Abstract
Rationale
Cannabis users display a constellation of withdrawal symptoms upon drug discontinuation, including sleep disturbances, irritability, and possibly memory deficits. In cannabinoid-dependent rodents, the CB1 antagonist rimonabant precipitates somatic withdrawal and enhances forskolin-stimulated adenylyl cyclase activity in cerebellum, an effect opposite that of acutely administered ∆9-tetrahydrocannabinol (THC), the primary constituent in cannabis.
Objectives
Here, we tested whether THC-dependent mice undergoing rimonabant-precipitated withdrawal display short-term spatial memory deficits, as assessed in the Morris water maze. We also evaluated whether rimonabant would precipitate adenylyl cyclase superactivation in hippocampal and cerebellar tissue from THC-dependent mice.
Results
Rimonabant significantly impaired spatial memory of THC-dependent mice at lower doses than those necessary to precipitate somatic withdrawal behavior. In contrast, maze performance was near perfect in the cued task, suggesting sensorimotor function and motivational factors were unperturbed by the withdrawal state. Finally, rimonabant increased adenylyl cyclase activity in cerebellar, but not in hippocampal, membranes.
Conclusions
The memory disruptive effects of THC undergo tolerance following repeated dosing, while the withdrawal state leads to a rebound deficit in memory. These results establish spatial memory impairment as a particularly sensitive component of cannabinoid withdrawal, an effect that may be mediated through compensatory changes in the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marijuana (cannabis sativa) is the most widely used illicit substance in the USA. The high prevalence of marijuana use along with the most recent SAMHSA report indicating that marijuana use rose in 2009 (SAMHSA 2010) raises concern about dependence to this drug. A cannabinoid withdrawal syndrome has been described in humans in which abrupt discontinuation following chronic smoked marijuana or oral ∆9-tetrahydrocannabinol (THC) produces sleep disturbances, disrupted cognition, decreased appetite, restlessness, irritability, sweating, chills, and nausea (Budney et al. 2001, 2008; Jones and Benowitz 1976; Jones et al. 1976; Vandrey et al. 2005, 2008). The recent findings that cognitive symptoms (Budney et al. 2008) and difficulty concentrating (Mennes et al. 2009) associated with withdrawal contributed to relapse in marijuana users suggest that cognitive impairment represents a significant symptom of withdrawal.
Marijuana and its chief psychoactive component, THC, activate cannabinoid1 receptors (CB1) (Maldonado and Rodriguez de Fonseca 2002), which are found in high concentrations in cerebellum, hippocampus, and other brain regions associated with memory and cognitive function (Herkenham et al. 1991; Matsuda et al. 1993). Accordingly, it is well documented that marijuana or THC impairs memory function (Harrison et al. 2002; Lichtman et al. 2002; McHale and Hunt 2008; Pope et al. 2001; Solowij et al. 2002). Acute administration of THC or other cannabinoid receptor agonists disrupt performance in a wide range of laboratory animal models of learning and memory (Brodkin and Moerschbaecher 1997; da Silva and Takahashi 2002; Hampson and Deadwyler 2000; Lichtman and Martin 1996; Mallet and Beninger 1998; Varvel and Lichtman 2002). However, few controlled published reports have evaluated whether cannabinoid-dependent animals show memory impairment during withdrawal. The CB1 antagonist rimonabant has proven useful in investigating cannabinoid dependence in laboratory animals, as it precipitates a variety of somatic symptoms of withdrawal in mice, rats, and dogs treated subchronically with THC (Aceto et al. 1996; Cook et al. 1998; Hutcheson et al. 1998; Lichtman et al. 2001b; Tsou et al. 1995). Rimonabant has been reported to impair delayed non-match-to-sample performance in rats treated chronically with the cannabinoid receptor agonist WIN55,212-2, though it is unclear whether the decrement in performance was due to impaired working memory or somatic withdrawal symptoms interfering with responding (Hampson et al. 2003).
The primary objective of the present study was to test whether a state of precipitated cannabinoid withdrawal disrupts short-term memory. To test this hypothesis, mice received a mild subchronic THC dosing regimen, were challenged with rimonabant, and then assessed for short-term spatial memory in the Morris water maze. An important aspect of this paradigm is the ability to distinguish between sensorimotor/motivational deficits and impaired memory through the employment of a cued platform task in which the platform is made visible. A second objective of this work was to test whether rimonabant was more potent in precipitating memory impairment than somatic THC withdrawal signs by comparing its dose–response relationships in eliciting these effects. The final goal of the present study was to elucidate the biochemical changes associated with cannabinoid withdrawal symptoms. Rimonabant elicits increases in adenylyl cyclase activity in cannabinoid-dependent mice, an effect opposite that of acute cannabinoids (Hutcheson et al. 1998; Rubino et al. 2000b, c). Therefore, we investigated whether superactivation of adenylyl cyclase in the hippocampus or cerebellum is related to memory deficits in THC-dependent mice.
