Abstract
Rationale
Increased food consumption following ∆9-tetrahydrocannabinol-induced cannabinoid type 1 receptor agonism is well documented. However, possible non-∆9-tetrahydrocannabinol phytocannabinoid-induced feeding effects have yet to be fully investigated. Therefore, we have assessed the effects of the individual phytocannabinoids, cannabigerol, cannabidiol and cannabinol, upon feeding behaviors.
Methods
Adult male rats were treated (p.o.) with cannabigerol, cannabidiol, cannabinol or cannabinol plus the CB1R antagonist, SR141716A. Prior to treatment, rats were satiated and food intake recorded following drug administration. Data were analyzed for hourly intake and meal microstructure.
Results
Cannabinol induced a CB1R-mediated increase in appetitive behaviors via significant reductions in the latency to feed and increases in consummatory behaviors via increases in meal 1 size and duration. Cannabinol also significantly increased the intake during hour 1 and total chow consumed during the test. Conversely, cannabidiol significantly reduced total chow consumption over the test period. Cannabigerol administration induced no changes to feeding behavior.
Conclusion
This is the first time cannabinol has been shown to increase feeding. Therefore, cannabinol could, in the future, provide an alternative to the currently used and psychotropic ∆9-tetrahydrocannabinol-based medicines since cannabinol is currently considered to be non-psychotropic. Furthermore, cannabidiol reduced food intake in line with some existing reports, supporting the need for further mechanistic and behavioral work examining possible anti-obesity effects of cannabidiol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While Cannabis sativa has been used on the Indian subcontinent and in China for thousands of years as a medicine, its use has been a source of controversy in Western medicine since its introduction in the nineteenth century due to widespread recreational use and abuse (O’Shaughnessey 1843; Wang et al. 2008). C. sativa’s pharmacological actions and psychotropic properties include sedation, analgesia, hypothermia, catalepsy, and euphoria (Martin et al. 1981) alongside ravenous eating (Abel 1975).
Since the original identification of the cannabinoid type 1 and 2 receptors (CB1 and CB2R; Devane et al. 1988; Matsuda et al. 1990; Munro et al. 1993) and confirmation of an endogenous cannabinoid system following the discovery of the endogenous cannabinoids [eCBs; anandamide (AEA; Devane et al. 1992) and 2-AG (Mechoulam et al. 1995; Sugiura et al. 1995)], research has largely focused on the effects of ∆9-tetrahydrocannbinol (∆9THC; Gaoni and Mechoulam 1964). Indeed, only limited research has considered the effects of the numerous other phytocannabinoids (pCBs) also present (Izzo et al. 2009). More recently, research has begun to examine the effects of these individual pCBs (for a review of cannabinoid pharmacology, see Izzo et al. 2009). Currently, a range of possible cannabinoid-based therapies are being considered for a number of disorders (e.g., neurological and neurodegenerative, multiple sclerosis, and anti-obesity (Glass 2001; Pryce et al. 2003; Van Gaal et al. 2005); for a review, see Amar 2006). Interestingly, this new research has made it apparent that these pCBs are likely to act at sites other than CB1 and CB2R due to their low binding affinities at these receptor subtypes (with the exceptions of ∆9-tetrahydrocannabivarin (∆9THCV) and cannabinol (CBN); Petrosino et al. 2009). Importantly, the currently available literature gives no indication of non-∆9THC pCB psychoactivity (for reviews, see Amar 2006; Izzo et al. 2009).
Specifically in terms of feeding, and unlike the other pCBs, ∆9THC has been relatively well studied. Indeed, some time ago, it became apparent that CB1R sites in the central nervous system (CNS; Herkenham et al. 1991) were responsible for ∆9THC-mediated increases in feeding (Williams and Kirkham 2002a, b; Williams et al. 1998). ∆9THC-induced CB1R-mediated hyperphagia following a prefeed process is classically described by increases in consumption during the first hour of testing due to significant decreases in the latency to feed without concomitant increases in meal size and duration (Williams and Kirkham 2002b; Williams et al. 1998). ∆9THC-induced hyperphagia has been shown to be CB1R-mediated in experiments which co-administered ∆9THC alongside the CB1R antagonist SR141716A (Rinaldi-Carmona et al. 1999), even though in the same paradigm SR141716A alone was unable to alter feeding patterns (Williams and Kirkham 2002b). Similar alterations to feeding patterns have also been observed following exogenous AEA administration where AEA not only reduced the latency to feed but also increased meal size and duration (Hao et al. 2000; Jamshidi and Taylor 2001; Williams and Kirkham 2002a). As such, it has been suggested that CB1R-mediated alterations to feeding patterns can be divided into consummatory (those which control intake quantity) and appetitive (those which control feeding pattern) behaviors (Farrimond et al. 2011).
Recently in our lab, we have described alterations to feeding behaviors induced by a variety of non-∆9THC pCBs when administered as standardized cannabis extracts, i.e., botanical drug substances (BDS). Following the administration of a ∆9THC-rich standardized extract (high-∆9THC BDS, 67 % ∆9THC and 6.5 % other pCBs), we observed a reduction in ∆9THC-induced hyperphagia when our extract was compared with the purified ∆9THC alone (Farrimond et al. 2010a). Interestingly, in subsequent trials, we demonstrated that a ∆9THC-free extract analogue (non-∆9THC pCB content matched to the high-∆9THC BDS; Farrimond et al. 2012) and a second standardized extract which contained little ∆9THC (low-∆9THC BDS, 6.9 % ∆9THC and 14.2 % other pCBs; Farrimond et al. 2010b) administered at ∆9THC doses below those previously observed to alter feeding patterns could both increase feeding in male rats. Importantly, the ∆9THC-free extract analogue altered feeding behaviors by reducing the latency to feed and increasing the quantity of food consumed during both the first hour of testing and the first meal in the same manner as the high-∆9THC BDS and purified ∆9THC did, but without increases in first meal duration. However, the low-∆9THC BDS significantly increased appetitive behaviors, only inducing hyperphagia as a result of a highly significant decrease in the latency to feed but without concomitant increases in meal size and duration. These data have led us to suggest that non-∆9THC pCBs can not only modulate the feeding effects of ∆9THC but also induce alterations to feeding behaviors by themselves. However, our previous data shed no light on the specific contributions made by the individual pCBs found in our BDS to changes in feeding behaviors.
