Abstract
Rationale
Although high anxiety is commonly associated with drug addiction, its causal role in this disorder is unclear.
Objectives
In light of strong evidence for dissociable neural mechanisms underlying heroin and cocaine addiction, the present study investigated whether high anxiety predicts the propensity of rats to lose control over intravenous cocaine or heroin self-administration.
Methods
Sixty-four rats were assessed for anxiety in the elevated plus-maze, prior to extended access to intravenous cocaine or heroin self-administration.
Results
High-anxious rats, identified in the lower quartile of the population, showed a greater escalation of cocaine, but not heroin, self-administration compared with low-anxious rats selected in the upper quartile of the population. Anxiety scores were also positively correlated with the extent of escalation of cocaine self-administration.
Conclusions
The present data suggest that high anxiety predisposes rats to lose control over cocaine—but not heroin—intake. High anxiety may therefore be a vulnerability trait for the escalation of stimulant but not opiate self-administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is substantial evidence to suggest a major role for anxiety in substance abuse disorders. In humans, high anxiety is associated with drug addiction (e.g., Lejuez et al. 2008), possibly as a consequence of chronic drug exposure (Sherman et al. 1989; Sinha 2001; Lejuez et al. 2008), by contributing to drug craving or greater rates of relapse. Epidemiological and clinical studies suggest that anxiety may confer a vulnerability to drug addiction (for example, Norton 2001; Lejuez et al. 2008), since individuals with high anxiety have been suggested to use drugs as a coping, or self-medication, strategy in order to reduce central arousal and to regulate affective distress (see Khantzian 1985 for detailed discussion), thereby promoting the transition from recreational drug use to loss of control, a hallmark of addiction (DSM-IV-R, APA 2000).
In preclinical studies, high anxiety as measured in the elevated plus maze (EPM) (Pellow et al. 1985) predicts increased propensity to develop conditioned place preference for cocaine (Pelloux et al. 2009), as well as higher oral intake levels (Walker et al. 2009), and increased motivation to self-administer cocaine (Homberg et al. 2002). Additionally, exposure to, or withdrawal from, cocaine and heroin increases anxiety (Rogerio and Takahashi 1992; Schulteis et al. 1998; Zhou et al. 2003; Le Merrer et al. 2006; Ambrose-Lanci et al. 2010; Salas-Ramirez et al. 2010).
However, the role of anxiety in promoting the loss of control over drug intake has not been explicitly evaluated. We therefore investigated whether high anxiety in rats can predict individual differences in the propensity to lose control over drug self-administration (SA), a key feature of drug addiction according to the DSM-IV (APA 2000). The study also addressed recent evidence from clinical (Lejuez et al. 2008) and preclinical studies (Cruz et al. 2011) that any relationship between high anxiety and loss of control over drug intake may depend upon the self-administered drug itself, namely stimulants or opiates. Thus, Cruz et al. (2011) recently demonstrated that rats that had been subjected to social defeat stress were more likely to escalate cocaine, but not heroin, SA, thereby suggesting that the aetiologies of cocaine and opiate addiction could be distinguishable (Badiani et al. 2011). We therefore compared the relationship between high anxiety trait and the subsequent vulnerability to lose control over cocaine or heroin self-administration.
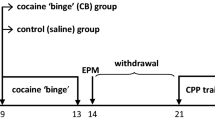
High- (HA) and low-anxious (LA) rats were identified using the EPM (Pellow et al. 1985) and given the opportunity to self-administer either cocaine or heroin intravenously. After an acquisition period, rats were subjected to long-access exposure to the drug to facilitate the escalation of drug intake (Ahmed and Koob 1998; Ahmed et al. 2000). Individual differences in the propensity to escalate drug SA in this paradigm is considered to represent loss of control over drug intake, one of the major criteria for drug addiction in humans (APA 2000).
Materials and methods
Subjects
Sixty-four adult out-bred male Lister-Hooded rats (Charles River Laboratories, Kent, UK), weighing approximately 280 g at the beginning of the experiment, were housed in pairs under a reversed 12 h light/dark cycle (lights on at 7:00 pm.). One week before EPM assessment, rats were placed on a restricted diet of 20 g per day lab chow (Purina), sufficient to maintain growth throughout the experiment. Water was available ad libitum, and food was given at the same time every afternoon. Following intravenous surgery, animals were housed individually and were habituated to these new conditions for 7 days before the beginning of any new testing. Experiments were performed between 7:00 am and 9:00 pm, 6–7 days a week and were conducted in accordance with the United Kingdom 1986 Animals (Scientific Procedures) Act (Project License PPL 80/1767).