Methods
Subjects
The subjects consisted of male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) that were 7–12 weeks of age at the start of the study. All subjects were housed in a temperature-controlled (20–22°C) environment, with a 12-h light/dark cycle. Food and water were available ad libitum in the home cages. All experiments were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Drugs and chemicals
THC and rimonabant were provided by the National Institute on Drug Abuse (Bethesda, MD, USA) and WIN55,212-2 was purchased from Sigma Aldrich (St. Louis, MO, USA). THC and rimonabant were dissolved in a 1:1 mixture of absolute ethanol and alkamuls-620 (Rhone-Poulenc, Princeton, NJ, USA) and diluted with saline to a final ratio of 1:1:18 (ethanol, alkamuls, and saline, respectively). THC was administered via the s.c. route of administration and rimonabant was given by the i.p. route of administration. All injections were given in volume of 10 μl/g. Trizma base, MgCl2, and NaCl were purchased from Fisher Scientific (Pittsburgh, PA, USA). Ethylene glycol-bis(2-aminoehtylether)-N, N, N′, N′-tetraacetic acid (EGTA), adenosine 3′,5′-cyclic monophosphate (cAMP), adenosine 5′-triphosphate (ATP), guanosine 5′-triphosphate (GTP), bovine serum albumin (BSA), forskolin, papaverine, phosphocreatine, creatine phosphokinase, and dithiothreitol were purchased from Sigma Aldrich. [α-32P]ATP and [3H]cAMP were purchased from Perkin-Elmer (Shelton, CT, USA). Ecolite scintillation fluid was purchased from MP Biomedical (Irvine, CA, USA).
Procedures
Morris water maze
The water maze consisted of a large circular galvanized steel pool (1.8 m diameter, 0.6 m height). A white platform (10 cm diameter) was placed inside, and the tank was filled with water (22°C) until the top of the platform was submerged 1 cm below the water surface. A sufficient volume of white paint (Proline—Latex Flat) was added to make the water opaque and render the platform virtually invisible.
The mice were trained to perform a repeated acquisition version of a spatial memory Morris water maze task in which they were required to find a hidden platform that was placed in a new location each day (Niyuhire et al. 2007). Subjects first received a single 5-min acclimation session in which no platform was present, followed by 8 days of training in a fixed-platform task (four trials per day, 10 min between each trial). Repeated acquisition training consisted of five consecutive 2-min trials, in which the platform was placed in new location each day. After locating the platform, the subjects were allowed to remain on it for 30 s and were then gently removed. Training continued until the subject reached the platform in less than 30 s on two of the last three trials on the last three of 4 days.
When training criteria were met, mice continued to receive daily repeated acquisition trials, but were given an injection of vehicle or THC (10 mg/kg) 1 h after the fifth trial on four consecutive days. On the fifth day, the mice received their respective injections in the morning, and 4 h later were assessed in the repeated acquisition task. Thirty minutes after the fifth trial, the mice were injected with either vehicle or rimonabant (0.3, 1, 3, or 10 mg/kg), and were returned to the tank at 60 min when they received a 60-s probe trial (i.e., the platform was removed from the tank). The selection of these doses of rimonabant was based on previous studies assessing somatic signs of withdrawal in THC-dependent mice challenged with rimonabant (Cook et al. 1998; Lichtman et al. 2001b; Wilson et al. 2006). The distance that the mice swam (i.e., path length) to first enter the target location and the percentage of time spent in the target zone (defined as the area immediately surrounding the target location that comprised 8% of the total surface area) as well as a control zone, of equal area, on the opposite side of the tank were assessed. An automated tracking system (Columbus Instruments, Columbus, OH, USA) connecting to a video camera was used to quantify these dependent variables. A quasi-Latin square within subject design was used to evaluate the dose–response relationship of rimonabant in precipitating withdrawal with a minimum of 72 h between each cycle of subchronic dosing. Two different cohorts of mice were used for a sample size of 11–16 mice/group.
In order to control for possible sensorimotor or motivational impairments, mice were trained to perform in a cued version of the task in which the platform location was made visible by placing a black rubber stopper (height, 3 cm; radius, 1.5 cm) on top of the submerged platform, which extended approximately 2 cm above the surface of the water. Following a washout period for at least 1 week, 15 mice from the above experiment were trained in this manner for 3 days, during which time they learned to find the platform in less than 30 s. The mice were then treated with THC (10 mg/kg) for five consecutive days, but on the final day were given an injection of vehicle or rimonabant (10 mg/kg) 4.5 h after their morning injection. Thirty minutes later, each subject was tested for its ability to swim to the visible platform. An additional group of mice (n = 5) was trained in the repeated acquisition spatial memory Morris water maze task to test the acute effects of THC during the probe trial, using the day 5 treatment conditions described above. The treatment order was counter-balanced and at least a 1-week washout period was imposed to limit the possibility of THC tolerance.