To date, there has only been limited research into the effects of the non-∆9THC pCBs on feeding behaviors. In 1976, Sofia and Knobloch reported that CBN (50.0 mg/kg, i.p. injection) reduced food intake in rats, an effect that has yet to be recapitulated (Sofia and Knobloch 1976). However, one might expect that CBN could elicit hyperphagia because, like ∆9THC, it exhibits CB1R agonist properties (Felder et al. 1995). In contrast, cannabidiol (CBD) exerts a superfluity of intracellular effects in vitro [e.g., modulation of Ca2+ homeostasis (Ryan et al. 2009) and AEA reuptake and FAAH inhibition (De Filippis 2008; Izzo et al. 2009)] and has been employed in a small number of feeding studies. Wiley et al. (2005) reported that CBD (3, 10, 30, and 100 mg/kg, i.p.) did not affect food intake in mice, a result confirmed by Scopinho et al. (2011) who demonstrated that CBD (1, 10, or 20 mg/kg, i.p.) did not affect feeding in rats. Similar data, in mice, were also recently described by Riedel (2009) (10.0 mg/kg, i.p.). Conversely, however, Sofia and Knobloch (1976) reported a CBD-induced (50 mg/kg, i.p.) reduction in feeding in rats. Very recently, these data have been supported by the observation that CBD (2.5 and 5 mg/kg, i.p.) can reduce body weight gain in relatively young (260 ± 20 g at the start of testing) rats over a period of 2 weeks, a finding which suggests either reduced food consumption or increased activity over the test period (Ignatowska-Jankowska et al. 2010). As such, data describing the effects of CBD on feeding remain inconclusive, and the mechanisms by which it could increase or decrease intake and/or body weight remain to be elucidated.
To our knowledge, the possible effects of cannabigerol (CBG) on feeding have yet to be examined, although such investigation is warranted since CBG shows partial agonism at CB1R and/or CB2R sites (Pertwee 2008), possible antagonism at CB1R sites (Cascio et al. 2010), phospholipase A2 activation (Evans et al. 1987), and/or AEA reuptake inhibition (Ligresti et al. 2006). Therefore, it is conceivable that CBG administration could induce either hyper- or hypophagic effects.
Considering the poor side effect profile of current ∆9THC-based anti-anorectic agents (e.g., hallucinations; BNF 2006), and given the drive to produce new anti-obesity agents which do not cause unwanted side effects [viz., SR141716A (EMA 2009) or MK-0364 (Clark 2009)], it is clear that further research examining the possible feeding effects of pCB could prove therapeutically useful. Furthermore, considering the myriad of protocols thus far used to test possible feeding effects of pCBs, direct comparisons of these data are limited. To address this, we have administered CBD, CBG, and CBN individually using the same prefeed protocol that we have successfully used to highlight the hyperphagic actions of ∆9THC. Furthermore, in order to assess possible CB1R mediation of any observed CBN effects, we have also performed a CBN and SR141716A co-administration trial. We present an analysis of hourly intakes and critical meal parameters following drug administration.
General methods
Animals
Thirty adult, male Lister-hooded rats (p > 40, 200–250 g at the start of testing; Harlan UK Ltd., UK) were maintained in a temperature-controlled environment (21–22 °C) under a 12:12-h light/dark cycle (red light on at 1030 hours). Given the distinct pharmacological profiles of CBD, CBG, and CBN (reviewed in Farrimond et al. 2012), direct comparisons between the drugs on feeding behavior would yield little pertinent data; thus, rats were split into three groups of ten animals, with each group acting as its own control and receiving a different test substance (see Table 1). Normal laboratory chow (PCD Mod C, Special Diet Services, Witham, UK) was available ad libitum, but on test days was removed for a 3-h period and replaced with a prefeed mash for a 2-h period (see “Prefeed procedure”) which was followed by 1 h of food deprivation immediately after drug administration. Fresh tap water was available ad libitum. All procedures were performed in compliance with the requirements of the United Kingdom Animals (Scientific Procedures) Act 1986.
Test environment
All tests were performed during the dark light phase under low-intensity red light (~4 lx). Testing took place in standard plastic cages, each fitted with a modified food hopper connected, via a strain gauge weighing device, to a computer running data acquisition and analysis software (The Feeding and Drinking Monitor, v. 2.16, TSE Systems GmbH, Bad Homburg, Germany) which permitted continuous monitoring of food intake. In addition, each cage was fitted with a CCTV camera positioned above each cage to allow an unimpeded view of rat behavior (distance from cage to camera, approximately 10 cm). Food intake data were analyzed to provide information on hourly food intakes as well as critical meal parameters such as latency to onset of meals, individual meal size, and duration. For the purposes of this study, a meal was defined as any feeding episode causing a change in food weight of ≥0.1 g, lasting at least 1 min and separated by at least 15 min from any subsequent episode. These criteria have been previously used to facilitate the visualization and interpretation of drug effects on feeding behavior and to distinguish prolonged eating episodes from more transient, exploratory contacts with food (Williams and Kirkham 2002a). Consecutive feeding events separated by intervals of <15 min were considered to be part of a single meal. Due to these criteria, in some instances, animals have or have not chosen to consume meals during different test hours. Therefore, some analysis of variance (ANOVA) results have different degrees of freedom and F values than might be expected.
Drugs
Fresh solutions of CBG, CBD, and CBN (GW Pharmaceuticals, Salisbury, UK) were prepared 15 min before administration on each test day. All pCBs were dissolved in a sesame seed oil vehicle (Sainsbury’s Supermarkets Ltd., London, UK), and the doses specified in Table 1 were administered. The presented pCB doses were based on multiples of 1× (low), 10× (medium), and 100× (high) times the concentrations present in a low-∆ 9THC cannabis extract that we have previously shown to induce increases in appetitive behaviors (Farrimond et al. 2010b). Phytocannabinoids were delivered orally via a syringe placed into the rat’s cheek pouch (p.o.).
