Abstract
Rationale
The perception that smoking relieves negative affect contributes to smoking persistence. Endogenous opioid neurotransmission, and the μ-opioid receptor (MOR) in particular, plays a role in affective regulation and is modulated by nicotine.
Objectives
We examined the relationship of MOR binding availability in the amygdala to the motivation to smoke for negative affect relief and to the acute effects of smoking on affective responses.
Methods
Twenty-two smokers were scanned on two separate occasions after overnight abstinence using [11C]carfentanil positron emission tomography imaging: after smoking a nicotine-containing cigarette and after smoking a denicotinized cigarette. Self-reports of smoking motives were collected at baseline, and measures of positive and negative affect were collected pre- and post- cigarette smoking.
Results
Higher MOR availability in the amygdala was associated with motivation to smoke to relieve negative affect. However, MOR availability was unrelated to changes in affect after smoking either cigarette.
Conclusions
Increased MOR availability in amygdala may underlie the motivation to smoke for negative affective relief. These results are consistent with previous data highlighting the role of MOR neurotransmission in smoking behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco smoking is the leading preventable cause of death worldwide (WHO 2009). Despite this, 20% of adults in the United States continue to smoke (CDC 2009). The perception that smoking relieves negative mood or affect has long been considered a key motive for continued smoking (Kassel et al. 2007; Shiffman 1993). Indeed, negative mood symptoms following a quit attempt predict smoking relapse (Lerman et al. 2002; Strasser et al. 2005b). However, self-report measures of mood and smoking motives have their limitations, and are not always in agreement with real-time monitoring in smokers (Shiffman et al. 1997; Shiffman and Prange 1988). Assessment of the neurobiological mechanisms that underlie smoking motives related to negative affect regulation could provide insights for treating nicotine dependence (Lerman and Audrain-McGovern 2010).
The endogenous opioid system, and the μ-opioid receptor (MOR) in particular, plays a role in both affective regulation and nicotine dependence (Mague and Blendy 2010; Ribeiro et al. 2005; Walters et al. 2005). There is a high density of MORs in the amygdala and throughout the limbic system (Pfeiffer et al. 1982), and MOR neurotransmission may mediate affective regulation (Ribeiro et al. 2005). Human positron emission tomography (PET) imaging studies have demonstrated positive associations of MOR availability (decreased endogenous opioid neurotransmission) in the amygdala and limbic system with increases in negative affect ratings following negative mood induction (Zubieta et al. 2003), whereas decreases in negative affective ratings after a pain challenge were associated with lower MOR binding availability (Zubieta et al. 2001).
Of relevance to nicotine dependence, preclinical studies have shown that acute nicotine administration modulates release of endogenous opioids (Boyadjieva and Sarkar 1997; Houdi et al. 1991; Marty et al. 1985) and chronic nicotine exposure upregulates MORs (Wewers et al. 1999). In mice, MOR activation is required for nicotine reward (Berrendero et al. 2002; Walters et al. 2005) as well as the anxiolytic effects of nicotine (Balerio et al. 2005). Furthermore, smokers exhibit increased MOR availability in amygdala relative to non-smoking controls (Ray et al. 2011), and a variant in the gene encoding the MOR in humans (OPRM1 A118G) is associated with the relative reinforcing value of nicotine (Perkins et al. 2008; Ray et al. 2006) as well as with smoking cessation outcomes (Lerman et al. 2004; Munafo et al. 2007; Ray et al. 2007). The MOR antagonist naltrexone mitigates increases in craving and negative affect in abstinent smokers viewing smoking cues (Hutchison et al. 1999), and there is evidence that naltrexone may be an effective smoking cessation treatment among certain subpopulations of smokers (Epperson et al. 2010; O'Malley et al. 2006; Walsh et al. 2008). These data highlight the dual role of the opioid system in affect regulation and nicotine dependence, and suggest a potential neurobiological mechanism that may underlie the motivation to smoke for negative affect relief.