Somatic withdrawal signs
Naïve mice were treated with 10 mg/kg THC or vehicle once per day for 4 days in their home cages. On the fifth day, the subjects were brought to the laboratory where they received their final respective treatment in the morning. The subjects were administered vehicle or rimonabant (1, 3, or 10 mg/kg; i.p.) 4.5 h later and were placed in clear plastic cages. The number of paw flutters (a lateral forepaw clapping behavior) and headshakes were scored from 4.75 to 5.25 h by an observer who was blinded to treatment condition as previously described (Cook et al. 1998; Lichtman et al. 2001b; Wilson et al. 2006)
Adenylyl cyclase assay
Mice were given daily s.c. injections of either THC (10 mg/kg) or vehicle for 5 days. On the fifth day, subjects were challenged with an i.p. injection of either rimonabant or vehicle 4.5 h following the final THC or vehicle injection. The mice were euthanized by cervical dislocation at 5.25 h after the final injection, which represents the time point immediately after subjects would have completed their testing in the water maze. As previously described (Selley et al. 2004), cerebellum or hippocampus tissue was homogenized in 50 mM Tris–HCl, 3 mM MgCl2, and 1 mM EGTA, and membranes collected by centrifugation at 50,000×g at 4°C. The resulting pellet was homogenized as above, centrifuged at 50,000×g and the resulting pellet was homogenized in 50 mM Tris–HCl, 3 mM MgCl2, 0.2 mM EGTA and 100 mM NaCl (assay buffer). Membranes (13–15 μg protein) were incubated for 15 min at 30°C in the absence (basal) or presence of 1 μM forskolin with and without 1 or 10 μM WIN55,212-2 (WIN), a CB1 receptor agonist, in assay buffer containing [α-32P]ATP (1.5 μCi), 0.2 mM dithiothreitol, 0.01% BSA, 50 μM ATP, 50 μM GTP, 50 μM cAMP, 0.2 mM papaverine, 5 mM phosphocreatine, and 20 U/ml creatine phosphokinase; final volume 100 μl. These conditions result in a total amount of [α-32P]cAMP that is less than 1% of the total [α-32P]ATP added to each sample. The reaction was terminated by boiling for 3 min and [3H]cAMP (10,000 dpm) was added as an internal standard to each sample. [α-32P]cAMP was isolated using the dual column (Dowex and alumina) method (Salomon 1979). The eluate was dissolved in Ecolite scintillation fluid and radioactivity was determined by liquid scintillation spectrometry. Data are expressed as mean ± SEM as a percentage of the control group (repeated dose of vehicle and challenged with vehicle).
Statistical analysis
Data were analyzed with one- or two-factor analysis of variance (ANOVA) tests. Significant ANOVA results were followed by Dunnett’s test in which each dose of rimonabant was compared with the vehicle condition or Tukey test for multiple comparisons. In addition, planned comparisons were conducted using Bonferroni-adjusted t tests. All analyses were conducted with Statview for Windows version 5.0 (SAS Institute Inc.).
Results
Rimonabant precipitates short-term memory impairment in mice treated subchronically with THC
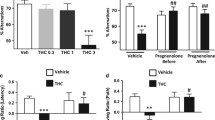
Both vehicle and THC-dependent mice that were given their daily injection 1 h after their daily training session performed well during the acquisition phase of the task by reaching the platform in less than 30 s on two of their last three trials (data not shown). On the test day, subjects were administered their respective vehicle or THC injection 4 h before the repeated acquisition session and continued to perform well during acquisition, indicating that subchronic THC drug treatment did not elicit residual deficits in learning the platform location. In contrast, mice given an acute injection of THC 4 h before acquisition displayed profound deficits during the retention test (Fig. 1b, inset), suggesting that subchronic THC administration resulted in tolerance to the memory disruptive effects of THC. Rimonabant given 30 min after the five acquisition trials dose-dependently impaired performance during the probe trial in THC-dependent mice, but not in non-dependent mice. Rimonabant significantly increased the distance THC-treated mice swam (i.e., path length; Fig. 1a) to reach the target location, F(6,86) = 8.8, p < 0.001. Dunnett’s post hoc analysis revealed that 1 (p < 0.05), 3 (p < 0.01), and 10 (p < 0.01) mg/kg rimonabant increased path lengths in THC-dependent mice as compared to THC-dependent mice challenged with vehicle. In addition, 1 (p < 0.01), 3 (p < 0.002), and 10 (p < 0.002) mg/kg rimonabant increased path lengths in mice treated subchronically with THC compared to mice treated subchronically with vehicle and challenged with vehicle. However, vehicle treatment (p = 0.45) or 10 mg/kg rimonabant (p = 0.78) did not affect the path lengths in non-dependent mice as compared to THC-dependent mice challenged with vehicle. The path lengths in the non-dependent mice did not differ between rimonabant challenge and vehicle challenge (p = 0.35). As previously reported (Varvel et al. 2005), rimonabant significantly increased swim speeds regardless of subchronic drug administration, F(6, 86) = 7.2, p < 0.001 (Table 1), thus, the latency to target zone data are not shown.