In a second experiment, because of the likelihood of CB1R involvement in any observed CBN effects, we co-administered 26.0 mg/kg CBN with SR141716A (1.0 mg/kg) and compared these data with that collected previously following CBN alone administration. SR141716A was administered in a 1:1:18 vehicle made as 1 part ethanol (Fisher Scientific UK Ltd., Loughborough, UK), 1 part cremophor (Sigma-Aldrich, St. Louis, MO, USA), and 18 parts 0.9 % sodium chloride (Fisher Scientific UK Ltd.) saline via subcutaneous injection.
Both administration methods (p.o. and s.c.) were calculated to have an injection volume of 1.0 mL/kg. Each group of animals received their drug treatments according to a Latin square design, counterbalanced for phytocannabinoid dose, with at least 48 h between successive treatments. All drug groups used vehicle controls as part of the Latin square design. Drug administration began only after animals had been habituated to housing conditions, oral dosing, s.c. injections, and all subsequent test procedures.
Throughout these tests, no nonspecific behavioral effects of any drug at any dose were evident.
Prefeed procedure
In all experiments, rats were transferred from home cages to individual test cages immediately after dark onset (1030 hours) and presented with 30 g of a wet mash diet for 120 min as a prefeed. Any remaining wet mash and spillage was recovered after 120 min and weighed. Animals were fully habituated to the prefeed procedure before testing began, and drug administration did not begin until prefeed intakes were stable as assessed by a non-significant ANOVA.
Procedure
Following removal of the prefeed at 1230 hours, all drugs were administered to the rats according to a Latin square design. Rats were then deprived of food until 1330 hours to allow for drug assimilation. At 1330 hours, 30 g of normal laboratory chow was placed into the food hoppers. Subsequent, hourly food intake (calculated from: starting food mass − (remaining food mass + spillage)) was measured for four consecutive hours.
Statistical analysis
Hourly food intake was analyzed using two-way ANOVA with four dose levels (vehicle, low, medium, and high) and four time points (hours 1, 2, 3, and 4); where appropriate, this analysis was followed by individual one-way ANOVA tests for each time point and Bonferroni post hoc tests. The data collected for each meal parameter following test substance administration were separately analyzed using one-way ANOVA with four dose levels (vehicle, low, medium, and high), with Bonferroni post hoc tests performed where appropriate. Following SR141716A plus CBN co-administration, the same hourly intake and meal parameter data were analyzed by further one-way ANOVA with three drug levels (vehicle, CBN alone, and CBN plus SR141716A), Bonferroni post hoc tests carried out when appropriate. All tests were performed using IBM SPSS Statistics 19 (International Business Machines Corp., Armonk, USA).
Results
Cannabinol alone and cannabinol co-administered with SR141716A
Before testing began, prefeed intakes were stabilized for both CBN-only administration [F(12,122) = 1.277, p = 0.242] and CBN-SR141716A co-administration [F(4,49) = 1.538, p = 0.207]. During testing, animals consumed 19.40 (±0.57) and 19.50 (±0.46) g of prefeed per day, respectively. Furthermore, upon rearrangement of prefeed intakes by dose, no significant differences were apparent between any prefeed intakes for any individual dose of either CBN alone, CBN plus SR141716A, or their respective vehicle treatments [F(5,57) = 0.113, p = 0.989].
Hourly intake
Two-way analysis of variance failed to show significant effects of either dose [F(3,60) = 0.973, p = 0.411] or time [F(3,20) = 0.807, p = 0.505]; however, there was a significant time-by-dose interaction [F(9,60) = 2.704, p = 0.010]. Subsequent analysis of effects for each individual hour showed that CBN significantly increased chow consumption during the first hour [F(3,34) = 7.663, p = 0.001] (Fig. 1a, white bars) from a vehicle-treated intake of 0.86 ± 0.51 to 2.87 ± 0.45 g at the 26.0-mg/kg dose. Post hoc analysis revealed that intake following 26.0 mg/kg CBN was significantly greater than after vehicle treatment (p = 0.010); no other doses induced significant hyperphagic effects. During the second hour of testing, a marginal effect was apparent [F(3,34) = 2.391, p = 0.088] (Fig. 1a, light gray bars), which is most likely due to the small increases in feeding seen following the 2.60-mg/kg dose compared with vehicle treatment; intakes increased from 0.86 ± 0.51 g following vehicle treatment to 1.44 ± 0.44 g at the 2.6-mg/kg dose. However, post hoc tests show no significant differences between chow intakes following any CBN treatment when compared with vehicle treatments (p ≥ 0.938 in all cases) during hour 2. Significant increases were observed in all cumulative combinations of hourly intake [hours 1 and 2: F(3,34) = 3.590, p = 0.025; hours 1–3: F(3,34) = 4.635, p = 0.009; all four hours: F(3,34) = 3.509, p = 0.027] (Fig. 1b, light gray, gray, and black bars, respectively).
Mean hourly chow (a), cumulative hourly chow (b), and meal 1 chow consumption (c) and meal pattern (d) following administration of CBN (0, 0.26, 2.60, and 26.00 mg/kg, p.o.). CBN administration significantly increased hour 1 intake (a) (white bars) and chow consumption over all cumulative hourly arrangements (b). Furthermore, following CBN administration, significant increases in chow consumption during the first meal (c) and highly significant decreases in the latency to feed (d) were observed. No statistical analyses have been performed on second meal data as animals consumed too few second meals. Meal 2 bars are included for reference only. In (d), meal duration is represented by the length of the bar and is provided numerically above each bar (min ± SEM). Chow intake (a–c) is represented as the mean intake ± SEM. *p ≤ 0.05 (in Bonferroni post hoc test following a significant one-way ANOVA result versus vehicle-treatment). Solid line denotes a significant Bonferroni post hoc test following a significant one-way ANOVA on meal 1 latency
Co-administration of SR141716A with CBN blocked the previously observed CBN-mediated increases in hour 1 intake (p = 0.696; Fig. 2a, white bars). Furthermore, SR141716A co-administered with CBN also blocked the marginally significant increase in chow consumption observed during the second hour of testing [F(2,27) = 2.099, p = 0.114] (Fig. 2a, light gray bars) and during each consecutive cumulative hourly arrangement of the animals’ intakes [hours 1 and 2: F(2,27) = 1.351, p = 0.227; hours 1–3: F(2,27) = 0.974, p = 0.392; all 4 hours: F(2,27) = 2.112, p = 0.142] (Fig. 2b, light gray, gray, and black bars, respectively). However, during the third hour of testing, intakes recorded for the two vehicle-treated conditions (those animals which received both the sesame oil and 1:1:18 ethanol/cremophor/saline vehicles) varied such that SR141716A plus CBN co-administration vehicle-treated animal intakes were ~2 g higher than their CBN vehicle-treated counterparts (0.1 g). As such, during the third hour of CBN plus SR141716A treatment, a significant reduction in chow consumption compared with the control was apparent [F(2,27) = 3.940, p = 0.033] such that SR141716A plus CBN-treated animals displayed significantly reduced intakes versus vehicle-treated animals (p = 0.038).