In this secondary analysis of data from a previous [11C]carfentanil PET imaging study (Ray et al. 2011), we examined the relationship of MOR availability to self-reported smoking for negative affect relief. Twenty-two smokers were scanned on two separate occasions (after smoking a nicotine containing cigarette and after smoking a matching denicotinized cigarette). We focused on the amygdala as our primary region of interest and included other limbic system structures (i.e., the thalamus, anterior cingulate cortex, caudate nucleus, insula, and ventral striatum) in a secondary exploratory analysis. Based on prior data described above, we hypothesized that increased MOR binding availability would be associated with increases in self-reported motivation to smoke for negative affect relief. In a secondary analysis, we tested the hypothesis that acute negative affect relief from smoking would be associated with decreased MOR availability (increased endogenous opioid neurotransmission).
Methods and materials
Participants
Participant selection and study design were described previously (Ray et al. 2011). Briefly, smokers were required to report consumption of ≥10 non-menthol cigarettes per day for at least the past 6 months and provide a baseline breath carbon monoxide (CO) reading >10 ppm. The study was approved by the University of Pennsylvania Institutional Review Board, the Food and Drug Administration, and the Environmental Health and Radiation Safety Committee; all participants provided written informed consent. Individuals with a history of or current neurological or DSM-IV Axis I psychiatric or substance disorders (except nicotine dependence), and those taking psychotropic medications were excluded. Of 26 eligible smokers, one smoker was deemed ineligible at PET scan 1 due to noncompliance with the overnight abstinence requirement, one withdrew before the first scan, and two were excluded from the final dataset because a different scanner was used for their sessions, leaving 22 participants with complete data.
Procedures
Following eligibility screening, participants completed measures of smoking history and demographics. The modified version of the Horn–Waingrow Reasons for Smoking (RFS) Scale (Horn and Waingrow 1966) was used to assess smoking motives. Based on our prior work on smoking motives (Lerman et al. 1996; Lerman et al. 1998), we analyzed the three items comprising the RFS subscale “smoking for negative affect reduction” (e.g., “When I feel uncomfortable or upset about something, I light up a cigarette”). This subscale has been shown to correlate with self-monitored smoking behavior (Shiffman and Prange 1988; Tate and Stanton 1990) and has a stable factor structure and satisfactory test–retest reliability (Costa et al. 1980). RFS items were rated on a Likert scale (0 = “not at all characteristic of me” to 3 = “very much characteristic of me”); answers to the three items were summed to provide a Negative Affect Reduction summary score (NAR score). Participants also completed the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al. 1991) and the Diener and Emmons Mood Form (Diener and Emmons 1984). This form consists of nine items assessing how strongly subjects are experiencing positive and negative moods; items are rated on a scale of 0 (not at all) to 6 (very much). OPRM1 A118G (rs1799971) genotype was determined using Taqman SNP genotyping assays (Applied Biosystems) as previously described (Ray et al. 2011).
On each of two separate visits, 60-min [11C]carfentanil PET scans were performed after 14 h of overnight abstinence from smoking (verified by expired CO <10 ppm), as previously described (Ray et al. 2011). Participants completed a urine drug screen at each session to verify abstinence from psychotropic medications. To standardize nicotine exposure, and to avoid nicotine withdrawal, all participants smoked a Quest® research cigarette (Vector Tobacco) 15 min prior to the PET scan, using a standardized puffing procedure (Strasser et al. 2005a). As reported previously (Ray et al. 2011), the scan order was fixed: the nicotine cigarette (0.6 mg nicotine) was smoked before scan 1 and the placebo cigarette (denicotinized; 0.05 mg nicotine) was smoked before scan 2. Participants and data analysts were blind to the cigarette type. Participants also completed the Diener and Emmons Mood Form before and after smoking the cigarette. Female participants were scanned during their early follicular phase (2–9 days after menses) to minimize the effects of rising levels of estrogen on the endogenous opioid system (Mills et al. 2004). The time interval between the two scans was 1–4 weeks (as female participants were scanned during their early follicular phase).
