Abstract
Rationale and objective
Intravenous infusions of nicotine appear to exert little primary reinforcing effects in adult rats but, instead, maintain self-administration behavior at least, in part, by increasing the intrinsic reinforcing effects of drug-paired sensory stimuli. The present study examined instead the impact of a motivationally neutral cue on self-administration.
Methods
Adult male Long-Evans rats were permitted to self-administer nicotine (0.015 mg/kg IV given over 30 s, 2 h/day) or saline presented with or without a sensory stimulus (light, white noise). Fixed and progressive ratio reinforcement schedules of nicotine reinforcement were tested. Experiment 2 determined whether noncontingent nicotine or mecamylamine (nicotinic antagonist) would induce lever pressing for either sensory stimulus. Experiment 3 tested whether the white noise stimulus alone could maintain responding after repeated pairing with self-administered nicotine. Finally, the sensory stimuli were assessed for possible aversive properties.
Results
Nicotine infusions alone were at best weakly reinforcing. The white noise stimulus, presented alone, was neither reinforcing nor aversive, whereas the white light appeared marginally reinforcing. Both stimuli, however, facilitated intravenous nicotine self-administration. Neither nicotine nor mecamylamine challenge rendered the white noise reinforcing. The white noise, after being self-administered with nicotine, failed to maintain self-administration behavior on its own.
Conclusions
Even a motivationally neutral sensory stimulus, lacking detectable primary or secondary reinforcing properties, can facilitate self-administration of nicotine. Possibly, drug-paired stimuli provide a "response marker" or serve as a temporal bridge between the operant response and drug effect. Motivationally neutral stimuli may therefore serve to isolate primary reinforcing effects of nicotine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although nicotine is considered to play a central role in tobacco dependence (Stolerman and Jarvis 1995; United States Department of Health and Human Services 1988), it tends to act as a weak primary reinforcer in animals (Chaudhri et al. 2007; Donny et al. 2003; Manzardo et al. 2002). For example, nicotine appears to be consistently reinforcing only at unit doses of 0.06 mg/kg or higher in rats, when the drug is not paired with a sensory stimulus (Caggiula et al. 2002; Chaudhri et al. 2005, 2007; Donny et al. 2003; Palmatier et al. 2006). Such unit doses far exceed the 0.001–0.003 mg/kg obtained from a single cigarette puff in human smokers (Matta et al. 2007).
Intravenous nicotine is typically self-administered together with drug-paired sensory cues. The latter are frequently reinforcing in their own right (Stewart 1960; Stewart and Hurwitz 1958) and can also acquire secondary reinforcing properties (Cohen et al. 2005; Palmatier et al. 2007a). Nicotine increases the efficacy of a variety of primary and secondary nondrug reinforcers (Brunzell et al. 2006; Chaudhri et al. 2006c; Clarke and Kumar 1984; Olausson et al. 2004a, c; Palmatier et al. 2007a; Raiff and Dallery 2008), and in rodent self-administration studies, nicotine synergizes with drug-paired cues through a process termed "reinforcement enhancement" (Caggiula et al. 2002, 2009; Donny et al. 2003). Both response-contingent and noncontingent administration of nicotine enhances responding for such stimuli (Caggiula et al. 2009; Chaudhri et al. 2006a, b; Donny et al. 2003; Palmatier et al. 2006, 2007a, b). Hence, reinforcement enhancement appears to be a major facilitator of nicotine self-administration.
The reinforcement-enhancing effects of nicotine are most evident for strong conditioned or unconditioned reinforcers (Chaudhri et al. 2006a; Palmatier et al. 2007b). However, in a different study, active lever responding for intravenous nicotine was enhanced 4- to 5-fold by the addition of an apparently neutral compound sensory stimulus (Caggiula et al. 2002; see Fig. 4 therein). This observation suggests that even motivationally neutral stimuli may, in some situations, enhance nicotine self-administration, although it is also possible that in this experiment, the sensory stimulus had acquired secondary reinforcing properties through repeated association with self-administered nicotine.
