette liquid are contained within one unit (cartridge). The cartridge is replaced as required, and the user has no contact with the e-cigarette liquid. Second-generation e-cigarettes are multiple-piece devices which do not resemble cigarettes. The batteries tend to be larger; atomisers are more sophisticated, and they contain fluid-filled cartridges (‘tanks’) which are re-filled from bottles of e-cigarette liquid. Whilst surveys of users and small-scale clinical studies suggest their potential for smoking cessation and harm reduction (Caponnetto et al. 2013; Dawkins et al. 2013; Etter and Bullen 2011; Polosa et al. 2011), their ability to deliver nicotine is under-explored.
Nicotine delivery via tobacco smoke is absorbed within 10–20 s in high concentration through the lungs into the bloodstream, reaching the brain in the same high concentration within 10 s (Royal College of Physicians 2000). Within 10 min of smoking, blood nicotine peaks at 15–30 ng/ml (Hukkanen et al. 2005; McEwen et al. 2008). Nicotine absorbed via commercially available NRT, by contrast, is much slower, either never reaching the peak achieved via tobacco smoking or taking much longer to do so depending on dose (Evans et al. 2006; Hukkanen et al. 2005; McEwen et al. 2008). Few studies have explored blood nicotine delivery via the e-cigarette, although two early studies reported ineffective nicotine delivery with naïve users using first-generation e-cigarettes. Bullen et al. (2010) observed a peak serum nicotine level of only 1.3 ng/ml in 19.6 min, and Vansickel et al. (2010) reported that two different brands of e-cigarettes failed to raise blood nicotine levels significantly over a 45-min period.
Three subsequent lines of evidence, however, indicate that naïve e-cigarette users may not puff effectively for nicotine delivery. For example, e-cigarette and tobacco cigarette puffing characteristics differ (Trtchounian et al. 2011); there is a learning curve to efficient vaping (McQueen et al. 2011), and regular users tend to use the second rather than first-generation devices (Dawkins et al. 2013; Foulds et al. 2011). In order to determine whether user experience and/or characteristics of the e-cigarette itself might influence blood nicotine delivery, Vansickel and Eissenberg (2013) carried out a pragmatic test in eight experienced e-cigarette users who used their own preferred devices and strength of nicotine cartridges. The brands used varied between individuals but were, generally, second-generation devices with nicotine strength e-liquid ranging from 9 to 24 mg/ml. Mean plasma nicotine levels increased significantly from 2 ng/ml at baseline to 10.3 ng/ml within 5 min, reaching a maximum concentration of 16.3 ng/ml by the end of a 60-min ad libitum puffing period.
The findings of Vansickel and Eissenberg (2013) in experienced users clearly demonstrate that e-cigarettes can deliver measurable levels of nicotine, but that this may depend on the user's technique and/or device characteristics. The purpose of the present study was to replicate the study design of Vansickel and Eissenberg (2013) using a more systematic approach; all users were accustomed to using the e-cigarette, but the device was standardised (first generation, 18 mg/ml nicotine cartridge) across users. The overall aim was to measure the acute blood nicotine delivery profile and to evaluate subjective effects in regular e-cigarette users.
Methods
Design and ethical approval
A repeated measures design was used with one group of participants assessed at seven time points over a 2.5-h period (Vansickel and Eissenberg 2013). The study was granted ethical approval by the University of East London ethics Committee on 21 November 2012 and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Participants were recruited via advertisements for the study via the e-cigarette manufacturer's December 2012 newsletter, Facebook page and through e-mail distribution to the existing customer base. To be included in the study, participants had to be (1) regular e-cigarette users (using the device for at least 1 month and using at least one 18-mg nicotine cartridge per day), (2) between the ages of 18 and 55, (3) a smoker or ex-smoker, (4) physically fit and willing to provide blood samples and (5) willing to abstain from smoking, vaping and the use of all nicotine-containing products for 12 h prior to testing. Participants were excluded if they (1) were non-smokers, (2) were not current e-cigarette users, (3) reported any physical/medical condition (including asthma, diabetes, epilepsy, heart or neurological condition) or psychiatric condition (including depression, anxiety or psychosis), (4) had any history of high or low blood pressure, (5) had any history of fainting or feeling faint associated with providing blood samples and (6) were pregnant or lactating females. All potential participants were given an initial telephone screening interview in order to confirm that they met the eligibility requirements for participation and fully understood the nature of the study. They were then sent an information sheet (via e-mail or post) and an appointment date.