Elevated plus-maze (EPM) and selection of high (HA) and low (LA) anxious rats
Rats were assessed for anxiety on the EPM (Pellow et al. 1985). The apparatus consisted of two 50 × 10 cm open arms (OA) and two 50 × 10 cm closed arms (CA) surrounded by a 40-cm wall. The four arms were equidistant from each other and converged on a 10 × 10 cm central platform. The LUX settings were as follows—70–80 for the open arms; 10–14 for the closed arms; and 40–50 for the central platform, providing an OA/CA ratio of 6–7. Behavioral measures on the EPM took place from 10 am to 2 pm, that is, from 3 h after the beginning of the dark phase. All animals were placed in an identical manner on the maze, facing the same open arm. Trials took place for 5 min only and were recorded and manually scored by the same investigator.
During the 5-min test, time spent in the OA and CA of the maze was recorded. An animal was considered to be in one particular arm of the maze if all four limbs were placed in that arm. The cocaine and heroin experiments were carried out separately, with 32 animals assessed on the EPM prior to drug exposure. For each experiment, rats were ranked according to the percentage of time spent on the OA [time OA/(time OA + time CA)] of the maze. The lower and upper quartiles of the population were selected as high- and low-anxious rats, respectively (HA and LA; n = 8 per group). An intermediate group (‘middle anxious’ or MA; n = 8) was also chosen to enable dimensional analyses and thereby a more comprehensive consideration of the relationship between trait-like anxiety and the propensity to escalate cocaine or heroin SA. Consequently, 24 animals were selected to undergo intravenous catheterization and drug SA. Three animals were removed from each experiment due to catheter patency failure or adverse reactions to drug SA. The final group sizes for each experiment were: HA, n = 7; MA, n = 6; and LA, n = 8.
Drugs
Heroin and cocaine were purchased from McFarlan-Smith (UK) and dissolved in 0.9% sterile saline. Infusion doses were based on the hydrochloride salt form of each drug.
Implantation of intravenous catheters
Rats were anesthetized with a xylazine/ketamine mixture (Ketalar, 90 mg/kg, i.p., Bury St. Edmunds, UK; Rompun, 6.7 mg/kg, i.p., Bury St. Edmunds, UK) and implanted with chronic intravenous jugular catheters (CamCaths®, St John’s Innovation Centre, Cowley Road, Cambridge, UK) according to a standardized procedure described previously (Belin and Everitt 2008).
Self-administration
Rats were tested in 12 operant chambers (29.5 × 32.5 × 23.5 cm; Med Associates, St. Albans, VT) equipped with two 4-cm-wide retractable levers, as previously described (Belin and Everitt 2008). Briefly, the two levers were 12 cm apart and 8 cm from the grid floor. Above each lever was a cue light (2.5 W, 24 V), and a white house light (2.5 W, 24 V) was located on the top of the opposite wall. The floor of the chamber was covered with a metal grid with bars 1 cm apart and was located 8 cm above an empty tray. The testing chamber was placed within a sound- and light-attenuating box, equipped with a ventilation fan that also screened external noise. SILASTIC tubing shielded with a metal spring extended from each animal’s intravenous catheter to a liquid swivel (Stoelting, Wood Dale, IL) mounted on an arm fixed outside the operant chamber. Tygon tubing extended from the swivel to a Razel infusion pump (Semat Technical, Herts, UK) located adjacent to the external chamber. As before, operant chambers were controlled using the Whisker control system. There was no lever-pressing training prior to drug SA, nor were priming infusions given in the present study.
The heroin and cocaine SA procedures comprised two phases: an initial acquisition phase that lasted 5 days, during which animals were allowed access to cocaine (250 μg/100 μl/infusion) or heroin (40 μg/100 μl/infusion) for only 1 h a day (short-access sessions or ShA) on a fixed-ratio 1 schedule. The active lever was designated as the right or left lever in a counterbalanced manner, in order to control for any side-bias. Pressing the active lever resulted in drug infusion and a 20-s retraction of both active and inactive levers along with presentation of a light cue above the active lever (this time-out had to be increased for several rats as the escalation phase progressed to prevent overdose). Pressing the inactive lever had no effect. This was immediately followed by the escalation phase. Sessions were increased to 6 h a day (long-access sessions or LgA) for 12 days. Throughout the procedure, SA sessions were carried out 6–7 days a week. As 12 operant chambers were used for each experiment, 12 subjects underwent testing in the morning (approximately 7:30 am to 1:30 pm) and 12 subjects underwent testing in the afternoon (approximately 2:30 pm to 8:30 pm). Anxiety groups were distributed evenly between these two sessions.