Rimonabant disrupts short-term spatial memory of THC-dependent mice in the Morris water maze. Mice were administered THC (10 mg/kg) or vehicle once daily for five consecutive days. On day 5, subjects were given their daily respective injection 4 h before acquisition, and treated with rimonabant or vehicle (V) 30 min after acquisition. Each data point represents the average performance of subjects during a probe trial 1 h after repeated acquisition training. a The path length to target was dose-dependently increased by rimonabant in THC-dependent mice. However, rimonabant (10 mg/kg) did not affect memory in mice treated subchronically with vehicle. b The percentage of time spent in the target zone during probe trials is dose-dependently decreased by rimonabant in THC-dependent mice. Rimonabant did not affect time spent in the target zone in non-dependent mice. Inset: acute THC (10 mg/kg) administered to non-dependent mice 4 h before acquisition impaired spatial memory performance. c Representative swim traces in the probe trial given 1 h after the repeated acquisition session. Non-dependent mice challenged with either vehicle (top left) or rimonabant (10 mg/kg; top right) as well as THC-dependent mice challenged with vehicle (bottom left) spent much of their time swimming in the target zone. THC-dependent mice challenged with rimonabant (10 mg/kg; bottom right) displayed no bias to any region. The target zone is represented by the shaded circle which indicates the area where the platform was located during the acquisition trials, and the control zone, directly opposite, is represented by an open circle. *p < 0.05; **p < 0.01 vs. mice treated subchronically with THC and challenged with vehicle (Dunnett’s test). # p < 0.01; ## p < 0.002 vs. mice treated subchronically with vehicle and challenged with vehicle (adjusted Bonferroni t tests). All values are expressed as mean ± SEM; n = 11–16 mice/group
Strikingly, rimonabant eliminated the spatial bias for the target zone in THC-dependent mice, but not in the vehicle control group, F(6, 86) = 8.0, p < 0.001 (Fig. 1b). In THC-dependent mice, the Dunnett’s test revealed that 3 (p < 0.05) and 10 (p < 0.01) mg/kg rimonabant decreased time spent in the target zone compared to mice challenged with vehicle (Fig. 1b). THC-dependent mice challenged with 3 (p < 0.002) or 10 (p < 0.002) mg/kg rimonabant also significantly differed from non-dependent mice that were challenged with vehicle. In contrast, time spent in the control zone was unaffected by rimonabant challenges in both THC-dependent mice and mice treated repeatedly with vehicle (p = 0.69; Table 1). As can be seen by the representative swim traces in Fig. 1c, THC-dependent mice that were challenged with rimonabant (10 mg/kg) displayed no bias to the target zone or surrounding area. In contrast, non-dependent mice treated with vehicle or rimonabant (10 mg/kg) 30 min before the probe trial spent a significant duration of time swimming in the target zone and surrounding area. On the other hand, a single injection of THC (10 mg/kg) given 4 h before acquisition training impaired probe trial performance in non-dependent mice regardless of whether rimonabant was administered 30 min after acquisition (Fig. 1b, inset).
Assessment of non-mnemonic water maze performance
In order to assess whether rimonabant impaired Morris water maze performance of THC-dependent mice during the probe trial because of sensorimotor or motivational deficits, we evaluated the subjects in a cued procedure in which the platform was made visible. Rimonabant (10 mg/kg) did not disrupt performance in the cued Morris water maze task in either THC-dependent or non-dependent mice. Both groups of mice had similar latencies to target (Fig. 2a; p = 0.25), path lengths to target (Fig. 2b; p = 0.83), and swim speeds (Fig. 2c; p = 0.40).
THC-dependent mice challenged with rimonabant (RIM) displayed good performance in a cued water maze platform task in which the platform is made visible. Mice treated subchronically with THC (10 mg/kg) or vehicle (Veh) once daily for 5 days had similar (a) escape latencies to platform (b), path lengths to platform, and swim speeds (c) when challenged with rimonabant (10 mg/kg). Each data point represents a single trial in which mice were required to locate a platform that was made explicitly visible. All values are expressed as mean ± SEM; n = 7–11 mice/group
Decreased rimonabant potency in precipitating somatic withdrawal behavior
In the next experiment, we examined the potency of rimonabant to precipitate somatic withdrawal signs in THC-dependent mice. Naïve mice were given a daily injection of THC (10 mg/kg) for 5 days and challenged with rimonabant (0, 1, 3, or 10 mg/kg) 4 h after the final THC injection on day 5 as described for the Morris water maze experiments. A significant effect of treatment was found for paw flutters, F(5, 26) = 4.4, p < 0.01 (Fig. 3a). A trend but no significant effect was found for head shakes, F(5, 26) = 2.4, p = 0.06 (Fig. 3b). In THC-treated mice, only the 10 mg/kg dose of rimonabant significantly increased paw flutters (p < 0.05) compared to the other groups. In contrast, rimonabant (10 mg/kg) did not significantly affect either measure in mice treated subchronically with vehicle.
Effect of rimonabant challenge on somatic signs of withdrawal following a low THC dosing regimen. Rimonabant precipitated a significant increase in paw flutters (a), but not head shakes (b), in THC-dependent mice. All values are expressed as mean ± SEM; n = 6/group. *Significantly different from non-dependent mice that were challenged with vehicle (p < 0.05)
Rimonabant elicits a superactivation of adenylyl cyclase activity in cerebellar membranes from THC-dependent mice
The effects of subchronic THC administration vs. vehicle on WIN55,212-2-induced inhibition of forskolin-stimulated adenylyl cyclase activity were evaluated in hippocampal and cerebellar membrane preparations. Forskolin stimulated adenylyl cyclase activity in mice that received subchronic vehicle and were challenged with vehicle was 138 ± 6.2% and 172 ± 8.1% in hippocampus and cerebellum, respectively. The data presented in Fig. 4 are expressed as a percentage of forskolin-stimulated adenylyl cyclase activity obtained from these control mice. WIN55,212-2 (10 μM) significantly inhibited forskolin-stimulated adenylyl cyclase activity in cerebellar membranes from control mice, F(2,20) = 3.8, p < 0.05, and THC-dependent mice, F(2,20) = 4.3, p < 0.05. However, WIN55,212-2 did not significantly inhibit forskolin-stimulated adenylyl cyclase activity in hippocampal membranes of either non-dependent or THC-dependent mice (p > 0.05).