A total of 14 participants (three women) aged between 22 and 54 years (mean = 37) completed the study. Nine described themselves as ‘White British’; one, as ‘Black British’; two, as ‘Asian’; one, as ‘White Spanish’; and one, as ‘Mongolian’. Ten were educated to a degree level or higher, and four had completed GCSE/O levels. Six participants described themselves as current smokers, and eight, as ex-smokers. All were current first-generation e-cigarette users.
Materials
The e-cigarette
The ‘SKYCIG’ is a two-piece first-generation cartomiser e-cigarette. Individually sealed boxed starter kits and 18-mg Crown Tobacco Bold cartridges were provided by the manufacturer. E-cigarettes were fully charged prior to the assessment session. According to the manufacturer's website, the cartridges contain nicotine, propylene glycol and ‘common natural and artificial flavourings’.
Baseline assessment measurements
Demographic and smoking history information was taken at baseline (age, gender, ethnicity, occupational status, smoking and vaping history). In order to assess baseline current or former dependence on tobacco, the six-item scale Fagerström Test of Cigarette Dependence (FTCD; Fagerström 2012) was employed. Scores can range from 0 to 10 with a higher score indicating greater cigarette dependence.
Tobacco-related withdrawal symptoms were assessed using the Mood and Physical Symptoms Scale (MPSS; West and Hajek 2004) which measures the presence and severity of five symptoms (depressed, irritable, restless, hungry and poor concentration) and urge to smoke. These were each rated ‘at this moment in time’ at four time points during the study. Each withdrawal symptom is rated on a scale from 1 (not at all) to 5 (extremely). Total score can, therefore, range from 5 to 25 with a higher score indicating a higher severity of withdrawal symptoms. Urge to smoke is rated from 1 (not at all strong) to 7 (extremely strong).
For assessing direct (positive) effects of the e-cigarette (such as ‘hit’, ‘satisfaction’, ‘pleasant’, ‘feels like smoking’, etc.), an 11-item visual analogue scale was used based on the study of Blank et al. (2008). Participants rate each item by placing a cross through a 200-mm line where the far left indicates ‘not at all’, and the far right indicates ‘extremely’. Scores are derived by measuring from the far left to the point of the line in millimetre and then halving to achieve a score out of 100. A higher score, therefore, indicates a stronger/more positive effect.
Finally, in assessing (adverse) side effects of the e-cigarette, we used a 21-item visual analogue scale based on study of Vansickel and Eissenberg (2013) (confused, dizzy, headache, pounding heart, light-headedness, nausea/feeling sick, nervous, salivation, sweaty, weak, mouth irritation, throat irritation, aching jaws, vomiting, flatulence/bloating, stomach ache, heartburn, diarrhoea, hiccups, cold hands/feet, palpitations). As above, participants rate each item by placing a cross through a 200-mm line where the far left indicates not at all, and the far right indicates extremely. Scores are derived as above. A mean overall ‘side effects’ score is computed here by adding the scores for each item and dividing by 21.
Clinical procedure
Participants were asked to abstain from all tobacco/nicotine products overnight (for 12 h) prior to a morning testing session (Vansickel and Eissenberg 2013). Upon arrival at the lab, written informed consent was taken, and participants provided an expired air CO sample to verify compliance with the instruction to remain abstinent from smoking (using a calibrated Bedfont Micro Smokerlyzer; CO of <10 ppm required).
Baseline questionnaires (see above) were then completed before a qualified phlebotomy nurse inserted a venous cannula into the forearm and took a baseline 6-ml blood sample. Each participant was then presented with a new boxed e-cigarette fitted with an 18-mg Crown Tobacco Bold flavoured cartridge and instructed to take 10 puffs (within 5 min, inter-puff interval was not measured). Questionnaires were completed at 5 min, and 6 ml of blood was collected at 10 min after the start of this 10-puff period. This was then followed by 60 min of ad lib puffing with 6 ml of blood taken every 15 min (another four times). During this time, each participant made a note of the number of puffs taken under observation of the researcher. Questionnaires were then completed again at the end of the 60-min ad lib vaping period which was followed by a 60-min rest period during which time participants did not use the e-cigarette. A final 6-ml blood sample was then taken, and questionnaires were completed at the end of this 60-min rest period (total of seven blood samples taken) before the venous cannula was removed. Each participant was reimbursed for his/her time and travel with £50 cash and the SKYCIG Starter Kit (retails at £49.99).