Data and statistical analyses
For each experiment, the escalation score was calculated for each animal as the slope of drug intake (milligrams per kilogram) over the 12-day LgA period. A positive slope indicates an overall increase in intake over time, while a negative slope indicates a decrease in intake over time.
In the present study, the average escalation slope observed for cocaine and heroin SA was 1.55 ± 0.44 and 0.27 ± 0.026, respectively, representing an average escalation ratio (drug intake during the last 6-h session/intake during the first 6-h session) of 132% ± 9.6% for cocaine and 208% ± 11% for heroin. The magnitude of escalation seen in the present study is therefore comparable to that observed in previous studies (Ahmed and Koob 1998; Ahmed et al. 2000).
Data are expressed as means ± SEM and were subjected to ANOVA with tests of significance performed at α = 0.05. All SA data are presented as the daily averaged cocaine or heroin intake for each group, calculated as the individual amount of drug self-administered (milligrams) within each SA session divided by the bodyweight of the animal (kilograms). The amount of drug self-administered for each animal was based on the number of infusions taken and the known concentration of cocaine or heroin per infusion. Such an approach accounts for differences in bodyweight between subjects and is therefore a reliable method of determining drug intake. Upon confirmation of significant main effects, differences were analyzed using a Newman–Keuls or Dunnett’s post hoc test. Correlation data were assessed using parametric analyses and the Pearson’s rho coefficient. All data were analyzed using the StatSoft Statistica 9 statistics package.
Results
HA and LA rats were selected as the lower and upper quartiles, respectively, of a population, based on the percentage time spent in the EPM open arms (OA) (Fig. 1a, b). For both heroin and cocaine experiments, HA rats (n = 7) spent less than 10% of the 5-min session on the OA of the EPM, significantly less than the 35% of time spent on these arms by LA rats (n = 8) [F 1,13 = 57.02 and F 1,13 = 68.54, respectively, P < 0.001] (Fig. 1a, b). Similarly, LA rats showed greater novelty-seeking compared with HA rats as revealed by their higher end-exploring rates on the OA (SOM Fig. 1) [F 1,13 = 20.80 P < 0.001 and F 1,13 = 34.62, P < 0.001, respectively].
High-anxious rats are vulnerable to escalate cocaine, but not heroin self-administration. High- (HA, n = 7) and low-anxious (LA, n = 8) rats were selected in the lower and upper quartiles of two separate cohorts of Lister-Hooded rats (n = 32), based on the percentage time spent in the open arms (OA) of the elevated plus-maze (EPM) (a, b). When introduced to the heroin escalation paradigm, HA rats did not differ from LA rats regarding the acquisition of self-administration (SA) as measured during five 1-h (short-access or ShA) daily sessions (c, left). There were also no differences in the tendency to escalate heroin SA over 12 6-h extended access (long-access or LgA) daily sessions (c, right), or within the first hour of each LgA session (e). Similarly, HA and LA rats did not differ in their propensity to acquire cocaine SA (d, left). However, these groups did differ in their vulnerability to escalate cocaine SA as revealed by a robust increase in the total daily cocaine intake of HA rats, which was not apparent in the LA group (d, right). This increase was also seen in the first hour of each LgA session (f). Dashed lines (c, d) represent the average drug intake for the entire population during the first day of extended access. Data are expressed as the mean ± SEM of the percentage time spent in the OA of the EPM (a, b), or drug intake (milligrams per kilogram) over 5 days of ShA and 12 days of LgA SA sessions (c, e, d, f). The dashed reference line is used to demonstrate more clearly the progressive escalation of drug SA over sessions and represents the average intake for all rats on day 1 of LgA. Asterisk, different from intake on the first day of LgA (LgA d1), Dunnett’s post hoc test, P ≤ 0.02; number sign, different from LA, P < 0.001
Despite inter-individual differences in anxiety, the two drug cohorts acquired heroin and cocaine SA within the first three sessions with no significant difference in the acquisition of drug SA (Fig. 1c, left and d, left). However, although an increase in daily SA was observed for both drugs when the entire cohort was considered (n = 21 for each drug) [F 11,220 = 36.72, P < 0.001 and F 11,220 = 3.86, P < 0.01 for heroin and cocaine, respectively], escalation of heroin SA developed more rapidly than it did for cocaine, with daily intake differing from the first day of extended access from day 3 onward. Conversely, cocaine intake only differed from day 1 on days 9–12. This difference was also demonstrated by the much higher escalation ratio for heroin SA of 208%, calculated as the drug intake during the last 6-h session/intake during the first 6-h session, compared with 132% for cocaine. The same profile was observed when only the first hour of extended access was considered. Thus, daily intake increased for both heroin and cocaine over time [F 11,220 = 11.19, P < 0.001 and F 11,220 = 3.75, P < 0.001, respectively], an increase that was statistically significant when compared with the first day of extended access from day 3 for heroin and day 9 for cocaine.