Mean hourly chow (a), cumulative hourly chow (b), and meal 1 chow consumption (c) and meal pattern (d) following administration of CBN and SR14171A (0 and 26.00 mg/kg CBN, p.o., and 1.0 mg/kg SR141716A, s.c.). The response recorded previously (see Fig. 1) following the highest dose of CBN (26.00 mg/kg, p.o.) is compared with those following CBN (26.00 mg/kg, p.o.) and SR141716A (1.0 mg/kg, s.c.) co-administration. The co-administration of SR141716A with CBN blocked CBN-mediated increases in hour 1 intake (a) (white bars), meal 1 size (c), and duration (d) and the latency to feed (d). No statistical analyses have been performed on second meal data as animals consumed too few second meals. Meal 2 bars are included for reference only. In (d), meal duration is represented by the length of the bar and is provided numerically above each bar (min ± SEM). Chow intake (a–c) is represented as the mean intake ± SEM. *p ≤ 0.05 (in Bonferroni post hoc test following a significant one-way ANOVA result versus vehicle treatment). Solid line denotes a significant Bonferroni post hoc test following a significant one-way ANOVA on meal 1 latency
Alterations to meal pattern
Following CBN administration, the observed increase in hour 1 food consumption (Fig. 1a, white bars) was due to a significant dose-dependent increase in the size of the first meal [F(3,34) = 4.377, p = 0.011] (Fig. 1c) and a reduction in the latency to the first meal [F(3,34) = 5.217, p = 0.005] (Fig. 1d, light gray bars) which shifted feeding into the first hour of the test. However, post hoc Bonferroni tests revealed no significant differences in meal one size following any individual dose of CBN versus vehicle treatments, while the latency to meal 1 was significantly reduced from 96.7 ± 26.7 to 10.8 ± 4.6 min (at a dose of 26.0 mg/kg CBN, p = 0.038). In conjunction with the dose-dependent increase in the size of the first meal, its duration was also significantly increased in a dose-dependent manner [F(3,34) = 2.963, p = 0.047] (Fig. 1d, light gray bars). Consistent with the previously reported abolition of the CBN effect upon hourly intake, SR141716A blocked the CBN-induced increases in meal 1 size (p = 0.374: Fig. 2c), meal 1 duration (p = 1.000; Fig. 2d), and the latency to feed (p = 1.000; Fig. 2d), supporting a CB1R-mediated mechanism for CBN. Analysis of second, third, and inter-meal interval parameters is not included as less than four rats consumed second or third meals in any given dose group during this test.
Cannabidiol administration
After habituation to test procedures, prefeed intakes were stabilized [F(6,69) = 1.282, p = 0.279] and CBD administration commenced. During the test period, animals consumed 16.57 ± 0.46 g of prefeed per test day.
Hourly intake
Here, two-way ANOVA failed to show a significant effect of CBD treatment [F(3,108) = 1.380, p = 0.253] or any dose-by-time interaction [F(9,108) = 1.412, p = 0.192]. However, a significant effect of time was seen [F(3,36) = 7.338, p = 0.001], indicating that chow intake did alter over the course of the experiment. However, one-way ANOVA for each individual hour showed that chow consumption following CBD administration did not vary significantly from those observed for vehicle treatments during any individual hour [hour 1: F(3,37) = 0.394, p = 0.758; hour 2: F(3,37) = 2.088, p = 0.120; hour 3: F(3,37) = 0.868, p = 0.467; hour 4: F(3,37) = 0.481, p = 0.698] (Fig. 3a, white, light gray, gray, and black bars, respectively). Cumulative food intakes in hours 1 and 2 (0.77 ± 0.19 g) and 1–3 (2.31 ± 0.26 g) also showed no significant variation from vehicle treatments induced by CBD administration [F(3,39) = 1.837, p = 0.158, and F(3,39) = 1.033, p = 0.390, respectively] (Fig. 3b, light gray and gray bars). However, importantly, CBD induced significant dose-dependent reductions in total food intake over the total 4-h test period [F(3,39) = 3.343, p = 0.030] (Fig. 3b, black bars). Vehicle-treated animals consumed 4.06 ± 0.44 g of chow which was reduced following administration of the highest CBD dose (4.40 mg/kg) to 2.59 ± 0.36 g in 4 h.
Mean hourly chow (a), cumulative hourly chow (b), and meal one chow consumption (c) and meal pattern (d) following administration of CBD (0.00, 0.044, 0.44, and 4.40 mg/kg, p.o.). CBD administration significantly reduced chow intake over the period of the test (b). No statistical analyses have been performed on second meal data as animals consumed two few second meals. Meal 2 bars are included for reference only. In (d), meal duration is represented by the length of the bar and is provided numerically above each bar (min ± SEM). Chow intake (a–c) is represented as the mean intake ± SEM. *p ≤ 0.05 (Bonferroni post hoc test following a significant one-way ANOVA result versus vehicle treatment). Solid line denotes a significant Bonferroni post hoc test following a significant one-way ANOVA on meal 1 latency
Alterations to meal pattern
While CBD administration significantly reduced the total amount of food consumed in all meals combined, it had no effect on all other meal parameters. Specifically, no significant effects of CBD administration were observed for the latency to meal 1 [121.8 ± 10.8 min: F(3,37) = 1.635, p = 0.196] (Fig. 3d), the intake during (1.88 ± 0.21 g) or duration (7.8 ± 0.9 min) of meal 1 [F(3,37) = 0.570, p = 0.638, and F(3,37) = 0.523, p = 0.670] (Fig. 3c, d, respectively), the cumulative intakes or durations of meals 1 and 2 combined [3.04 ± 0.25 g: F(3,37) = 0.957, p = 0.424; 13.4 ± 1.5 min: F(3,37) = 1.250, p = 0.307, respectively], or total duration of all consumed meals [17.1 ± 2.1 min: F(3,37) = 1.523, p = 0.226]. Please note that the quoted values are the averages ± SEM collapsed by dose. Analysis of the second, third, and inter-meal interval parameters is not included as less than four rats consumed second or third meals in any given dose group during this test.