WIN55,212-2 (WIN) significantly inhibited forskolin-stimulated adenylyl cyclase activity in cerebellar membranes, but not in hippocampal membranes, from mice subchronically treated with vehicle (Veh) or THC. Data are expressed as a percent of non-dependent mice that were challenged with vehicle, n = 7–8. *Significantly different than control mice (p < 0.05; Dunnett’s test)
To examine whether precipitated withdrawal is associated with increased forskolin-stimulated adenylyl cyclase activity, mice were given daily injections of vehicle or THC, challenged with vehicle or rimonabant (10 mg/kg) 4 h later, and then sacrificed at 4.75 h, corresponding to the time point that the probe trial would have been completed as well as in a previous report (Hutcheson et al. 1998). Rimonabant significantly increased forskolin-stimulated adenylyl cyclase activity in cerebellar tissue from THC-dependent mice, F(3,20) = 5.1, p < 0.01 (Fig. 5). However, no differences were found between the vehicle challenged and rimonabant challenged non-dependent mice. In contrast, rimonabant challenge did not significantly affect forskolin-stimulated adenylyl cyclase activity in hippocampal tissue, regardless of subchronic treatment (p > 0.05).
Rimonabant challenge enhances forskolin-stimulated adenylyl cyclase activity in cerebellum, but not in hippocampus, from THC-dependent mice. Challenge with rimonabant (Rim), but not vehicle (Veh), following subchronic THC administration significantly increased forskolin-stimulated adenylyl cyclase activity in cerebellum compared to the non-dependent mice that were challenged with vehicle (p < 0.05). No significant differences were observed in hippocampus. Data are expressed as a percent of the non-dependent mice that were challenged with rimonabant, n = 7–8. *Significantly different than mice treated subchronically with vehicle and challenged with rimonabant (p < 0.05; Tukey’s post hoc test)
Discussion
The results of the present study demonstrate that rimonabant precipitated spatial memory deficits in mice given a daily injection of THC (10 mg/kg) on five consecutive days, as assessed in the Morris water maze. During probe tests, THC-dependent mice undergoing precipitated withdrawal displayed longer swim path lengths and longer latencies to first reach the target location, and spent less time in the target zone than mice in the control groups. Rimonabant precipitated memory deficits in THC-dependent mice at lower doses than those required to precipitate somatic signs (i.e., paw tremors), suggesting that memory disruption is a particularly sensitive measure of cannabinoid withdrawal. Finally, rimonabant challenge to mice treated with this relatively mild THC subchronic dosing regimen elicited significant increases in forskolin-stimulated adenylyl cyclase activity in cerebellum, an effect opposite of the acute effects of THC. Of importance, this brain area has been proposed to mediate cannabinoid somatic withdrawal signs (Tzavara et al. 2000).
The observation that THC-dependent mice treated with rimonabant exhibited near-perfect performance in the cued task, in which the platform was made visible, suggests that sensorimotor or motivational deficits are unlikely to account for the poor performance during the probe trial. Moreover, the increased potency of rimonabant in precipitating memory deficits compared to precipitating paw tremors in THC-dependent mice suggests that somatic withdrawal responses did not interfere with water maze performance. Thus, it is unlikely that rimonabant elicited other actions in the THC-dependent mice that interfered with performance during the memory phase of the Morris water maze task, separately from mnemonic effects.
Another interpretation of the present studies is that rimonabant administered 30 min after the five acquisition trials interfered with probe trial performance because of state-dependent learning. However, the fact that rimonabant did not affect performance in the vehicle group indicates that antagonizing CB1 receptors per se does not impair spatial memory. This finding argues against a simple explanation of state-dependent learning. Instead, the state of THC withdrawal appears sufficient to impair short-term spatial memory. In the present study, an acute injection of THC (10 mg/kg) administered 4 h before the acquisition session profoundly disrupted probe trial performance in non-dependent mice (Fig. 1b, inset), but THC-dependent mice displayed excellent memory of the platform location (Fig. 1). A similar pattern of results was reported previously in THC-dependent rats assessed in the delayed non-match to sample operant (DNMS) task (Deadwyler et al. 1995). Rimonabant also disrupted DNMS performance in rats treated chronically with the full cannabinoid agonist, WIN 55,212-2 (Hampson et al. 2003). These results indicate that the memory disruptive effects of THC (Fig. 1b, inset) undergoes tolerance following repeated dosing (Fig. 1a, c; THC-dependent mice challenged with vehicle) while the withdrawal state elicits a rebound deficit in memory (Fig. 1a, c; THC-dependent mice challenged with rimonabant).
The observation that rimonabant did not affect spatial memory in non-dependent mice is consistent with previous results from our laboratory in which this drug did not affect performance in the working spatial memory task (i.e., Niyuhire et al. 2007; Varvel et al. 2006). However, rimonabant disrupts performance in a delayed extinction Morris water maze task (Varvel et al. 2005) as well as new reference memory learning (Robinson et al. 2008). Interestingly, rimonabant has been reported to enhance performance in other memory tasks, including delayed radial-arm maze (Lichtman 2000; Wise et al. 2007; Wolff and Leander 2003), social recognition (Terranova et al. 1996), and sequential memory (Deadwyler and Hampson 2008) tasks. Thus, the effects of rimonabant in various assays of learning and memory appear to be specific to the demands of the task. While rimonabant can enhance memory when the demands of the task require that specific information be retained, it can impair performance in certain tasks that require behavioral flexibility, such as extinction.