Currently, rodent studies of intravenous nicotine self-administration almost invariably employ rapid infusions (e.g., 1 s duration), whereas, in human smokers, arterial nicotine levels rise only gradually, peaking around 20–25 s following a cigarette puff (Rose et al. 1999). Recently, we established that 30-s infusions of nicotine are at least as reinforcing as more rapid infusions and are reliably self-administered across a wide dose range of 0.003–0.06 mg/kg/infusion; throughout this study, nicotine infusions were paired with a white light cue which itself did not appear to be reinforcing (Sorge and Clarke 2009).
The present study addressed several questions. The first was whether rats will reliably self-administer slow infusions (30 s) of nicotine in the absence of a sensory cue. We chose a dose of nicotine (0.015 mg/kg/inf) that produces near-maximal self-administration in the presence of a visual cue (Sorge and Clarke 2009). The second question was whether a response-contingent, but initially motivationally-neutral, sensory stimulus could enhance nicotine self-administration; having found this to be the case, we then tested whether acute noncontingent nicotine administration was able to impart positive reinforcing effects to this stimulus. Here, we tested several different modes of nicotine administration that have proven effective in previous studies (Donny et al. 2003; Liu et al. 2007; Palmatier et al. 2007b). In parallel, we also tested a centrally-active nicotinic receptor antagonist (mecamylamine); by inhibiting neuronal nicotinic receptor function, the intention was to mimic nicotinic receptor desensitization that can occur after acute nicotine administration (Picciotto et al. 2008). We further tested whether repeated association with nicotine would impart secondary reinforcer characteristics to one of the two sensory stimuli (white noise). Lastly, we investigated whether our sensory stimuli (white light, white noise) possessed aversive properties.
Materials and methods
Subjects
The subjects were 114 experimentally naive male Long-Evans rats (Charles River Laboratories, St. Constant, QC) weighing 300–350 g at surgery, housed in humidity-controlled (50–60%) rooms in the McGill University Animal Research Centre. They were housed in a normal-cycle (lights ON 0700 h, OFF 1900 h) room, since reverse-cycle housing was unavailable. Thus, all behavioral testing was done during the light phase of the cycle between 0900 h and 1700 h daily due to resource restrictions. Animals were housed three to a cage with food and water available ad libitum except during testing. All procedures were carried out according to the guidelines of the Canadian Council on Animal Care and were approved by an animal ethics committee at McGill University.
Surgery
Rats were implanted with intravenous (IV) silastic catheters (ID 0.51 mm, OD 0.94 mm; Fisher Scientific, Montreal, QC) in the right jugular vein under general anesthesia (ketamine 80 mg/kg and xylazine 16 mg/kg). The catheter was secured to the vein with silk sutures and was passed subcutaneously to the top of the skull where it was connected to a modified cannula (C313G-5UP; Plastics One, Roanoke, VA) mounted to the skull with jeweler's screws and dental cement. Following surgery, dipyrone (100 mg/kg; Vetoquinol, QC) was administered for pain management. A plastic blocker was placed over the opening of the cannula when not in use, and catheters were flushed on alternating days with 0.1 ml of saline or a solution of heparin (0.2 mg/ml), enrofloxacin (15 mg/kg; Baytril), and saline. Animals were allowed 5–7 days of recovery following surgery.
Apparatus
Training and testing were performed in commercially available Med Associates operant conditioning chambers (ENV-008CT, Med Associates, Lafayette, IN) housed individually in custom-made melamine cubicles. Each box was equipped with two retractable levers (ENV-112CM) located 10 cm apart and 8 cm above the stainless steel bar floor. One lever was designated "active" and the other "inactive"; responses on the latter were recorded but had no consequence. Levers were counterbalanced within each group of subjects. Visual stimuli were provided by a white stimulus light (2.5-cm diameter, 28 V, 100 mA, ENV-221M) situated 3 cm above each lever. Auditory stimuli were provided by a white noise amplifier and loudspeaker (ENV-225SM) situated on the wall opposite the levers.