CBG administration
Prefeed intakes were stabilized before testing began [F(9,99) = 1.395, p = 0.202]. On each test day, animals receiving CBG consumed 18.94 ± 0.44 g.
Hourly intake
Two-way ANOVA failed to show any significant effect of dose [F(3,72) = 0.872, p = 0.460], time [F(3,24) = 2.135, p = 0.122], or time-by-dose interaction [F(3,72) = 0.990, p = 0.456]. CBG administration induced no significant changes from vehicle-treated animal intakes during any hour of the test [hour 1: F(3,33) = 0.739, p = 0.537; hour 2: F(3,33) = 2.105, p = 0.121; hour 3: F(3,33) = 1.278, p = 0.300; and hour 4: F(3,33) = 1.473, p = 0.242] (Fig. 4a, white, light gray, gray, and black bars, respectively) or in any cumulative hourly arrangement of chow intakes [hour 1: F(3,33) = 0.739, p = 0.537; hours 1 and 2: F(3,33) = 0.810, p = 0.498; hours 1–3: F(3,33) = 0.834, p = 0.486; and total intake: F(3,39) = 1.563, p = 0.215] (Fig. 4b, white, light gray, gray, and black bars, respectively).
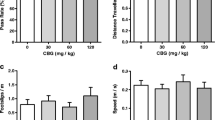
Mean hourly chow (a), cumulative hourly chow (b), and meal 1 chow consumption (c) and meal pattern (d) following administration of CBG (0.00, 0.176, 1.76, and 17.60 mg/kg, p.o.). The administration of CBG induced no significant deviations from vehicle treatments for any measure. No statistical analyses have been performed on second meal data as animals consumed too few second meals. Meal 2 bars are included for reference only. In (d), meal duration is represented by the length of the bar and is provided numerically above each bar (min ± SEM). Chow intake (a–c) is represented as the mean intake ± SEM. *p ≤ 0.05 (Bonferroni post hoc test following a significant one-way ANOVA result versus vehicle treatment). Solid line denotes a significant Bonferroni post hoc test following a significant one-way ANOVA on meal 1 latency
Alterations to meal pattern
In conjunction with hourly intake quantities, CBG administration had no effect on meal patterns. Indeed, meal 1 intake remained constant at 2.20 ± 0.23 g [F(3,29) = 0.488, p = 0.694[(Fig. 4c), the latency to the first meal at 110.9 ± 14.4 min [F(3,29) = 0.597, p = 0.622] (Fig. 4d), and the duration of the first meal at 12.4 ± 1.9 min [F(3,26) = 0.123, p = 0.945] (Fig. 4d). Analysis of the second, third, and inter-meal interval parameters is not included as less than six rats consumed second or third meals in any given dose group during this test.
Discussion
In this study, the effects of CBD, CBG, and CBN on the feeding patterns in adult male rats were investigated. Here, the results obtained demonstrate that CBN can stimulate feeding and alter meal patterns in rats, while CBD significantly reduces intake. CBG had no effect upon feeding patterns using the experimental paradigm employed here. It should be noted, however, that non-significant differences in intake under vehicle control conditions exist between our three experiments, and as a consequence, these differences may limit the extent of interpretation of the drug effects.
CBN induced a dose-dependent increase in chow consumption during the first hour, as illustrated by a significant increase in intake versus vehicle-treated intakes at its highest dose. This significant increase in first hour intake can be attributed to the significant decreases in the latency to feed which altered the temporal arrangement of feeding such that the first meal occurred in the first, rather than the second, hour of testing (Fig. 1). Furthermore, CBN increased the size and duration of the first meal, but, importantly, and unlike ∆9THC (Farrimond et al. 2010a), also increased the total amount of food consumed during the test period. Indeed, in the present case, total chow intake following CBN administration was significantly increased by ~60 % compared with vehicle treatments during the test period, whereas previously, we observed a non-significant change of ~2 % following ∆9THC administration compared with vehicle treatments over the same 4-h period (Farrimond et al. 2010a). CBN’s effects upon appetitive aspects of feeding (i.e., decreased latency to feed resulting in increased intake during the first hour of testing) and increases in the total amount of chow consumed mirror the behavioral effects of administration of the eCB, AEA, which have been shown to be CB1R-mediated (Hao et al. 2000; Jamshidi and Taylor 2001; Koch and Matthews 2001; Williams and Kirkham 1999, 2002a). Indeed, radioligand binding has demonstrated that CBN is a CB1R agonist (Rhee et al. 1997), which justified our co-administration of the CB1R antagonist, SR141716A, with CBN. This co-administration duly blocked first hour and first meal intake increases and the reduction in the latency to feed. These results conclusively demonstrate that the changes to feeding patterns seen following CBN administration alone were CB1R-mediated. Indeed, we have demonstrated that SR141716A blocked CBN-induced changes to all meal parameters and hour 1 intake and observed no significant effect of CBN alone administration in any subsequent hour. Therefore, even though during the third hour of SR141716A CBN co-administration testing there were differences in third hour vehicle-treated intakes between the CBN alone and SR141716A CBN co-administration trials, such differences do not hinder the analysis of our data.
It is interesting that CBN and ∆9THC administration induced different changes to feeding patterns, even though both have been shown to affect feeding via a solely CB1R-mediated mechanism (see Williams and Kirkham 2002b for CB1R involvement in ∆9THC-mediated hyperphagia). In this test, we have seen that the hourly effects of CBN administration do not exhibit the reduction in second hour chow consumption which is characteristic of ∆9THC-mediated modulation of feeding patterns. This lack of compensatory effect has led to increased total chow consumption in this study. Indeed, ∆9THC has previously been found to significantly increase intakes during the first hour of testing, but be followed by a significant reduction in feeding in the second hour; at the highest administered ∆9THC dose (2.68 mg/kg, p.o.), intakes during hour 2 were ~17 % of vehicle-treated intakes (Farrimond et al. 2010a). The reason for this lack of compensatory mechanism may suggest that CBN remained at higher concentrations in the brain for an increased period of time compared with ∆9THC (due to the comparatively higher administered doses) or could be due to the differences in the psychotropic properties of the two pCBs. Due to CBN’s lack of observed psychotropic side effects, it is possible to administer CBN at higher doses than ∆9THC without disruptions to feeding patterns caused by nonspecific behaviors (i.e., motor incoordination). These comparatively higher doses of CBN may have led to increased feeding behaviors with a longer duration of action.