Rimonabant (10 mg/kg) increased paw flutters in the THC-treated mice indicating that somatic signs of withdrawal are found when the present THC dosing regimen (10 mg/kg dose daily for 5 days) is used. While a trend (p = 0.06) was observed for head shakes, no significant effect was revealed. The present findings confirm previous results that rimonabant (10 mg/kg) precipitates paw flutters in mice that have been administered THC (10 mg/kg) for five (Lichtman et al. 2001a) or six (Schlosburg et al. 2009) consecutive days. Some studies have reported that repeated exposure to low doses of THC significantly increased head shakes; yet, others have found a pattern of results similar to those observed here in which significant increases in head shakes were not observed in THC-dependent mice (Huang et al. 2009; Schlosburg et al. 2009; Wilson et al. 2006). In general, paw flutters are the principal somatic sign of withdrawal defining THC dependence in mice as they are the most consistently observed, quantifiable, and dose-responsive measure, whereas head shakes are indicative of withdrawal when high THC dosing regimens are used (Cook et al. 1998; Hutcheson et al. 1998; Lichtman et al. 2001a, b). It is also worth noting that somatic signs of withdrawal in THC-dependent mice are also species and strain specific (Lichtman and Martin 2005).
The present data support and extend previous findings using cell culture and primary neuronal cultures demonstrating a “superactivation” of adenylyl cyclase activity following an acute challenge with rimonabant in agonist-pretreated cells (Rhee et al. 2000). In whole animals, rimonabant precipitated withdrawal was accompanied with an overshoot in adenylyl cyclase activity in cerebellar membranes (Hutcheson et al. 1998; Tzavara et al. 2000). We sought to determine whether rimonabant superactivation of cerebellar adenylyl cyclase activation would also occur following a mild THC dosing regimen that led to rimonabant-impaired deficits in spatial memory. The results of the present study reveal that adenylyl cyclase superactivation in cerebellum occurs at lower subchronic THC doses (10 mg/kg THC once a day for 5 days) than those previously reported (Hutcheson et al. 1998; Tzavara et al. 2000). Additionally, as previous studies have shown (Hutcheson et al. 1998; Rubino et al. 2000a), no changes of adenylyl cyclase in the hippocampus were observed in the present study. However, it is plausible that functional changes might occur at other time points in subregions of the hippocampus or other brain structures (e.g., amygdala, cingulate cortex, and nucleus accumbens). The present study also did not find evidence that rimonabant elicited inverse agonist effects, as previously demonstrated (Landsman et al. 1997; Sim-Selley et al. 2001). It should be noted that studies reporting inverse agonist activity of rimonabant generally employ much higher concentrations of this drug than necessary to antagonize cannabinoid receptor agonists at the CB1 receptor. For example, Sim-Selley et al. (2001) found that rimonabant was more than 7,000 fold more potent in inhibiting WIN55,212-2-stimulated GTP-gamma-S binding than in inhibiting basal GTP-gamma-S binding.
A substantial body of evidence has extended the functional role of the cerebellum from being primarily involved in motor control to include non-motor cognitive behaviors. The cerebellum plays a role in processing spatial information and in the acquisition of procedural components of spatial tasks (Dahhaoui et al. 1992a; Flament et al. 1996; Joyal et al. 1996; Petrosini et al. 1996). Specifically, cerebellar lesions or Purkinje cell loss can disrupt spatial learning and spatial memory without disrupting motor behavior (Dahhaoui et al. 1992b; Joyal et al. 1996; Leggio et al. 1999; Martin et al. 2003). Tetrodoxin inactivation in discrete regions of the cerebellum has been shown to disrupt the formation and consolidation of fear memory (Sacchetti et al. 2002). Indeed, the cerebellum and its associated circuitry has been shown to play a major role in classical Pavlovian conditioning (for review see Thompson and Steinmetz 2009). Thus, biochemical changes in the cerebellum observed in THC-dependent mice undergoing precipitated withdrawal may not only mediate somatic withdrawal signs but also may contribute to short-term memory impairment.
Considerable evidence from human studies indicates that heavy marijuana use is associated with deficits in memory and attention (Bolla et al. 2002; Eldreth et al. 2004; Grant et al. 2003; McHale and Hunt 2008; Nestor et al. 2008; Schwartz et al. 1989; Solowij et al. 2002). Human studies also suggest that abstinence in cannabis-dependent subjects leads to impaired cognitive function (Harrison et al. 2002; Pope et al. 2001). For example, Pope et al. (2001) found that cannabis users undergoing abstinence performed poorly in memorizing word lists for up to 1 week, but performed as well as controls by day 28, though it is unclear whether this deficit is a consequence of withdrawal or residual drug effects. In survey studies, daily cannabis users reported that cognitive symptoms, difficulty concentrating, as well as other symptoms associated with withdrawal significantly contribute to relapse. (Budney et al. 2008; Mennes et al. 2009). These findings suggest that disrupted cognition is a relevant aspect of cannabis withdrawal. It is difficult to relate disrupted performance in the water maze directly to human cognition. Nonetheless, the present findings demonstrating that THC-dependent mice undergoing precipitated withdrawal have increased path lengths and decreased time spent in the target zone support the premise that impaired memory may represent a cannabis withdrawal symptom. Controlled laboratory studies will be required to establish whether these deficits are related to residual effects of the drug, long term consequences of cannabis use, or a consequence of withdrawal.