HA and LA rats did not differ in the acquisition of heroin (Fig. 1c, left) or cocaine (Fig. 1d, left) SA [Fs1,1hin] measured during five 1-h daily sessions. However, high anxiety did predict a greater escalation of cocaine—but not heroin—intake during LgA sessions. Although there were no significant differences between HA and LA rats during the 6-h (Fig. 1e, right) [effect of session, F11,143 = 30.93, P < 0.001; and group × session interaction, F 11,143 <1], or in the first hour of each session of the heroin escalation phase [effect of session, F 11,143 = 13.1, P < 0.001; and group × session interaction, F 11,143 <1], HA rats showed a significantly greater increase in cocaine SA, compared with LA rats during the 12 LgA sessions [effect of session, F 11,143 = 5.28, P < 0.001; group × session interaction, F 11,143 = 2.35, P < 0.05], a difference that was also observed for the first hour of each session (Fig. 1f, right) [F 11,143 = 4.99, P < 0.001; group × session interaction, F 11,143 = 3.62, P < 0.001]. Post hoc analyses revealed that, while HA rats increased their daily intake of cocaine during both the entire 6-h session and in the first hour of days 9–12 when compared with day 1 of extended access, LA rats did not show an escalation of cocaine SA at all.
This specific relationship between high anxiety and loss of control over cocaine, but not heroin, SA was further supported by a correlational analysis of HA, MA, and LA subjects (Fig. 2). This dimensional analysis revealed that anxiety levels were not related to individual propensity to escalate heroin SA (Fig. 2a), while a clear negative relationship between anxiety levels as measured in the EPM and the escalation slope for cocaine [R = −0.44, P < 0.05] was observed.
High anxiety predicts loss of control over cocaine, but not heroin, self-administration. a, b Parametric correlation analyses between percentage time spent in the open arms (OA) of the elevated plus-maze (EPM) as a measure of anxiety, and the slope of escalation of heroin (a) or cocaine (b) self-administration (SA) as an index of loss of control over drug intake. While no relationship was observed between high anxiety and loss of control over heroin SA (a), anxiety was highly correlated with the escalation of cocaine SA (b) [R = −0.44, P < 0.05]. Dots represent individual data points; with white dots representing LA rats, grey dots representing MA rats, and black dots representing HA rats
The increased propensity to escalate cocaine SA attributed to HA was not due solely to a lack of escalation in LA rats since HA rats displayed a much higher escalation level than the overall population (n = 21), which did show a statistically significant escalation of drug intake [effect of session, F 11,198 = 4.20, P < 0.001; Fig. 1d, right]. The increased propensity of HA rats to escalate cocaine SA was also not due to drug-induced disinhibition of instrumental responding, as HA and LA rats did not differ with respect to the discrimination of active and inactive levers, both during the acquisition [effect of lever, F 1,13 = 50.74, P < 0.001; group × lever interaction, F 1,13 <1], and extended access phases [effect of lever, F 1,13 = 114.1, P < 0.001; group × lever interaction, F 1,13 <1] (data not shown). In addition, HA rats did not differ from LA rats with respect to their latency to the first active lever press of each LgA session for both heroin (Fig. 3a) [effect of group, F 1,7 = 2.25, P = 0.18 and group × session interaction, F 22,154 <1] and cocaine (Fig. 3c) [effect of group, F 1,12 = 2.88, P = 0.11 and group × session interaction, F 22,264 <1] SA. In addition, there were no differences between HA and LA rats in the latency to the first inactive lever press during heroin and cocaine SA sessions (Fig. 3b, d, respectively) [effect of group, F 1,12 = 3.39, P = 0.09; group × session interaction, F 22,264 <1 for heroin and effect of group, F 1,7 <1; group × session interaction, F 22,154 <1 for cocaine]. Thus, the increased vulnerability to escalate cocaine SA shown by HA rats appears unrelated to a differential sensitivity to the anxiogenic properties of cocaine.