It is also intriguing that CBN’s significant hyperphagic effects only manifested at a dose of 26 mg/kg versus vehicle treatments and not at any lower dose. While CBN is an agonist at CB1R, its disassociation constant (K i ) is approximately five times greater than that of ∆9THC [CBN = 211.2 nM (Rhee et al. 1997) versus ∆9THC = 39.5 nM (Bayewitch et al. 1996)]. Therefore, CBN’s lower affinity at CB1R could explain the observed difference in effective doses when compared with ∆9THC where a maximal effect in this paradigm is seen at 2.68 mg/kg (Farrimond et al. 2010a). Furthermore, CBN’s lower affinity for CB1R could also explain why no evidence supports the psychotropic effects of CBN which are commonly associated with CB1R activation. However, we must accept that the gross visual analysis of behaviors we used here does not preclude the possibility of nonspecific behavioral side effects. Therefore, while we believe that it is be highly unlikely that the administered CBN had any nonspecific behavioral side effects for the previously mentioned reasons, we suggest that further experiments be performed using a battery of behavioral tests (e.g., balance bars) which would fully determine any psychoactive properties of CBN.
Previously, we administered CBN (0.26 mg/kg) with ∆9THC (0.27 mg/kg) and various other pCBs and observed significant hyperphagia (Farrimond et al. 2010b). When CBN was administered alone at 0.26 mg/kg in this study, and purified ∆9THC alone at 0.34 mg/kg previously (Farrimond et al. 2010a), we observed no significant alterations to the feeding patterns. This comparison clearly suggests that ∆9THC and CBN interact synergistically in some way to induce changes to feeding patterns at doses which have previously been shown to be ineffective when administered alone. Further studies are required to fully characterize the behavioral adjustments induced by CBN ∆9THC co-administration and any mechanisms via which CBN and ∆9THC may interact to alter feeding patterns. Co-administration of ∆9THC and CBN could have the therapeutic advantage that similar increases in feeding could be induced by doses of ∆9THC below those currently used which induce unwanted side effects.
The data presented here contradict, to an extent, those previously published by Sofia and Knobloch (1976) where a significant reduction in feeding at a CBN dose twice as high as the highest presented here (50.0 mg/kg, i.p.) was reported. However, not only were Sofia’s experiments conducted over a considerably different timescale (daily food intake measurements over a 6-day period rather than a 4-h test), but the route of administration (i.p.) would have caused a more rapid increase in plasma/brain concentrations of CBN which would have reached a higher maximum concentration than via the p.o. administration employed here.
It must also be noted that repeated ∆9THC administration has previously been linked to sensitization effects in rat models. Both Cadoni et al. (2001) and Rubino et al. (2001) have observed that if rats are pretreated twice a day for 3 or 5 days, respectively, with ∆9THC, then, after a washout period, they react more strongly to further ∆9THC administration compared with untreated controls, an effect that can be removed by the administration of SR141716A. As such sensitization has not yet been demonstrated following CBN, CBD, or CBG administration and given the lower affinity of CBN for CB1R ∆9THC compared with ∆9THC, the distinct pharmacologies of both CBD and CBG, and since both Cadoni et al. (2001) and Rubino et al. (2001) demonstrated sensitization following i.p., not p.o., administration in non-feeding behavioral tests, it is unlikely that non-∆9THC pCB-induced behavioral sensitization is affecting the presented results.
Here, we also present results which demonstrate that CBD administration can induce significant reductions in chow consumption over a 4-h period. Specifically, CBD administration induced only subtle, non-significant reductions in animal intake during any individual hour of the test; however, together, this led to a significant reduction in total chow intake over the test period due to significant reductions in intake during all meals. It is worthy of mention that these apparent late-onset suppressive effects may reflect the relatively slow pharmacokinetic profile of CBD. Indeed, Deiana et al. (2012) recently showed that brain levels of CBD continued to rise progressively for 4 h following a 120-mg/kg oral dose. Despite these effects on hourly intakes, CBD administration had no significant effect on any other critical meal parameter. Such behaviors have been intimately linked to CB1R activation, and since it is currently thought that CBD is unlikely to interact with CB1R (Hill et al. 2012), these data may suggest that CBD can affect a feeding pathway which is unrelated to CB1R. Such data fit well with the reductions in chow consumption previously reported by Sofia and Knobloch in 1976 who also demonstrated a CBD-mediated reduction in chow consumption. Very recently, Ignatowska-Jankowska et al. (2010) have shown that CBD (2.5 and 5.0 mg/kg, i.p.) can reduce body weight gain in young rats, suggesting either reduced food intake or increased activity and therefore indirectly supporting the reductions observed by Sofia and Knobloch and the results presented here. However, data which describe the effects of CBD administration on feeding patterns are not yet conclusive. Recently, Wiley et al. (2005) and Scopinho et al. (2011) observed no effect of CBD on feeding patterns in food-restricted mice and normally fed and fasted rats, respectively. Wiley used 3.0–100.0 mg/kg CBD while Scopinho used 1.0–20.0 mg/kg CBD, both administered i.p. Therefore, it seems likely that the differences between the experimental protocols used by Wiley and Scopinho and those presented here could feasibly explain the differences in reported effects. Indeed, due to the route of administration (p.o.) used in our study, it is likely that peak cerebrospinal fluid concentrations were considerably lower than those achieved with the i.p. route used by Wiley and Scopinho. Furthermore, since neither Wiley nor Scopinho used prefed animals, it is likely that the differences in endocannabinergic tone between the models will have altered feeding behaviors and, consequently, the animals’ responses to CBD administration.