Blood nicotine analysis
Blood was collected in 6-ml lithium-heparinised vacutainers, stored on ice and then centrifuged and stored at 70 °C prior to analysis. The bioanalysis of nicotine from plasma samples was conducted by the Advanced Bioanalytical Service Laboratories Ltd., Welwyn Garden City, UK. Samples were analysed by HPLC interfaced with the AB/MDS Sciex 4000 Mass Spectrometer (Digard et al. 2013). Quantification of nicotine was by a peak area ratio. The determined lower limit of quantification for nicotine in the plasma using this method was 0.5 ng/ml.
Statistical analysis
Repeated measures analysis of variance (ANOVA) with simple contrasts to compare each time point to baseline was conducted for blood nicotine levels, urge to smoke and nicotine-related withdrawal symptoms.
Results
Baseline tobacco smoking and vaping-related information
Table 1 displays smoking and vaping-related information for the 14 participants. Baseline expired air test CO levels were all of <5 ppm, indicating that all participants had complied with the instruction to abstain from smoking. Compliance with the restriction on the other nicotine use was retrospectively confirmed when all the measured plasma nicotine levels were <2 ng/ml.
Plasma nicotine levels
Complete sets of blood were obtained from seven participants, a further two were able to provide blood on six out of the seven occasions, one provided five samples, two provided four samples, one provided three and one provided two. Reasons for failing to gain samples included blocked catheter lines (most commonly), a lost sample and participant requests to have the cannula removed. It proved difficult to obtain blood from the three female volunteers.
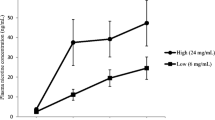
Mean plasma nicotine level at each time point (in nanogram per millilitre) is presented in Fig. 1 (based on the seven participants for whom nicotine levels were available at every time point). Plasma nicotine concentration rose from a mean of 0.74 ng/ml (standard error of the mean (SEM) = 0.12) at baseline to a mean of 6.77 ng/ml (SEM = 1.23) 10 min after 10 puffs and reached a mean maximum of 13.91 ng/ml (SEM = 2.12) by the end of the ad lib puffing period. ANOVA revealed a statistically significant increase from baseline to each and every follow-up time point (F 1, 6 > 28, p < 0.01 in all cases). There were large individual differences in plasma nicotine levels (n = 14). For example, 10 min after 10 puffs, the highest level achieved was 13.4 ng/ml, and the lowest was 2.50 ng/ml. Similarly, after 60 min of ad lib vaping, the highest plasma nicotine level achieved was 25.6 ng/ml, whilst the lowest was 4.35 ng/ml. Participants took an average of 29 ‘puffs’ during this hour of ad lib vaping (range, 11 to 63); however, the correlation between number of puffs taken and plasma nicotine (in nanogram per millilitre) at 60 min was only moderately and non-significantly correlated (r = 0.48, p = 0.16).
Urge to smoke and nicotine-related withdrawal symptoms
Mean urge to smoke and nicotine-related withdrawal symptoms are displayed in Figs. 2 and 3, respectively. There was a statistically significant reduction in urge to smoke from baseline to (a) the end of the 10-puff period and (b) the end of the 60-min ad lib vaping period (F 1, 12 > 12, p < 0.005 in both cases). By the end of the 60-min rest period, urge to smoke had increased and was no longer significantly lower than baseline (F 1, 12 = 2.52, p = 0.14). Nicotine-related withdrawal symptoms (as measured by the MPSS) also showed a statistically significant reduction from baseline to both (a) the end of the 10-puff period and (b) the end of the 60-min ad lib vaping period (F 1, 11 > 7, p < 0.05 in both cases), but not from baseline to the end of the 60-min rest period (F 1, 11 = 4.13, p < 0.07).