In Experiments 1 and 3, responding on the active lever resulted in a 30 s infusion which was associated with a 30 s visual or auditory stimulus or no stimulus, depending on the group assignment (see the following section). During each 30-s infusion, active lever responding was recorded but had no further consequence. In Experiment 2, responses on the active lever resulted in stimulus presentation only. In Experiment 4, the rats were permitted to terminate the visual or auditory stimulus. Here, the white light or white noise was turned on at the start of the session and a response on the active lever turned off the stimulus for 120 s during which time further presses on the active lever were recorded but had no consequence. At the end of the 120-s timeout, the stimuli were turned back on.
-
Experiment 1:
tests of nicotine self-administration with or without sensory stimuli
The purpose of this experiment was: (1) to determine whether slow (30-s) infusions of nicotine are self-administered in the absence of a sensory cue, and (2) to determine whether response-contingent neutral sensory stimuli can enhance nicotine self-administration. Without prior food training or restriction, rats were placed in operant conditioning chambers for 2 h/day and permitted to self-administer one of six combinations of drug and stimuli in a 2 × 3 design (n = 7–8), i.e., saline (SAL) or nicotine (NIC) combined with either white light (WL), white noise (WN), or no stimulus. Rats were trained for 15 days on a fixed ratio 1 (FR1) schedule of reinforcement then moved to an FR2 schedule for days 16–18 and finally placed on a progressive ratio (PR) schedule for one day. The progressive ratio used the following sequence of required number of presses: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, etc. In this experiment, nicotine was self-administered at a dose of 0.015 mg/kg/infusion over 30 s. The experiment was completed in two replicates and the results pooled.
-
Experiment 2:
responding for visual or auditory stimuli with or without noncontingent drug challenge
The aim of this experiment was to determine whether noncontingent administration of nicotine or of the centrally-active nicotinic receptor antagonist mecamylamine would enhance responding for the sensory stimuli (i.e., white light or white noise). Rats in this experiment were catheterized, allowed 5–7 days to recover, and then repeatedly exposed to IV nicotine in daily 2-hour sessions in the operant conditioning chambers for seven consecutive days (days 1–7) in the absence of cues or levers. In each session, nicotine was continuously infused to a total dose of 0.18 mg/kg. This dose was designed to approximate the mean total dose self-administered in Experiment 1 (NIC+WL group). Repeated nicotine infusions helped to habituate the rats to the infusion procedure and to provide experience with the drug prior to behavioral testing. Next, on days 8–12, rats were randomly allocated to two groups and permitted to press the active lever to obtain either a 30-s white light (n = 11) or 30 s of white noise (n = 10) on an FR1 schedule.
Test sessions continued in the same manner on days 13–25 except that each rat received one of five drug tests with two drug-free (control) sessions between each. The five test conditions were presented in random order: (1) NIC Pulse-IV infusions of nicotine of 0.003 mg/kg, a dose provided by 1–2 cigarette puffs (see Matta et al. 2007 for a review), in 0.1 ml given over 30 s, repeated every 120 s throughout the 120 min session; (2) NIC Cont, a continuous IV infusion of nicotine totaling 0.18 mg/kg over 120 min; (3) NIC acute nicotine 0.1 mg/kg SC injected immediately presession; (4) MEC Cont, continuous mecamylamine IV infusion of 1 mg/kg over 120 min; and (5) MEC acute mecamylamine 1 mg/kg SC immediately presession. Doses of nicotine either approximated the nicotine intake associated with self-administration (i.e., NIC+WL group in Experiment 1) or were intended to provide plasma levels relevant to human smokers (see “Discussion”).