It seems apparent from the available literature that the functional effect of CBD to induce significant decreases in chow intake could arise from the numerous intra- and extracellular mechanisms with which it is known to interact, but are likely to be unrelated to traditional CB1R-mediated feeding control. Such a theory is supported by CBD administration’s failure to affect any meal parameter or individual hourly intake in this test. Unfortunately, the relatively wide spectrum of cellular and molecular mechanisms that have been proposed but not definitively established in vivo makes such suggestions highly speculative, and further investigations that probe the discrete mechanisms potentially involved are required to confirm the mechanisms underlying the observed functional effects. Clearly, it would be most interesting to establish a non-CB1R-mediated feeding pathway that is modulated by a pCB, such as CBD, although the lack of pharmacological tools with which to block CBD’s putative AEA reuptake, FAAH inhibition, and antagonism of ∆9THC at CB1R separately renders such an experiment challenging to undertake.
We believe this to be the first time that possible CBG effects on feeding have been examined, although no significant CBG-mediated effects were observed. It is unlikely that CBG administration can exert any effects via direct CB1R binding since it has a very low affinity for CB1R [disassociation constant, 440 nM (Gauson et al. 2007), c.f. ∆9THC, 39.5 nM (Bayewitch et al. 1996)]. However, CBG is a known AEA reuptake inhibitor (Ligresti et al. 2006) such that CBG could induce increased brain concentrations of AEA which could conceivably produce similar effects to that seen following the administration of exogenous AEA. However, in the presented experimental paradigm, we had reduced eCB tone using a prefeed. Therefore, even if CBG was inducing functionally effective AEA reuptake blockade, little endogenous AEA would be present in the CNS and so reuptake blockade would be unable to potentiate CBG-mediated behavioral effects. Id est, were CBG to be tested using a food-restricted paradigm which elevated eCB production, then its putative effects on AEA reuptake inhibition may begin to induce significant effects on feeding patterns. As such, while the data we now present suggest that CBG administration has no effect on feeding patterns, different results may be found using a different experimental paradigm.
In summary, following the administration of CBN alone, we observed significant increases in appetitive, consummatory, and total intake behaviors. Thus, we suggest that a balance exists between endocannabinergic tone and pCB-mediated CB1R activation. This balance manifests as increasing feeding behaviors (appetitive, consummatory, and total chow intake increase) with increasing CB1R activation and decreasing feeding behaviors with decreasing CB1R activation. The data we have presented suggest that as CB1R activation is reduced, feeding behaviors decay and the weakest behaviors are lost first (increased total chow consumption < increased meal 1 duration < increased meal 1 chow intake < increased hourly intake and reduced latency to the first meal). Such a theory is supported by currently available literature since only recently have significant effects on ∆9THC-mediated meal pattern changes in rats been observed, but AEA-induced increases in total chow intake, appetitive, and consummatory behaviors have been demonstrated (see Farrimond et al. 2011 for a review). Furthermore, we have also demonstrated significant, short-term CBD-mediated reductions in feeding which, we suggest, are due to reduced consummatory behaviors following CBD administration. However, given CBD’s pharmacological profile, such effects are unlikely to be CB1R-mediated. Finally, we have observed that the administration of CBG induces no significant alterations to feeding patterns in the presented paradigm. A direct comparison between these three drug treatments is necessarily limited by the large variability in response seen under vehicle conditions, and as such, the robustness of the effects we describe here should be confirmed by a fully randomized replication of our study.
Conclusion
Using a prefeed paradigm, CBN induced significant CB1R-mediated hyperphagia in male rats via significant reductions in the latency to feed and significant increases in the food consumed during the first hour and meal, alongside significant increases in the total amount of food consumed when compared with vehicle-treatments. Conversely, CBD administration reduced total feeding over a 4-h period. Neither ∆9THCV nor CBG administration exerted effects on feeding behaviors in this paradigm.
As CBN has not so far been shown to have psychoactive properties, it could be a useful anti-anorexic agent since in this study CBN administration significantly increased intake over the total test period. Clearly, further experiments are required to fully characterize the effects of both chronic and acute CBN administration on food consumption and body weight. Moreover, the data we have presented here, when compared with some of our previous data (Farrimond et al. 2010b), suggest that CBN and ∆9THC, when co-administered, may synergistically induce powerful hyperphagic effects. Therefore, co-administration of CBN and ∆9THC may also exhibit anti-anorexic properties.
Given CBD’s well-documented non-psychotropic nature and its high tolerability in humans, further characterization of its effects on feeding reduction and the mechanisms via which CBD induces such effects is also clearly warranted. Such tests may provide an interesting insight into the subtle feeding effects of CBD we have observed here, and it would be particularly interesting to identify a non-eCB system-mediated mechanism of action of CBD in relation to feeding behaviors.