Image acquisition, reconstruction, and preprocessing
Scans were acquired on a GSO-based Phillips GPET Plus brain PET scanner, a custom-built model which operates without septa to increase the sensitivity of the instrument (Karp et al. 2003). Six sequential 10-min-long scans were acquired starting immediately after slow bolus injection (over 10 min) of 3–15 mCi [11C]carfentanil, so as not to exceed 0.03 μg/kg. The dose of [11C]carfentanil administered was based on published PET studies (Greenwald et al. 2007; Smith et al. 2006; Zubieta et al. 2005). The resulting six images consisted of 128 slices of 2-mm slice thickness (2 × 2 mm2 pixels) and an image matrix size of 128 × 128 pixels. The raw PET data were converted to Neuroimaging Informatics Technology Initiative format and preprocessed using SPM8 (Wellcome Trust Center for Neuroimaging, London, United Kingdom) Briefly, the data were first oriented to the AC-PC line and normalized to the MNI T1-weighted template using a 12-parameter affine registration algorithm in SPM8. Nonbrain areas were removed using a brain mask (Ray et al. 2011)
Regions of interest
Left and right hemisphere masks were defined as shown in Fig. 1 for the amygdala and the exploratory regions of interest (ROIs) (thalamus, anterior cingulate cortex, caudate nucleus, insula and ventral striatum) using the Harvard–Oxford probabilistic map distributed with FSL software (Smith et al. 2004). All ROIs except the ventral striatum were defined using a maximal probability threshold of 25%. The ventral striatum/nucleus accumbens ROI was defined using the accumbens label at 0% threshold. Any overlap with the caudate nucleus ROI was removed from the ventral striatum mask. Similarly, ventral striatum was subtracted from the caudate nucleus to create non-overlapping regions of interest.
Quantification of MOR binding (BPND)
MOR availability was calculated with the multilinear reference tissue method MRTM2 (Ichise et al. 2003) with a fixed value for the tissue to plasma efflux rate constant (k 2 ′) in the reference region (occipital cortex). The distribution volume ratio (DVR) is calculated from the ratio of the regression coefficients (Ichise et al. 2003) and the binding potential is given by BPND = DVR − 1. A k 2′ value of 0.1237 min−1 was assigned on the basis of a previous [11C]carfentanil study (Hirvonen et al. 2009). The MRTM2 BPND values for each ROI were exported for further statistical analysis using Stata software.
Statistical methods
Descriptive statistics were obtained for all variables. Multiple linear mixed-models (random effects regression) of MOR BPND were estimated incorporating the NAR score as the predictor and including the following covariates: session, OPRM1 genotype (A/A = 1, */G =0), sex, FTND score, and hemisphere (L = 1, R = 2). These covariates were chosen to control for potential confounding factors in this analysis: OPRM1 genotype was previously reported to be significantly associated with MOR BP in this sample (Ray et al. 2011), and other studies have demonstrated associations between MORs and sex (Zubieta et al. 1999) as well as nicotine dependence (Zhang et al. 2006). Next, multiple linear mixed-models (random effects regression) of MOR BPND were estimated using positive or negative affect difference scores (post- minus pre-cigarette) as the predictor, controlling for the same covariates. The alpha value was set at 0.05 for the bilateral amygdala based on our a priori hypothesis; other ROIs were deemed exploratory and therefore no correction for multiple hypothesis testing was applied.
Results
Descriptive data
Of the 22 smokers, six (27%) were female, and all were of European ancestry. Participants reported a mean age of 31.3 years (SD = 10.7), mean smoking rate of 17.6 cigarettes per day (SD = 6.3), and mean FTND score of 4.68 (SD = 2.01). The genotype distribution for OPRM1 A118G (rs1799971) was 12 A/A, nine A/G, and one G/G; for purposes of analysis, A/G and G/G groups were combined and denoted as */G. The mean NAR score was 4.23 (SD = 2.18); Chronbach’s alpha estimate for this three-item scale was 0.78.