Separate analysis of the independent items of the MPSS revealed significant reductions from baseline to both the end of the 10-puff period and the 60-min ad lib period for irritability (F 1, 12 > 12, p < 0.005), restlessness (F 1, 12 > 8, p < 0.01) and poor concentration (F 1, 12 > 5.5, p < 0.05), but not for depression or hunger (F 1, 12 ≤ 1, ns).
Direct effects of the e-cigarette
Mean visual analogue scale ratings for hit and satisfaction after the 10-puff period were 74.07 (SEM = 7.15) and 70.86 (SEM = 6.28), respectively. Responses to the items ‘tastes like smoking’ and feels like smoking were somewhat lower at 50.82 (SEM = 6.80) and 62.75 (SEM = 5.07), respectively.
Adverse effects of the e-cigarette
Reporting of adverse side effects associated with the use of the e-cigarette tested here was very low; the total mean score was 12.81 (SEM = 1.45). ‘Light-headedness’ showed the highest mean (41.36; SEM = 7.37), followed by ‘throat irritation’ (27.25; SEM = 7.53). Table 2 presents mean side effect scores for individual items and total score at the end of the 10-puff period (results were very similar and not higher at the end of the 60-min ad lib vaping period).
Discussion
Whilst surveys and small-scale clinical studies suggest a successful replacement of smoking with e-cigarettes due to the presence of nicotine (Dawkins et al. 2013; Etter and Bullen 2011; Polosa et al. 2011; Siegel et al. 2011), effects of vaping on blood nicotine levels are relatively unexplored. In the current study, a significant increase in plasma nicotine level was observed 10 min after taking 10 puffs on the e-cigarette (from 0.74 to 6.77 ng/ml). Plasma nicotine levels continued to increase thereafter, reaching a mean maximum of 13.91 ng/ml at the end of the 60-min ad lib puffing period. These findings demonstrate that reliable plasma nicotine concentrations can be achieved via the use of a first-generation e-cigarette among regular users. That e-cigarettes can deliver nicotine in addition to providing the sensory stimulation associated with smoking is likely to be an important determinant in their effectiveness for quitting or replacing smoking.
The findings are broadly consistent with those described by Vansickel and Eissenberg (2013), although the slightly higher plasma nicotine concentration reported in the earlier study may reflect differences in devices used—second generation (Vansickel and Eissenberg) versus first generation (current study). That earlier studies found minimal or no nicotine delivery (Bullen et al. 2010; Vansickel et al. 2010) in naïve users is arguably due to their inexperience, although it is also possible that the more recent positive findings reflect an improvement in device characteristics over the past few years.
There was coherence in plasma nicotine kinetics for 75–80 % of the users, with a couple of notable outliers at the lower and higher end of the range. This could reflect (1) the inherent pharmaceutical quality of the nicotine solution cartridges as provided in the e-cigarette boxed sets (not chemically confirmed), (2) genetic differences in nicotine metabolism or (3) different inhalation techniques. A precursor study could investigate aspects concerning quality control in advance of any further clinical study (Sanchez-Medina et al. 2007; Gao et al. 2008), whilst measurement of the nicotine metabolite, cotinine, would enable the exploration of individual differences in metabolism.
In relation to inhalation technique, it is unclear whether the wide range of nicotine delivery is due to an inefficient puffing technique in those receiving very low levels or deliberate titration to receive a dose that suits the individual. If the former is the case, such individuals may be prone to relapse to smoking if they are unable to receive adequate doses of nicotine. In the case of the latter, if e-cigarettes do allow such titration, this may help to explain their appeal and the reports of successful substitution of tobacco smoking (Dawkins et al. 2013; Caponnetto et al. 2013), though it would also imply that the nicotine content may not be important for everyone. Either way, assessment of e-cigarette puffing topography could more accurately characterise the relationship between puffing characteristics (e.g. puff duration, intensity and inter-puff interval) and blood nicotine delivery. If blood nicotine delivery does depend on a particular puffing technique, and if nicotine delivery is necessary for successful smoking cessation/replacement, an understanding of optimal puffing behaviour would be informative to both e-cigarette manufacturers and users.