Abbreviations
- 2-AG:
-
2-Arachidonoylglycerol
- ∆9THC:
-
∆9-Tetrahydrocannabinol
- ∆9THCV:
-
∆9-Tetrahydrocannabivarin
- AEA:
-
Anandamide
- ANOVA:
-
Analysis of variance
- BDS:
-
Botanical drug substance
- CB1R:
-
Cannabinoid type 1 receptor
- CB2R:
-
Cannabinoid type 2 receptor
- CBD:
-
Cannabidiol
- CBG:
-
Cannabigerol
- CBN:
-
Cannabinol
- CNS:
-
Central nervous system
- eCB:
-
Endocannabinoid
- pCB:
-
Phytocannabinoid
References
Abel E (1975) Cannabis: effects on hunger and thirst. Behav Biol 15:255
Amar B (2006) Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol 105:1–25
Bayewitch M, Rhee MH, Avidor-Reiss T, Breuer A, Mechoulam R, Vogel Z (1996) (−)-Delta9-tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor-mediated inhibition of adenylyl cyclase. J Biol Chem 271:9902–9905
BNF (2006) British National Formulary. BMJ Publishing Group, London
Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G (2001) Behavioural sensitization after repeated exposure to Delta 9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology 158(3):259–266
Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG (2010) Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol 159:129–141
Clark RT (2009) Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the Fiscal Year Ended December 31, 2008 United States Securities and Exchange Commission. Merck & Co., Inc., Washington
De Filippis Dea (2008) Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB1 receptors and fatty acid amide hydrolase. Neurogastroenterol Motil 20:919–927
Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S, Platt B, Riedel G (2012) Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), ∆9-tetrahydrocannabivaron (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacol 219:859-873
Devane W, Dysarz F, Johnson MR, Melvin LS, Howlett A (1988) Determination and characterisation of a cannabinoid receptor in the rat brain. Mol Pharmacol 34:605–613
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
EMA (2009) Procedural steps taken and scientific information after the authorisation Acomplia. In: Agency EM (ed) EMEA/H/C/000666/A20/0012. European Union, Brussels
Evans AT, Formukong E, Evans FJ (1987) Activation of phospholipase A2 by cannabinoids: lack of correlation with CNS effects. FEBS Lett 211:119–122
Farrimond J, Hill A, Whalley B, Williams C (2010a) Cannabis constituents modulate 9-tetrahydrocannabinol-induced hyperphagia in rats. Psychopharmacology 210:97–106
Farrimond JA, Whalley BJ, Williams CM (2010b) A low Δ9tetrahydrocannabinol cannabis extract induces hyperphagia in rats. Behav Pharmacol 21:769–773
Farrimond JA, Mercier MS, Whalley BJ, Williams CM (2011) Cannabis sativa and the endogenous cannabinoid system: therapeutic potential for appetite regulation. Phytother Res 25:18
Farrimond JA, Whalley BJ, Williams CM (2012) Non-∆9tetrahydrocannabinol phytocannabinoids are effective modulators of rat feeding patterns in vivo. Behav Pharmacol 23:113--117
Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, Lai Y, Ma AL, Mitchell RL (1995) Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol 48:443–450
Gaoni Y, Mechoulam R (1964) Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86:1646–1647
Gauson L, Stevenson L, Thomas A, Baillie G, Ross R, Pertwee R (2007) Cannabigerol behaves as a partial agonist at both CB1 and CB2 receptors. Symposium on the Cannabinoids. International Cannabinoid Research Society, Burlington, Vermont, USA, 206 pp
Glass M (2001) The role of cannabinoids in neurodegenerative diseases. Progress in Neuro-psychopharmacology and Biological Psychiatry 25:743–765
Hao S, Avraham Y, Mechoulam R, Berry EM (2000) Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet-restricted mice. Eur J Pharmacol 392:147–156
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583
Hill A, Williams C, Whalley B, Stephens G (2012) Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther 133:79–97
Ignatowska-Jankowska B, Jankowski M, Swiergiel A (2010) Cannabidiol decreases body weight gain in rats: involvement of CB2 receptors. Neurosci Lett 490:82–84
Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30:515–527
Jamshidi N, Taylor DA (2001) Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 134:1151–1154
Koch JE, Matthews SM (2001) 9-Tetrahydrocannabinol stimulates palatable food intake in Lewis rats: effects of peripheral and central administration. Nutr Neurosci 4:179–187
Ligresti A, Moriello A, Starowicz K, Matias I, Pisanti S, De Petrocellis L, Laezza C, Portella G, Bifulco M, Di Marzo V (2006) Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther 318:1375
Martin BR, Balster RL, Razdan RK, Harris LS, Dewey WL (1981) Behavioral comparisons of the stereoisomers of tetrahydrocannabinols. Life Science 29:565–574
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65
O'Shaughnessey WB (1843) On the Preparations of the Indian hemp, or gunjah (Cannabis indica): their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Provincial Medical Journal 5
Pertwee RG (2008) The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol 153:199–215
Petrosino S, Ligresti A, Di Marzo V (2009) Endocannabinoid chemical biology: a tool for the development of novel therapies. Curr Opin Chem Biol 13:309–320
Pryce G, Ahmed Z, Hankey D, Jackson S, Croxford J, Pocock J, Ledent C, Petzold A, Thompson A, Giovannoni G, Cuzner M, Baker D (2003) Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 126:2191–2202
Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, Breuer A, Mechoulam R (1997) Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J Med Chem 40:3228–3233
Riedel G, Fadda P, McKillop-Smith S, Pertwee R, Platt B, Robinson L (2009) Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol 156:1154–1166
Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C et al (1999) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350(2–3):240–244
Rubino T, Viganò D, Massi P, Parolaro D (2001) The psychoactive ingredient of marijuana induces behavioural sensitization. Eur J Neurosci 14(5):884–886
Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B (2009) Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci 29:2053–2063
Scopinho AA, Guimaraes FS, Correa F, Resstel L (2011) Cannabidiol inhibits the hyperphagia induced by cannabinoid-1 or serotonin-1A receptor agonists. Pharmacol Biochem Behav 98:268–272
Sofia RD, Knobloch LC (1976) Comparative effects of various naturally occurring cannabinoids on food, sucrose and water consumption by rats. Pharmacol Biochem Behav 4:591–599
Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K (1995) 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 215:89–97
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S (2005) Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe Study. Lancet 365:1389–1397
Wang T, Collet J, Shapiro S, Ware M (2008) Adverse effects of medical cannabinoids: a systematic review. Can Med Assoc J 178:1669
Wiley J, Burston J, Leggett D, Alekseeva O, Razdan R, Mahadevan A, Martin B (2005) CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol 145:293–300
Williams CM, Kirkham TC (1999) Anandamide induces overeating: mediation by central cannabinoid CB1 receptors. Psychopharmacology 143:315–317
Williams CM, Kirkham TC (2002a) Observational analysis of feeding induced by Delta9-THC and anandamide. Physiology and Behaviour 76:241–250
Williams CM, Kirkham TC (2002b) Reversal of D9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav 71:333–340
Williams CM, Rogers PJ, Kirkham TC (1998) Hyperphagia in pre-fed rats following oral D9-THC. Physiol Behav 65:343–346
Acknowledgments
This research was supported in part by the University of Reading Research Endowment Trust Fund (to JAF). The authors thank Ms. Pam Rummings and her team for technical assistance and GW Pharmaceuticals for the kind gift of purified phytocannabinoids.
Ethical compliance
All procedures were performed in compliance with the requirements of the United Kingdom Animals (Scientific Procedures) Act 1986 and all other applicable laws and standards in the UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farrimond, J.A., Whalley, B.J. & Williams, C.M. Cannabinol and cannabidiol exert opposing effects on rat feeding patterns. Psychopharmacology 223, 117–129 (2012). https://doi.org/10.1007/s00213-012-2697-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2697-x