MOR availability in amygdala and motivation to smoke for negative affect relief
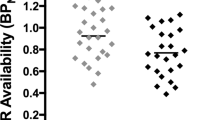
Stronger baseline motivations to smoke for negative affect relief were associated with higher levels of MOR availability in amygdala (β = 0.06, 95% confidence interval = 0.02–0.10, p = 0.007; Fig. 2). As previously reported (Ray et al. 2011), there were no effects of session on MOR BP in the amygdala (p = 0.73). The marginal mean MOR BPND values in the amygdala (adjusted for OPRM1 genotype, sex, and FTND score) were 1.18 in the nicotine session and 1.17 in the denicotinized session.
Association of NAR score with MOR BPND in the amygdala. Binding potentials for amygdala (adjusted for session, OPRM1 genotype, sex, FTND, and hemisphere) are plotted for each participant for both sessions. In the multiple regression models, NAR scores were significantly associated with MOR BPND in the amygdala (β = 0.06, p = 0.007). MOR BP ND μ-opioid receptor binding potential, NAR Negative Affect Reduction score
In the exploratory analysis, motivation to smoke for negative affect relief was associated with MOR BPND in the anterior cingulate cortex (p = 0.03), but not in the thalamus (p = 0.05), caudate nucleus (p = 0.27), insula (p = 0.59), or ventral striatum (p = 0.17).
MOR availability in amygdala and smoking-induced mood change
Smoking the research cigarettes did not produce significant changes in negative or positive mood (as measured using the Diener and Emmons Mood Form) in either session, and across sessions there was no association of positive or negative mood change with MOR availability. Decreased positive mood (post-cigarette minus pre-cigarette) after smoking a nicotine cigarette was associated with increased MOR BPND in the amygdala, but this effect was not significant (β = −0.14, 95% confidence interval = −0.29–0.01, p = 0.08); there was no significant association in the denicotinized cigarette session (p = 0.14). Change in negative affect in either session was not significantly associated with MOR BPND in the amygdala (p > 0.10).
Discussion
We report a strong association between MOR availability in the amygdala and motivation to smoke to relieve negative affect. However, contrary to our expectation, there were no significant changes in affect related to smoking the cigarettes prior to the scans, nor was the degree of change in negative affect after smoking related to MOR availability. Thus, we find that a general, pre-existing motivation for smoking relates to MOR binding availability, but that the experience of smoking, at least in this setting, does not.
Our finding of a positive correlation between MOR availability in the amygdala and motivation to smoke for negative affect relief is consistent with prior literature documenting the role of the MOR in both affect regulation and motivational behavior. The association of MOR availability in the amygdala with affective motives to smoke is consistent with functional neuroimaging evidence for a role of the amygdala in processing and regulating affective responses (Davidson and Irwin 1999; Diekhof et al. 2011; Kim et al. 2011). The amygdala is highly connected to cortical structures throughout the brain and is important in the integration and regulation of emotional responses (Pessoa 2010). Opioid neurotransmission in the amygdala has been implicated in assigning motivational salience to reward cues in rats (Mahler and Berridge 2009), and in humans changes in MOR availability are associated with emotional responses to stimuli (Ribeiro et al. 2005; Zubieta et al. 2003). Other than our previous report on this sample (Ray et al. 2011), only one other study has used PET imaging to examine acute effects of smoking on MOR availability (Scott et al. 2007). Scott and colleagues found a significant increase in MOR availability from the denicotinized to the nicotine condition, and the increase in MOR availability in the amygdala was related to decreased craving scores, but not to mood measures. Although we did administer a two-item craving questionnaire to participants before and after smoking the cigarettes, changes in craving were not related to MOR availability in our sample (data not shown). However, the differing results between these two studies may likely be attributed to differences in study design, including study population (six male smokers in the prior study versus 22 smokers of both genders in the current study), number of cigarettes smoked in each condition (two cigarettes 10 min apart versus a single pre-scan cigarette), and nicotine yield of the nicotine cigarette (1.01 mg versus 0.6 mg).