A greater tendency to escalate cocaine self-administration in high-anxious rats is not related to a differential sensitivity to the anxiogenic properties of the drug. High-anxious (HA) rats trained to self-administer heroin did not differ from low-anxious (LA) rats in relation to the latency to the first active lever press response during self-administration (SA) sessions (a). Inactive lever press latencies did not significantly differ between HA and LA rats self-administering heroin (b). HA rats did not differ from LA rats regarding the latency to the first active lever press response during cocaine SA (c). Similarly, no difference was observed between HA and LA rats for inactive lever press latency (d). The apparent difference between HA and LA rats regarding the latency to press the inactive lever on days 10–12 is due to the fact that in these sessions HA rats tended to press the inactive lever at least once, while LA rats rarely pressed the inactive lever at all (no lever press occurrence resulted in a latency equal to the duration of the session). Consequently, this difference is not meaningful and is an artifact of the experimental setup. Data are expressed as the mean ± SEM of the time taken to complete the first active (a and c) or inactive (b and d) lever press for each SA session
Discussion
The present study demonstrates a relationship between high anxiety and subsequent individual vulnerability to lose control over cocaine, but not heroin, intake in rats. Not only did HA rats display a greater escalation of cocaine SA compared with LA subjects, but the level of their trait-like anxiety as measured by the EPM predicted their subsequent rate of escalation of cocaine SA. In addition, HA rats also showed a greater intake of cocaine in the first hour of each LgA session. This observation both rules out increased sensitivity of HA rats to within-session tolerance (Ahmed and Koob 1998) and also demonstrates that HA rats are more sensitive to increased cocaine intake, a putative marker of drug dependence (Koob and Moal 1997). Importantly, this relationship between a high anxiety trait and cocaine SA was specific to the escalation period of cocaine SA. Thus, high anxiety was not associated with an increased propensity to acquire cocaine SA when compared with LA rats but was instead related to greater escalation of cocaine SA following extended access to the drug. This suggests that the neurobiological substrates responsible for a high anxiety trait interact with neuroadaptative changes triggered by prolonged cocaine exposure, resulting in enhanced escalation of drug SA.
The close relationship between trait-like anxiety and increased propensity to escalate cocaine SA may seem difficult to reconcile with reports that cocaine promotes anxiety-like behavior in both human users (Anthony et al. 1989; Bystritsky et al. 1991) and in animals (Blanchard and Blanchard 1999; Ettenberg et al. 1999; Blanchard et al. 1999, 2000). However, it has previously been shown that rats displaying high anxiety on the EPM have an increased tendency to drink an oral solution of cocaine (Walker et al. 2009), an enhanced motivation for the drug under a progressive-ratio schedule of reinforcement (Homberg et al. 2002; but see, Bush and Vaccarino (2007)), and acquire a cocaine conditioned place preference whereas LA rats do not (Pelloux et al. 2009). One explanation may be that the anxiogenic effects of cocaine in rats (Ettenberg et al. 1999) may only be evident in those individuals characterized as LA on the EPM (Rogerio and Takahashi 1992). It has also been suggested that these effects could limit the rewarding properties of the drug (David et al. 2001), with the implication being that the relative insensitivity of HA rats to cocaine-associated anxiogenesis could render them more vulnerable to escalation of drug intake. In the present study, however, there were no differences between HA and LA rats with respect to latencies to the first active lever press of each SA session, which has been taken to be an index of sensitivity to the anxiogenic properties of cocaine experienced in previous sessions (Maier et al. 2008). This indicates that, under the SA schedule used here, the propensity to escalate cocaine SA is unlikely to be related to a differential sensitivity of LA and HA rats to the anxiety-promoting properties of the drug.