In conclusion, THC-dependent mice undergoing precipitated withdrawal displayed deficits in spatial memory. Rimonabant also precipitated paw tremors, a somatic withdrawal sign, in THC-dependent mice. However, rimonabant was more potent in impairing memory than in eliciting somatic withdrawal signs. A significant increase in forskolin-stimulated adenylyl cyclase activity in cerebellum, but not in hippocampus, was also found in THC-dependent mice challenged with rimonabant. These results are consistent with the tenant that the cerebellum may be intricately involved in somatic and cognitive cannabinoid withdrawal signs, but they do not preclude the involvement of other brain regions mediating the spatial working memory impairment observed in THC-dependent mice undergoing precipitated withdrawal. Taken together, the results of the present study support the assertion that impaired memory is a significant consequence of cannabis withdrawal, which may be mediated through compensatory changes in the cerebellum.
References
Aceto M, Scates S, Lowe J, Martin B (1996) Dependence on D9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther 278:1290–1295
Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002) Dose-related neurocognitive effects of marijuana use. Neurology 59:1337–1343
Brodkin J, Moerschbaecher JM (1997) SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther 282:1526–1532
Budney AJ, Hughes JR, Moore BA, Novy PL (2001) Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry 58:917–924
Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z (2008) Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat 35:362–368
Cook SA, Lowe JA, Martin BR (1998) CB1 receptor antagonist precipitates withdrawal in mice exposed to Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 285:1150–1156
da Silva GE, Takahashi RN (2002) SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry 26:321–325
Dahhaoui M, Lannou J, Stelz T, Caston J, Guastavino JM (1992a) Role of the cerebellum in spatial orientation in the rat. Behav Neural Biol 58:180–189
Dahhaoui M, Stelz T, Caston J (1992b) Effects of lesion of the inferior olivary complex by 3-acetylpyridine on learning and memory in the rat. J Comp Physiol A 171:657–664
Deadwyler SA, Hampson RE (2008) Endocannabinoids modulate encoding of sequential memory in the rat hippocampus. Psychopharmacology (Berl) 198:577–586
Deadwyler SA, Heyser C, Hampson RE (1995) Complete adaptation to the memory disruptive effects of delta-9-thc following 35 days of exposure. Neurosci Res Commun 17:9–18
Eldreth DA, Matochik JA, Cadet JL, Bolla KI (2004) Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage 23:914–920
Flament D, Ellermann JM, Kim SG, Ugurbil K, Ebner TJ (1996) Functional magnetic resonance imaging of cerebellar activation during the learning of a visuomotor dissociation task. Hum Brain Mapp 4:210–226
Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T (2003) Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc 9:679–689
Hampson RE, Deadwyler SA (2000) Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci 20:8932–8942
Hampson RE, Simeral JD, Kelly EJ, Deadwyler SA (2003) Tolerance to the memory disruptive effects of cannabinoids involves adaptation by hippocampal neurons. Hippocampus 13:543–556
Harrison GP Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2002) Cognitive measures in long-term cannabis users. J Clin Pharmacol 42:41S–47S
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991) Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583
Huang P, Liu-Chen LY, Unterwald EM, Cowan A (2009) Hyperlocomotion and paw tremors are two highly quantifiable signs of SR141716-precipitated withdrawal from delta9-tetrahydrocannabinol in C57BL/6 mice. Neurosci Lett 465:66–70
Hutcheson DM, Tzavara ET, Smadja C, Valjent E, Roques BP, Hanoune J, Maldonado R (1998) Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol 125:1567–1577
Jones RT, Benowitz N (1976) The 30-day trip—clinical studies of cannabis tolerance and dependence. In: Braude MC, Szara S (eds) Pharmacology of marihuana. Raven, New York, pp 627–642
Jones RT, Benowitz N, Bachman J (1976) Clinical studies of cannabis tolerance and dependence. Ann NY Acad Sci 282:221–239
Joyal CC, Meyer C, Jacquart G, Mahler P, Caston J, Lalonde R (1996) Effects of midline and lateral cerebellar lesions on motor coordination and spatial orientation. Brain Res 739:1–11
Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI (1997) SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol 334:R1–R2
Leggio MG, Neri P, Graziano A, Mandolesi L, Molinari M, Petrosini L (1999) Cerebellar contribution to spatial event processing: characterization of procedural learning. Exp Brain Res 127:1–11
Lichtman AH (2000) SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol 404:175–179
Lichtman AH, Martin BR (1996) D9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacol 126:125–131
Lichtman AH, Martin BR (2005) Cannabinoid tolerance and dependence. Handb Exp Pharmacol (168):691–717
Lichtman AH, Poklis JL, Poklis A, Wilson DM, Martin BR (2001a) The pharmacological activity of inhalation exposure to marijuana smoke in mice. Drug Alcohol Depend 63:107–116
Lichtman AH, Sheikh SM, Loh HH, Martin BR (2001b) Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther 298:1007–1014
Lichtman AH, Varvel SA, Martin BR (2002) Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fatty Acids 66:269–285
Maldonado R, Rodriguez de Fonseca F (2002) Cannabinoid addiction: behavioral models and neural correlates. J Neurosci 22:3326–3331
Mallet PE, Beninger RJ (1998) The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta-9-tetrahydrocannabinol or anandamide. Psychopharmacol 140:11–19