-
Experiment 3:
testing for secondary reinforcement following repeated pairing of the white noise with nicotine
Three groups of rats (n = 7–8/group) were initially permitted to self-administer nicotine (0.015 mg/kg/inf) paired with 30 s of white noise on a FR1 schedule for a 7-day period. After this “Training” phase, the rats were switched to one of the following reinforcement contingencies: saline paired with white noise (SAL+WN), saline with no associated stimuli (SAL alone), or nicotine with no associated stimuli (NIC alone). Here, as throughout the experiment, the assignment of the active lever remained constant. After 4 days of the new contingencies (the “Switch” phase), the rats were briefly returned to the initial Training contingencies (“Retraining”) for 3 days. One additional group of rats (control, n = 8) was permitted to self-administer saline coupled with white noise throughout the entire experiment.
-
Experiment 4:
testing for possible aversive properties of the visual and auditory stimuli
Depending on group (n = 8/group), rats were permitted to lever press in order to turn off (for 120 s at a time) either a continuously illuminated white light or a continuous white noise. Three reinforcement schedules were tested: FR1 (days 1–7), FR5 (days 8–10), and PR (days 11–15).
Drugs
(−)-Nicotine hydrogen tartrate salt (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile 0.9% saline and the solution adjusted to pH 7.1–7.3 with NaOH. For IV self-administration, a stock solution of 1 mg/ml nicotine was frozen in aliquots and thawed to prepare fresh drug solution for self-administration each day. For SC administration, nicotine was given in a volume of 1 ml/kg. Mecamylamine HCl (Merck Research Laboratories, Rahway, NJ) was dissolved in saline and either given by constant IV infusion to a final dose of 1 mg/kg or given by acute SC injection (1 mg/kg). All doses of nicotine and mecamylamine are expressed as base.
Statistical analysis
Infusions and responses on active and inactive levers were analyzed by the SYSTAT v11 software (SPSS Inc., Chicago, IL). Analysis of variance (ANOVA) was used. Between-subjects factors were as follows: drug (nicotine or saline, Experiment 1), group (Experiment 3), and stimulus (light, white noise, or no stimulus). Within-subjects factors were lever (active, inactive), drug (Experiment 2), and phase (training, switch, retraining, Experiment 3). ANOVA p values were subjected to the Huynh-Feldt correction where appropriate. Extreme outliers (based on studentized residuals; see “Results”) were excluded from parametric analyses and graphs. Where heteroscedasticity (i.e., unequal variances) would have invalidated full parametric ANOVA, subsets of the data were selected as explained in “Results”. Comparisons between specific groups or conditions were made by Tukey's test or unprotected t tests. Throughout, a 2-tailed p value of less than 0.05 was considered significant.
Results
-
Experiment 1:
tests of nicotine self-administration with or without sensory stimuli
Performance on the FR1 schedule appeared relatively stable across the final 10 days (Fig. 1), which were collapsed for statistical analysis (Fig. 2a). The 3 days of FR2 testing were similarly pooled (Fig. 2b). The experimental design was thereby reduced to three factors: lever (active vs. inactive), stimulus (no cue vs. light vs. noise), and drug (nicotine vs. saline). For a given reinforcement schedule, variances differed markedly between conditions, so that it was not possible to perform a 3-way ANOVA incorporating all six groups. Instead, the nicotine and saline self-administering groups were analyzed in separate 2-way ANOVAs with lever and stimulus as within- and between-subject factors, respectively. One rat in the NIC + WL group was identified as an outlier (active lever responding, studentized residual >10) and was excluded throughout. On the PR schedule, one outlier in the SAL + WL group was excluded (active lever responding, studentized residual = 14).