The neural mechanisms underlying the interaction between high anxiety and escalation of cocaine SA are unclear, but previous reports of neurochemical differences between HA and LA rats may be relevant. In particular, HA rats show a reduction in electrically evoked dopamine release in the substantia nigra, medial prefrontal cortex, and amygdala (Homberg et al. 2002), as well as reduced levels of serotonin in the ventral striatum, compared with LA subjects (Schwarting et al. 1998). HA rats also have fewer benzodiazepine (BDZ) receptors both in the periphery (Rago et al. 1991) and in the frontal cortex (Harro et al. 1990). Previous reports have demonstrated that chronic cocaine SA produces complex effects on BDZ receptors in the prefrontal cortex, nucleus accumbens, and ventral tegmental area (Goeders 1991), while diminishing the anxiogenic effects of the BDZ inverse agonist FG 7142 (Waters and See 2011).
The present results also demonstrate that high anxiety does not interact with extended exposure to heroin to strongly facilitate its self-administration. In support of this finding, Cruz et al. (2011) demonstrated that social defeat stress facilitated both escalated and “binge-like” cocaine SA but had no effect on heroin SA. Similarly, the present results extend the recent demonstrations that a high-impulsivity trait predicts increased vulnerability to escalate (Dalley et al. 2007), and develop compulsive (Belin et al. 2008) cocaine SA but is not related to the escalation of, or relapse to, heroin SA (McNamara et al. 2010). These dissimilarities are likely to be related to the fact that, although heroin and cocaine share some common neurobiological targets within the reward network of the brain, thought to be the major mechanism of action of all addictive substances (Di Chiara and Imperato 1988; Wise 1996; Nestler 2005), there are also differences in the pharmacology or mechanisms of action of these two major classes of drugs that could be relevant to drug addiction (Johnson and North 1992; Bardo 1998; for review, see Badiani et al. (2011)). Studies investigating the reinforcing effects of drugs of abuse have shown that, although cocaine and heroin share an ability to enhance mesocorticolimbic dopaminergic activity, leading to an increase in dopamine levels in the nucleus accumbens (Wise 1996), there is also considerable evidence for dopaminergic-independent mechanisms of opiate reinforcement. For example, although dopamine receptor blockade or selective lesioning of dopaminergic neurons in the nucleus accumbens can block pyschostimulant SA, opiate SA is unaffected (Ettenberg et al. 1982; Pettit et al. 1984; Van Ree and Ramsey 1987). It could also be argued that the escalation model used for heroin may not be sufficiently sensitive to reveal inter-individual differences in the tendency to lose control over drug intake. However, we have previously shown that this is not the case, since we have demonstrated remarkable between-subject variability within this model, and rats differ greatly in their tendency to increase heroin intake in this paradigm (McNamara et al. 2010).
The findings here indicate that different etiological factors influence the development of opiate and stimulant dependence. In addition to the high anxiety trait studied in the present experiments, high impulsivity in rats also predicts an increased propensity to escalate cocaine (Dalley et al. 2007) but not heroin SA (McNamara et al. 2010). These two independent pre-clinical studies are consistent with the demonstration of heterogeneity in the personality traits of cocaine addicts, with one subset showing high anxiety and others showing high impulsive/sensation-seeking traits (Gunnarsdottir et al. 2000). Interestingly, it has been recently demonstrated that stimulant addicts and their siblings can also display both high impulsivity and trait-anxiety (Ersche et al., personal communication). From these studies, it may be concluded that several independent and possibly interacting behavioral traits contribute to individual predispositions to stimulant addiction and that they may differ from those that contribute to opiate addiction.
Several pre-clinical studies have now shown that different behavioral traits—some of which are unrelated or even negatively correlated—can predict similar outputs in various drug addiction paradigms. For example, high impulsivity (Dalley et al. 2007), anxiety (present study), and novelty preference (Belin et al. 2008, 2011), but not novelty-induced locomotor activity (Belin et al. 2008, 2011) have been shown to predict various features of addiction-like behavior in rats self-administering cocaine, yet there is little apparent correlation between them (Molander et al. 2011). Clearly, an overarching conceptual framework must be developed in order to facilitate the interpretation of these animal experimental data in a clinical context. It would be of considerable value to investigate whether there is an additive or interactive effect on cocaine escalation in rats demonstrating high levels of more than one of these behavioral traits. This is one avenue for future research. It should also be pointed out that only a single test of anxiety was conducted in the present study, namely, time spent in the open arms of the EPM. Other anxiety paradigms, including social defeat stress, or conditioned freezing, may provide further insights into the behavioral characteristics associated with a propensity to escalate cocaine SA.