Martin LA, Goldowitz D, Mittleman G (2003) The cerebellum and spatial ability: dissection of motor and cognitive components with a mouse model system. Eur J Neurosci 18:2002–2010
Matsuda LA, Bonner TI, Lolait SJ (1993) Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol 327:535–550
McHale S, Hunt N (2008) Executive function deficits in short-term abstinent cannabis users. Hum Psychopharmacol 23:409–415
Mennes CE, Ben Abdallah A, Cottler LB (2009) The reliability of self-reported cannabis abuse, dependence and withdrawal symptoms: multisite study of differences between general population and treatment groups. Addict Behav 34:223–226
Nestor L, Roberts G, Garavan H, Hester R (2008) Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage 40:1328–1339
Niyuhire F, Varvel SA, Martin BR, Lichtman AH (2007) Exposure to marijuana smoke impairs memory retrieval in mice. J Pharmacol Exp Ther 322:1067–1075
Petrosini L, Molinari M, Dell’Anna ME (1996) Cerebellar contribution to spatial event processing: Morris water maze and T-maze. Eur J Neurosci 8:1882–1896
Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2001) Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58:909–915
Rhee MH, Nevo I, Avidor-Reiss T, Levy R, Vogel Z (2000) Differential superactivation of adenylyl cyclase isozymes after chronic activation of the CB(1) cannabinoid receptor. Mol Pharmacol 57:746–752
Robinson L, McKillop-Smith S, Ross NL, Pertwee RG, Hampson RE, Platt B, Riedel G (2008) Hippocampal endocannabinoids inhibit spatial learning and limit spatial memory in rats. Psychopharmacology (Berl) 198:551–563
Rubino T, Massi P, Vigano D, Fuzio D, Parolaro D (2000a) Long-term treatment with SR141716A, the CB1 receptor antagonist, influences morphine withdrawal syndrome. Life Sci 66:2213–2219
Rubino T, Vigano D, Massi P, Spinello M, Zagato E, Giagnoni G, Parolaro D (2000b) Chronic delta-9-tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in some rat brain regions [in process citation]. Neuropharmacology 39:1331–1336
Rubino T, Vigano D, Zagato E, Sala M, Parolaro D (2000c) In vivo characterization of the specific cannabinoid receptor antagonist, SR141716A: behavioral and cellular responses after acute and chronic treatments. Synapse 35:8–14
Sacchetti B, Baldi E, Lorenzini CA, Bucherelli C (2002) Cerebellar role in fear-conditioning consolidation. Proc Natl Acad Sci USA 99:8406–8411
Salomon Y (1979) Adenylate cyclase assay. Adv Cyclic Nucleotide Res 10:35–55
Schlosburg JE, Carlson BL, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH (2009) Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J 11:342–352
Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P (1989) Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child 143:1214–1219
Selley DE, Cassidy MP, Martin BR, Sim-Selley LJ (2004) Long-term administration of Delta9-tetrahydrocannabinol desensitizes CB1-, adenosine A1-, and GABAB-mediated inhibition of adenylyl cyclase in mouse cerebellum. Mol Pharmacol 66:1275–1284
Sim-Selley LJ, Brunk LK, Selley DE (2001) Inhibitory effects of SR141716A on G-protein activation in rat brain. Eur J Pharmacol 414:135–143
Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J (2002) Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 287:1123–1131
Substance Abuse and Mental Health Services Administration (SAMHSA). (2010). Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586Findings). Rockville, MD
Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P (1996) Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacol 126:165–172
Thompson RF, Steinmetz JE (2009) The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience 162:732–755
Tsou K, Patrick S, Walker JM (1995) Physical withdrawal in rats tolerant to D9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. Eur J Pharmacol 280:R13–R15
Tzavara ET, Valjent E, Firmo C, Mas M, Beslot F, Defer N, Roques BP, Hanoune J, Maldonado R (2000) Cannabinoid withdrawal is dependent upon PKA activation in the cerebellum. Eur J Neurosci 12:1038–1046
Vandrey R, Budney AJ, Kamon JL, Stanger C (2005) Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depend 78:205–210
Vandrey RG, Budney AJ, Hughes JR, Liguori A (2008) A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, to both substances. Drug Alcohol Depend 92:48–54
Varvel SA, Lichtman AH (2002) Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther 301:915–924
Varvel SA, Anum EA, Lichtman AH (2005) Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 179:863–872
Varvel SA, Cravatt BF, Engram AE, Lichtman AH (2006) Fatty acid amide hydrolase (−/−) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. J Pharmacol Exp Ther 317:251–257
Wilson DM, Varvel SA, Harloe JP, Martin BR, Lichtman AH (2006) SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacol Biochem Behav 85:105–113
Wise LE, Iredale PA, Stokes RJ, Lichtman AH (2007) Combination of rimonabant and donepezil prolongs spatial memory duration. Neuropsychopharmacology 32:1805–1812
Wolff MC, Leander JD (2003) SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol 477:213–217
Conflicts of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding sources: This research was supported by the National Institute on Drug Abuse (R01DA015683, R01DA02396, R01DA003672, R01DA014227, P01DA009789, and T32DA07027).
Rights and permissions
About this article
Cite this article
Wise, L.E., Varvel, S.A., Selley, D.E. et al. ∆9-Tetrahydrocannabinol-dependent mice undergoing withdrawal display impaired spatial memory. Psychopharmacology 217, 485–494 (2011). https://doi.org/10.1007/s00213-011-2305-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2305-5