There are several potential interpretations of finding an association of MOR availability in the amygdala with smoking motivation but not with actual mood change after smoking a cigarette, suggesting future avenues of investigation. It is possible that those individuals with greater MOR availability may have experienced greater negative affect relief early in their smoking experience, and that the belief that cigarette smoking reduced negative affect persisted despite the development of tolerance to pharmacological effects of nicotine. In support of this, adolescents with higher expectancies for negative affect relief from smoking do report greater reductions of negative affect after smoking (Colvin and Mermelstein 2010), whereas expectancies for negative affect relief are not related to actual changes in negative affect in adult smokers (Perkins et al. 2011). Another potential explanation may relate to our use of research cigarettes yielding 0.6 mg of nicotine for the “nicotine cigarette” session, which was lower than the mean yield of our participants’ preferred brands (mean = 1.1 mg, SD = 0.5 mg). Prior studies have shown that low-nicotine cigarettes are rated as lower quality and less satisfying than participants’ preferred brands (Benowitz et al. 2006; Strasser et al. 2007). It is possible that the research cigarettes were less rewarding than the participants’ usual brand, and thus did not affect mood in the same fashion. In addition, even the denicotinized cigarette contains minute amounts of nicotine; Brody et al. (2009) found that smoking a denicotinized cigarette results in reduced, but still significant, activation of α4β2 nicotinic receptors, which may explain why the smoking manipulation in the current study had no effect. Alternatively, there is evidence to suggest that effects of smoking on mood may derive more from the sensory effects or ritual of smoking than from nicotine (Juliano et al. 2011; Perkins et al. 2008; Rose 2006). Our paced puffing procedure, although necessary to standardize nicotine exposure, did not allow participants to smoke at their own pace or to smoke to satiety, and thus may have affected mood differently than self-regulated smoking. Future investigations into smoking-related mood effects may benefit from assessments of mood change in a naturalistic environment using the participants’ preferred cigarettes.
Another possibility to consider is that it is μ-opioid system regulation of reward, rather than mood change, which drives the motivation to smoke to relieve negative affect. There is a growing body of literature suggesting that although smokers expect improvements in mood after smoking a cigarette, smoking does not reliably relieve negative affect due to anything other than nicotine withdrawal (Conklin and Perkins 2005; Perkins et al. 2010). Indeed, the absence of significant mood change in our study is consistent with this literature. However, a common polymorphism in the gene encoding the MOR (OPRM1 A118G) is associated with the relative reinforcing value of nicotine (Perkins et al. 2008; Ray et al. 2006). Interestingly, the A allele, which we previously demonstrated was associated with greater MOR availability in this sample (Ray et al. 2011), has also been associated with increased cigarette reward during negative versus positive mood (Perkins et al. 2008) as well as with increased reinforcement learning compared to G allele carriers (Lee et al. 2011). It is possible that even though smoking is not effective in actually relieving negative mood, smokers with greater MOR availability find cigarettes more rewarding under these circumstances and are more likely to develop a conditioned association between smoking and negative mood.
There are a few limitations which should be considered within our study. The NAR scale is a self-report measure and relies on accurate self-assessment of motivation rather than objective observation; however, this measure has been shown to correlate significantly with self-monitored smoking data (Shiffman and Prange 1988; Tate and Stanton 1990). Mean NAR scores in this study were lower than scores for non-depressed smokers in previous studies (Lerman et al. 1996; Lerman et al. 1998). This may be related to oversampling for OPRM1 */G genotype, which has been associated with reduced subjective nicotine reward (Ray et al. 2011) and reduced reinforcement learning (Lee et al. 2011). Also due to oversampling of OPRM1 */G, the distribution of OPRM1 genotypes in our sample is not representative of the normal smoking population. Smokers of menthol cigarettes were excluded from the study; therefore caution should be used when extrapolating to general smoking populations. We did not use a smoking topography device to measure puff volume or assess plasma nicotine levels at either session. Our use of a standardized puffing procedure mitigates differences in puff volume between smokers (Strasser et al. 2005a), and significant differences in plasma nicotine levels are obtained when smoking Quest® denicotinized cigarettes compared to the 0.6 mg cigarettes (Brody et al. 2009). Other studies have shown variation in MOR availability and in μ-opioid system response to stimuli between males and females (Zubieta et al. 1999; Zubieta et al. 2002). The number of female smokers included in the current study was too small to test for sex heterogeneity; however, sex was included as a covariate in all models and did not predict MOR availability. Finally, as with any PET imaging study, the reported binding potentials do not distinguish between number of available receptors, receptor affinity or endogenous opioid tone.