An interesting feature of the current study was the plasma nicotine levels in the three female subjects. Blood samples could only be collected for the first 10–30 min of the study due to problems with veins, but their plasma nicotine levels 10 min after the 10 puffs suggested that the peak plasma concentration is lower than that for the male participants and that the recovery period could be predictably back to baseline cravings at an earlier time than that of men. With such small numbers, it is not appropriate to conduct statistical tests, but the effect of gender certainly merits further exploration, particularly given that previous studies suggest that nicotine content may be more important for men, and sensorimotor aspects, more important for women smokers (Dawkins et al. 2013; Perkins et al. 1999).
In general, the device tested here fitted with an 18-mg/ml nicotine cartridge in regular e-cigarette users which delivers a range of blood nicotine concentrations on average comparable to that reported via commercially available doses of NRT (Evans et al. 2006; McEwen et al. 2008), oral snuff (2.5 g) and chewing tobacco (7.9 g; Hukkanen et al. 2005). The plasma profiles observed here, however, are a slightly different shape (less pronounced plasma max and more of a gradual plateau over 60 min) compared to previous profiles for NRT (Choi et al. 2003; McEwen et al. 2008; Molander and Lunell 2001; Schneider et al. 2001). This is a consequence of the dose escalation design used here, rather than a single dose followed over time, which reflects how vapers describe using e-cigarettes in practice (Dawkins et al. 2013).
The e-cigarette tested here also reduced urge to smoke and nicotine-related withdrawal symptoms (irritability, restlessness and poor concentration), which is consistent with previous reports (Bullen et al. 2010; Dawkins et al. 2012; Vansickel and Eissenberg 2013). Both urge to smoke and withdrawal symptoms increased again during the 1-h rest period (no vaping permitted), which is consistent with the reduction in plasma nicotine levels during this period. Levels of hit and satisfaction associated with using the e-cigarette were also fairly high, with mean scores of 74.07 and 70.86, respectively (with 100 representing the highest score of extremely). Consistent with the findings of two large survey studies (Etter and Bullen 2011; Dawkins et al. 2013), use of the e-cigarette was associated with very low reporting of side effects. Given that these participants were regular users, however, possibly with a vested interest in presenting e-cigarettes in a positive light, it is possible that there is over-reporting of positive effects and under-reporting of negative effects here. Light-headedness was most frequently reported which is consistent with a nicotine hit. Similar to other studies (e.g. Dawkins et al. 2013), some degree of throat irritation was reported which may be related to the nicotine hit in the throat and/or the effects of propylene glycol which acts as a humectant.
This small-scale clinical laboratory study reports for the first time significantly elevated blood nicotine levels and positive subjective effects in regular users after using a standardised first-generation e-cigarette. Nevertheless, there are some limitations. With full sets of blood available from only seven participants, the sample size is small; thus, findings may not be generalised to the other devices or to novice users. Females were also under-represented in this study, and their blood was difficult to obtain, so conclusions cannot be drawn in relation to e-cigarette nicotine delivery in women. Finally, positive subjective effects may have been over-estimated, and adverse effects may have been under-estimated in this sample of regular e-cigarette users who may have a vested interest in presenting the product in the most positive light.
To conclude, the current findings demonstrate reliable nicotine delivery after acute administration using a first-generation e-cigarette fitted with an 18-mg/ml nicotine cartridge in this small sample of regular users. Urge to smoke and nicotine-related withdrawal symptoms were also reduced with e-cigarette use, and side effects were consistent with those widely accepted for inhaled nicotine products. Taken together, these findings add to the growing body of evidence that e-cigarettes can reliably deliver nicotine in regular users, although there were some notable exceptions. Further clinical studies might usefully quantify inhalation technique and device characteristics in relation to nicotine delivery and explore the relationship with successful smoking cessation.