In summary, the results of this study demonstrate that high anxiety, as measured by the EPM, predicts loss of control over cocaine, but not heroin, intake. Since the interaction between anxiety and increased cocaine intake only manifested itself after several LgA sessions, it may be hypothesized that extended access to cocaine induces neuroadaptative changes that result in augmented responding for the drug in HA, but not LA, rats. Together with the demonstration that high impulsivity in rats is associated with an increased vulnerability to escalate cocaine, but not heroin, SA, the present results suggest that cocaine and heroin addictions do not share the same aetiological mechanisms (Badiani et al. 2011) and that high anxiety levels in heroin addicts (Lejuez et al. 2006, 2008) may not be entirely due to pre-existing anxiety traits.
References
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282(5387):298–300
Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22(4):413–421
Ambrose-Lanci LM, Sterling RC, Van Bockstaele EJ (2010) Cocaine withdrawal-induced anxiety in females: impact of circulating estrogen and potential use of delta-opioid receptor agonists for treatment. J Neurosci Res 88(4):816–824
Anthony JC, Tien AY, Petronis KR (1989) Epidemiologic evidence on cocaine use and panic attacks. Am J Epidemiol 129(3):543–549
APA (2000) Diagnostic and statistical manual of mental Disorders fourth edition, text revision (DSM-IV TR). American Psychiatric Association, Washington
Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011) Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 12(11):685–700
Bardo MT (1998) Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol 12(1–2):37–67
Belin D, Everitt BJ (2008) Cocaine-seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57(3):432–441
Belin D, Mar A, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320(5881):1352–1355
Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V (2011) High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36(3):569–579
Blanchard DC, Blanchard RJ (1999) Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev 23(7):981–991
Blanchard RJ, Kaawaloa JN, Hebert MA, Blanchard DC (1999) Cocaine produces panic-like flight responses in mice in the mouse defense test battery. Pharmacol Biochem Behav 64(3):523–528
Blanchard RJ, Hebert M, Dulloog L, Markham C, Figueira R, Nishimura O, Newsham K, Kaawaloa JN, Blanchard DC (2000) Cocaine-induced sniffing stereotypy changes in response to threat. Pharmacol Biochem Behav 66(2):249–256
Bush D, Vaccarino F (2007) Individual differences in elevated plus-maze exploration predicted progressive-ratio cocaine self-administration break points in Wistar rats. Psychopharmacology 194(2):211–219
Bystritsky A, Ackerman DL, Pasnau RO (1991) Low dose desipramine treatment of cocaine-related panic attacks. J Nerv Ment Dis 179(12):755–758
Cruz FC, Quadros IM, Hogenelst K, Planeta CS, Miczek KA (2011) Social defeat stress in rats: escalation of cocaine and “speedball” binge self-administration, but not heroin. Psychopharmacology (Berl) 215:165-175
Dalley JW, Fryer T, Brichard L, Robinson E, Theobald DE, Laane K, Pena Y, Murphy E, Shah Y, Probst K, Abakumova I, Aigbirhio F, Richards H, Hong Y, Baron J, Everitt BJ, Robbins TW (2007) Nucleus accumbens d2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315(5816):1267–1270
David V, Gold LH, Koob GF, Cazala P (2001) Anxiogenic-like effects limit rewarding effects of cocaine in balb/cbyj mice. Neuropsychopharmacology 24(3):300–318
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85(14):5274–5278
Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982) Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 78(3):204–209
Ettenberg A, Raven MA, Danluck DA, Necessary BD (1999) Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav 64(3):507–512
Goeders NE (1991) Cocaine differentially affects benzodiazepine receptors in discrete regions of the rat brain: persistence and potential mechanisms mediating these effects. J Pharmacol Exp Ther 259(2):574–581
Gunnarsdottir ED, Pingitore RA, Spring BJ, Konopka LM, Crayton JW, Milo T, Shirazi P (2000) Individual differences among cocaine users. Addict Behav 25(5):641–652
Harro J, Kiivet RA, Lang A, Vasar E (1990) Rats with anxious or non-anxious type of exploratory behaviour differ in their brain cck-8 and benzodiazepine receptor characteristics. Behav Brain Res 39(1):63–71
Homberg JR, van den Akker M, Raaso HS, Wardeh G, Binnekade R, Schoffelmeer AN, de Vries TJ (2002) Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci 15(9):1542–1550
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12(2):483–488