In conclusion, we have demonstrated a significant association of MOR availability in the amygdala with motivation to smoke to relieve negative affect; furthermore, this motivation was inconsistent with the actual experience of smoking. These data offer a potential biological mechanism underlying this particular smoking motive, and add to a growing body of literature highlighting inconsistencies between smokers’ expectations and real-time effects of smoking. Further research into the biological mechanisms influencing affective response to smoking and motivation to smoke may be useful in effecting improvements in smoking cessation treatment.
References
Balerio GN, Aso E, Maldonado R (2005) Involvement of the opioid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology (Berl) 181:260–269
Benowitz NL, Jacob P 3rd, Herrera B (2006) Nicotine intake and dose response when smoking reduced-nicotine content cigarettes. Clin Pharmacol Ther 80:703–714
Berrendero F, Kieffer BL, Maldonado R (2002) Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci 22:10935–10940
Boyadjieva NI, Sarkar DK (1997) The secretory response of hypothalamic beta-endorphin neurons to acute and chronic nicotine treatments and following nicotine withdrawal. Life Sci 61:PL59–PL66
Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG (2009) Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol 12:305–316
CDC (2009) Center for Disease Control: Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep 58:1227–1232
Colvin PJ, Mermelstein RJ (2010) Adolescents' smoking outcome expectancies and acute emotional responses following smoking. Nicotine Tob Res 12:1203–1210
Conklin CA, Perkins KA (2005) Subjective and reinforcing effects of smoking during negative mood induction. J Abnorm Psychol 114:153–164
Costa PT Jr, McCrae RR, Bosse R (1980) Smoking motive factors: a review and replication. Int J Addict 15:537–549
Davidson RJ, Irwin W (1999) The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 3:11–21
Diekhof EK, Geier K, Falkai P, Gruber O (2011) Fear is only as deep as the mind allows a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. NeuroImage 58:275–285
Diener E, Emmons RA (1984) The independence of positive and negative affect. J Pers Soc Psychol 47:1105–1117
Epperson CN, Toll B, Wu R, Amin Z, Czarkowski KA, Jatlow P, Mazure CM, O'Malley SS (2010) Exploring the impact of gender and reproductive status on outcomes in a randomized clinical trial of naltrexone augmentation of nicotine patch. Drug Alcohol Depend 112:1–8
Greenwald M, Johanson CE, Bueller J, Chang Y, Moody DE, Kilbourn M, Koeppe R, Zubieta JK (2007) Buprenorphine duration of action: mu-opioid receptor availability and pharmacokinetic and behavioral indices. Biol Psychiatry 61:101–110
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127
Hirvonen J, Aalto S, Hagelberg N, Maksimow A, Ingman K, Oikonen V, Virkkala J, Nagren K, Scheinin H (2009) Measurement of central mu-opioid receptor binding in vivo with PET and [11C]carfentanil: a test–retest study in healthy subjects. Eur J Nucl Med Mol Imaging 36:275–286
Horn D, Waingrow S (1966) Some dimensions of a model for smoking behavior change. Am J Public Health Nations Health 56(Suppl 56):21–26
Houdi AA, Pierzchala K, Marson L, Palkovits M, Van Loon GR (1991) Nicotine-induced alteration in Tyr–Gly–Gly and Met-enkephalin in discrete brain nuclei reflects altered enkephalin neuron activity. Peptides 12:161–166
Hutchison KE, Monti PM, Rohsenow DJ, Swift RM, Colby SM, Gnys M, Niaura RS, Sirota AD (1999) Effects of naltrexone with nicotine replacement on smoking cue reactivity: preliminary results. Psychopharmacology (Berl) 142:139–143
Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE (2003) Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112
Juliano LM, Fucito LM, Harrell PT (2011) The influence of nicotine dose and nicotine dose expectancy on the cognitive and subjective effects of cigarette smoking. Exp Clin Psychopharmacol 19:105–115