References
Blank MD, Sams C, Weaver MF, Eissenberg T (2008) Nicotine delivery, cardiovascular profile, and subjective effects of an oral tobacco product for smokers. Nicotine Tob Res 10:417–421
Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Langesen M (2010) Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control 19:98–103
Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R (2013) Efficiency and safety of an electronic cigarette (ECLAT) as tobacco cigarette substitute: a prospective 12-month randomized control design study. PLoS One 8(6):1–12
Choi JH, Dresler CM, Norton MR, Strahs KR (2003) Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine Tob Res 5:635–644
Dawkins L, Turner J, Hasna S, Soar K (2012) The electronic-cigarette: effects on desire to smoke, mood and cognition. Addict Behav 37:970–973
Dawkins L, Turner J, Roberts A, Soar K (2013) ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction 108:1115–1125
Digard H, Proctor C, Kulsekaran A, Malmqvist U, Richter A (2013) Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine Tob Res 15:255–261
Etter J-F, Bullen C (2011) Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction 106:2017–2028
Etter J-F, Stapleton JA (2006) Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 15:280–285
Evans SA, Blank M, Sams C, Weaver MF, Eissenberg T (2006) Transdermal nicotine-induced tobacco abstinence symptom suppression: nicotine dose and smokers' gender. Exp Clin Psychopharmacol 14(2):121–135
Fagerström KO (2012) Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res 14:75–78
Foulds J, Veldheer S, Berg A (2011) Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract 65:1037–1042
Gao J, Sanchez-Medina A, Pendry BA, Hughes MJ, Webb GP, Corcoran O (2008) Validation of a HPLC method for flavonoid biomarkers in skullcap (Scutellaria) and its use to illustrate wide variability in the quality of commercial tinctures. J Pharm Pharmacol Sci 11:77–87
Hukkanen J, Jacob P 3rd, Benowitz NL (2005) Metabolism and disposition kinetics of nicotine. Pharmacol Rev 75:79–115
McEwen A, West R, Gaiger M (2008) Nicotine absorption from seven current nicotine replacement products and a new wide-bore nicotine delivery device. J Smok Cessat 3:117–123
McQueen A, Tower S, Summer W (2011) Interviews with ‘vapers’: implications for future research with electronic cigarettes. Nicotine Tob Res 13:560–567
MHRA (2013). The regulation of nicotine containing products (NCPs) 12 June 2013. http://www.mhra.gov.uk/Safetyinformation/Generalsafetyinformationandadvice/Product-specificinformationandadvice/Product-specificinformationandadvice%E2%80%93M%E2%80%93T/NicotineContainingProducts/index.htm. Accessed 19 Aug 2013
Molander L, Lunell E (2001) Pharmacokinetic investigation of a nicotine sublingual tablet. Eur J Clin Pharmacol 56:813–819
Perkins KA, Donny E, Caggiula AR (1999) Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res 1:301–315
Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C (2011) Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Publ Health 11:786–798
Royal College of Physicians (2000) Nicotine addiction in Britain. Royal College of Physicians, London
Sanchez-Medina A, Etheridge CE, Hawkes GE, Hylands PJ, Pendry BA, Hughes MJ, Corcoran O (2007) Comparison of rosmarinic acid content in commercial tinctures produced from fresh and dried lemon balm (Melissa officinalis). J Pharm Pharmacol Sci 10:455–463
Schneider NG, Olmstead RE, Franzon MA, Lunell E (2001) The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clin Pharmacokinet 40:661–684
Siegel MB, Tanwar KL, Wood KS (2011) Electronic cigarettes as a smoking cessation tool: results from an online survey. Am J Prev Med 40:472–475
The NHS Information Centre (2011) Lifestyles statistics. Statistics on Smoking, England. http://www.hscic.gov.uk/pubs/smoking11. Accessed 19 Aug 2013
Trtchounian A, Williams M, Talbot P (2011) Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res 12:905–912
Vansickel AR, Eissenberg T (2013) Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res 15(1):267–270
Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE (2010) A clinical laboratory model for evaluating the acute effects of electronic ‘cigarettes’: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev 19:1945–1953
West R, Hajek P (2004) Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology 177:195–199
Acknowledgments
This study was funded and supported by SKYCIG Ltd. Thanks are due to Paula Booth (Psychology), Alexander Lyons, Angela Ng, Susan Harrison, Mark Newsum (Health, Sport and Bioscience) and Samila Payaniandy for their assistance with the study; to Dr. Mira Doig (ABS Labs) for the bioanalysis of nicotine from plasma; and to the participants for their time.
Conflict of interest
Dr. Lynne Dawkins has received funding to speak at research conferences and benefits in kind from e-cigarette companies. Professor Olivia Corcoran has no conflict of interests to declare. The sponsor had no role in the design and conduct of the study or in the preparation, review or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dawkins, L., Corcoran, O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology 231, 401–407 (2014). https://doi.org/10.1007/s00213-013-3249-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3249-8