Khantzian EJ (1985) The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 142(11):1259–1264
Koob GF, Moal ML (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278(5335):52–58
Le Merrer J, Cagniard B, Cazala P (2006) Modulation of anxiety by mu-opioid receptors of the lateral septal region in mice. Pharmacol Biochem Behav 83(3):465–479
Lejuez CW, Paulson A, Daughters SB, Bornovalova MA, Zvolensky MJ (2006). The association between heroin use and anxiety sensitivity among inner-city individuals in residential drug use treatment. Behav Res Ther 44(5), 5, 667–677
Lejuez CW, Zvolensky MJ, Daughters SB, Bornovalova MA, Paulson A, Tull MT, Ettinger K, Otto MW (2008) Anxiety sensitivity: a unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behav Res Ther 46(7):811–818
Maier EY, Ledesma RT, Seiwell AP, Duvauchelle CL (2008) Diazepam alters cocaine self-administration, but not cocaine-stimulated locomotion or nucleus accumbens dopamine. Pharmacol Biochem Behav 91(1):202–207
McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D (2010) Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology 212(4):453–464
Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, Dalley JW (2011) High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl) 215(4):721–731
Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8(11):1445–1449
Norton GR (2001) Substance use/abuse and anxiety sensitivity what are the relationships? Addict Behav 26:935-946
Pelloux Y, Costentin J, Duterte-Boucher D (2009) Anxiety increases the place conditioning induced by cocaine in rats. Behav Brain Res 197(2):311–316
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14(3):149–167
Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984) Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 84(2):167–173
Rago L, Adojaan A, Harro J, Kiivet RA (1991) Correlation between exploratory activity in an elevated plus-maze and number of central and peripheral benzodiazepine binding sites. Naunyn Schmiedebergs Arch Pharmacol 343(3):301–306
Rogerio R, Takahashi RN (1992) Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav 43(2):631–633
Salas-Ramirez KY, Frankfurt M, Alexander A, Luine VN, Friedman E (2010) Prenatal cocaine exposure increases anxiety, impairs cognitive function and increases dendritic spine density in adult rats: influence of sex. Neuroscience 169(3):1287–1295
Schulteis G, Yackey M, Risbrough V, Koob GF (1998) Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol Biochem Behav 60(3):727–731
Schwarting RK, Thiel CM, Muller CP, Huston JP (1998) Relationship between anxiety and serotonin in the ventral striatum. NeuroReport 9(6):1025–1029
Sherman JE, Zinser MC, Sideroff SI, Baker TB (1989) Subjective dimensions of heroin urges: influence of heroin-related and affectively negative stimuli. Addict Behav 14(6):611–623
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158(4):343–359
Van Ree JM, Ramsey N (1987) The dopamine hypothesis of opiate reward challenged. Eur J Pharmacol 134(2):239–243
Walker QD, Schramm-Sapyta NL, Caster JM, Waller ST, Brooks MP, Kuhn CM (2009) Novelty-induced locomotion is positively associated with cocaine ingestion in adolescent rats; anxiety is correlated in adults. Pharmacol Biochem Behav 91(3):398–408
Waters RP, See RE (2011) Chronic cocaine self-administration attenuates the anxiogenic-like and stress potentiating effects of the benzodiazepine inverse agonist, fg 7142. Pharmacol Biochem Behav 99(3):408–413
Wise RA (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251
Zhou Y, Spangler R, Ho A, Kreek MJ (2003) Increased crh mrna levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res Mol Brain Res 114(1):73–79
Acknowledgments
This work was supported by the United Kingdom Medical Research Council (Grant 9536855 to BJE and Grant G0701500 to JWD) and was conducted within the MRC/Wellcome Trust Behavioural and Clinical Neuroscience Institute. RD was financially supported by a Young Foreign Researcher grant from the University of Poitiers. DB is supported by an INSERM AVENIR grant.
Financial disclosures
The authors report no competing interests.
Authors contribution
DB and BJE were responsible for the study concept. RD, BJE, JWD and DB designed the experiments. RD, YP, ACM and AM performed the experiments. RD and DB performed statistical analyses and designed the figures. RD drafted the manuscript. DB, BJE, JWD and TWR provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 77 kb)
Rights and permissions
About this article
Cite this article
Dilleen, R., Pelloux, Y., Mar, A.C. et al. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology 222, 89–97 (2012). https://doi.org/10.1007/s00213-011-2626-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2626-4