Karp JS, Surti S, Daube-Witherspoon ME, Freifelder R, Cardi CA, Adam LE, Bilger K, Muehllehner G (2003) Performance of a brain PET camera based on anger-logic gadolinium oxyorthosilicate detectors. J Nucl Med 44:1340–1349
Kassel JD, Evatt DP, Greenstein JE, Wardle MC, Yates MC, Veilleux JC (2007) The acute effects of nicotine on positive and negative affect in adolescent smokers. J Abnorm Psychol 116:543–553
Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ (2011) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410
Lee MR, Gallen CL, Zhang X, Hodgkinson CA, Goldman D, Stein EA, Barr CS (2011) Functional Polymorphism of the Mu-Opioid Receptor Gene (OPRM1) Influences Reinforcement Learning in Humans. PLoS One 6:e24203
Lerman C, Audrain-McGovern J (2010) Reinforcing effects of smoking: more than a feeling. Biol Psychiatry 67:699–701
Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, Caporaso N (1996) Investigation of mechanisms linking depressed mood to nicotine dependence. Addict Behav 21:9–19
Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, Shields PG (1998) Depression and self-medication with nicotine: the modifying influence of the dopamine D4 receptor gene. Health Psychol 17:56–62
Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, Niaura R, Epstein L (2002) Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend 67:219–223
Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain-McGovern J, Restine S, Shields PG, Kaufmann V, Redden D, Benowitz N, Berrettini WH (2004) The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short-term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics J 4:184–192
Mague SD, Blendy JA (2010) OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend 108:172–182
Mahler SV, Berridge KC (2009) Which cue to "want?" Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29:6500–6513
Marty MA, Erwin VG, Cornell K, Zgombick JM (1985) Effects of nicotine on beta-endorphin, alpha MSH, and ACTH secretion by isolated perfused mouse brains and pituitary glands, in vitro. Pharmacol Biochem Behav 22:317–325
Mills RH, Sohn RK, Micevych PE (2004) Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci 24:947–955
Munafo MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC (2007) Association of the mu-opioid receptor gene with smoking cessation. Pharmacogenomics J 7:353–361
O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P (2006) A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med 166:667–674
Perkins KA, Giedgowd GE, Karelitz JL, Conklin CA, Parzynski CS (2011) Expectancy for negative affect relief due to smoking may not be predictive under acute mood situations. Exp Clin Psychopharmacol
Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA, Sayette MA (2010) Differences in negative mood-induced smoking reinforcement due to distress tolerance, anxiety sensitivity, and depression history. Psychopharmacology (Berl) 210:25–34
Perkins KA, Lerman C, Grottenthaler A, Ciccocioppo MM, Milanak M, Conklin CA, Bergen AW, Benowitz NL (2008) Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement owing to negative mood. Behav Pharmacol 19:641–649
Pessoa L (2010) Emergent processes in cognitive-emotional interactions. Dialogues Clin Neurosci 12:433–448
Pfeiffer A, Pasi A, Mehraein P, Herz A (1982) Opiate receptor binding sites in human brain. Brain Res 248:87–96
Ray R, Jepson C, Patterson F, Strasser A, Rukstalis M, Perkins K, Lynch KG, O'Malley S, Berrettini WH, Lerman C (2006) Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology (Berl) 188:355–363
Ray R, Jepson C, Wileyto EP, Dahl JP, Patterson F, Rukstalis M, Pinto A, Berrettini W, Lerman C (2007) Genetic variation in mu-opioid-receptor-interacting proteins and smoking cessation in a nicotine replacement therapy trial. Nicotine Tob Res 9:1237–1241
Ray R, Ruparel K, Newberg A, Wileyto EP, Loughead JW, Divgi C, Blendy JA, Logan J, Zubieta JK, Lerman C (2011) Human Mu Opioid Receptor (OPRM1 A118G) polymorphism is associated with brain mu-opioid receptor binding potential in smokers. Proc Natl Acad Sci U S A 108:9268–9273
Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK (2005) Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry 29:1264–1280
Rose JE (2006) Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 184:274–285
Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S, Zubieta JK (2007) Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology 32:450–457
Shiffman S (1993) Assessing smoking patterns and motives. J Consult Clin Psychol 61:732–742
Shiffman S, Hufford M, Hickcox M, Paty JA, Gnys M, Kassel JD (1997) Remember that? A comparison of real-time versus retrospective recall of smoking lapses. J Consult Clin Psychol 65:292–300
Shiffman S, Prange M (1988) Self-reported and self-monitored smoking patterns. Addict Behav 13:201–204
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219
Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK (2006) Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci 26:5777–5785
Strasser AA, Ashare RL, Kozlowski LT, Pickworth WB (2005a) The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav 82:320–329
Strasser AA, Kaufmann V, Jepson C, Perkins KA, Pickworth WB, Wileyto EP, Rukstalis M, Audrain-McGovern J, Lerman C (2005b) Effects of different nicotine replacement therapies on postcessation psychological responses. Addict Behav 30:9–17
Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA (2007) New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend 86:294–300
Tate JC, Stanton AL (1990) Assessment of the validity of the Reasons for Smoking scale. Addict Behav 15:129–135
Walsh Z, Epstein A, Munisamy G, King A (2008) The impact of depressive symptoms on the efficacy of naltrexone in smoking cessation. J Addict Dis 27:65–72
Walters CL, Cleck JN, Kuo YC, Blendy JA (2005) Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 46:933–943
Wewers ME, Dhatt RK, Snively TA, Tejwani GA (1999) The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res 822:107–113
WHO (2009) World Health Organization. WHO Report on the global tobacco epidemic.
Zhang L, Kendler KS, Chen X (2006) The mu-opioid receptor gene and smoking initiation and nicotine dependence. Behav Brain Funct 2:28
Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS (2005) Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 25:7754–7762
Zubieta JK, Dannals RF, Frost JJ (1999) Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry 156:842–848
Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA (2003) Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry 60:1145–1153
Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2001) Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293:311–315
Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2002) mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci 22:5100–5107
Acknowledgements
We thank the following individuals for their contributions to the study: Dr. Richard Freifelder, Dr. Joel Karp, Dr. Alexander Schmitz, and Rahul Poria for [11C]carfentanil synthesis; Dr. Daniel Pryma and Dr. Rodolfo Perini for serving as PET center injectors; and Dr. Janet Reddin and PET center technologists for PET acquisition and preprocessing at the PET center. This research was supported by National Institute on Drug Abuse Grants R21-DA027066 (to C.L. and J.A.B.) and U01-DA020830 (to C.L.), National Cancer Institute Grant P50-CA143187 (to C.L. and J.A.B.), and a grant from the Pennsylvania Department of Health. C.L. has served as a consultant for and/or received research support from Pfizer, AstraZeneca, Novartis, and GlaxoSmithKline. R.R. has received research support from Pfizer. This research was not supported by industry funds. The authors declare that they have full control of all primary data and they agree to allow the journal to review their data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falcone, M., Gold, A.B., Wileyto, E.P. et al. μ-Opioid receptor availability in the amygdala is associated with smoking for negative affect relief. Psychopharmacology 222, 701–708 (2012). https://doi.org/10.1007/s00213-012-2673-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2673-5