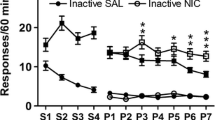
The mean (±SEM) number of infusions (a), active lever presses (b), and inactive lever presses (c) on successive days of training on FR1, FR2, and PR schedules. Depending on group assignment (see legend in panel a), rats had access to either nicotine (NIC) or saline (SAL) infusions combined with either a white light (WL), white noise (WN), or no stimulus; n = 6–8/group
The mean (+SEM) number of active and inactive lever presses per 120-min session on the FR1 (a), FR2 (b), and PR (c) schedules of reinforcement in Experiment 1. Depending on group, rats had access to either nicotine (NIC) or saline (SAL) infusions combined with a white light (WL), white noise (WN), or no stimulus. The FR1 schedule data were pooled across the final 10 days of testing; the FR2 schedule data were pooled across all 3 days of testing, n = 7–8/group. The inset represents the mean (+SEM) number of infusions taken in the 120-min session for each of the groups listed. Multiple comparisons were performed separately for the NIC and SAL groups and revealed significant group differences for only active lever pressing (Tukey's, *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001). Active and inactive lever pressing were compared to determine lever selectivity (paired t tests, #p < 0.05, ##p < 0.01, ###p < 0.005)
In the absence of sensory cues, nicotine infusions were self-administered significantly more than saline infusions on the FR1 (t = 2.49, df 8, p < 0.05) and FR2 schedules (t = 4.11, df 13, p < 0.005) with a similar trend on the PR schedule (p = 0.14). These data are shown in the Fig. 2 insets. However, there was little evidence for discriminated (i.e., active > inactive lever) responding for nicotine alone across all schedules (see following section). Generally, the light and white noise stimuli significantly increased response rates and/or number of infusions on all three reinforcement schedules but only in the groups permitted to self-administer nicotine (see Table 1 for ANOVA results). Findings for these nicotine groups are discussed next.
On the FR1 schedule, the two sensory stimuli produced comparable increases in FR1 active lever responding for nicotine, although only the effect of the light cue was significant (Tukey's test, p = 0.035 vs. p = 0.08, respectively). However, this enhancement did not appear selective for the active vs. inactive lever (Lever x stimulus interaction, Table 1). The light and white noise stimuli also significantly increased the number of nicotine infusions (Fig. 2a inset: Tukey's test, p = 0.001 and 0.01, respectively).
FR2 responding in the nicotine groups was also significantly increased by the cues with a preferential effect on active lever presses (Fig. 2b, Table 1). Here, both the light and the white noise were effective (Tukey's test, p < 0.001 and p < 0.05, respectively), and neither cue was significantly more effective than the other (p = 0.17). The cues also increased the number of nicotine infusions (Fig. 2b inset: Tukey's test, p < 0.0001 for each stimulus), but here, the light was significantly more effective (Tukey's test, p < 0.02).
PR responding for nicotine was also enhanced by the sensory cues. The sensory cues preferentially increased responding on the active lever (Fig. 2c, Table 1), and this facilitatory effect was significant for both the light and the white noise (Tukey's test, p < 0.01 and p < 0.001, respectively); in this regard, the light was not significantly more effective (Tukey's test, p = 0.23). Nicotine intake was enhanced by both light and white noise (Fig. 2c inset: Tukey's test, p < 0.0001 and p < 0.01, respectively), and here, the light cue was significantly more effective (Tukey's test, p < 0.02).
In the three groups permitted to self-administer saline rather than nicotine, the sensory cues did not significantly alter responding or number of infusions on any reinforcement schedule (Fig. 2; stimulus main effects and stimulus x lever interactions, all p > 0.2).
For the FR1 schedule, only two of the six groups responded significantly more on the active vs. inactive lever: NIC + WL and NIC alone (paired t tests, t(6) = 2.50, p < 0.05 and t(7) = 2.57, p < 0.05, respectively; Fig. 2a). For the FR2 and PR schedules, significant discrimination occurred only for the NIC+WL and NIC+WN groups (for FR2, t(6) = 4.67, p < 0.01, and t(7) = 2.78, p < 0.05, respectively, Fig. 2b; for PR, t(6) = 4.09, p < 0.01, and t(7) 4.92, p < 0.005; Fig. 2c).
-
Experiment 2:
responding for visual or auditory stimuli with or without noncontingent drug challenge
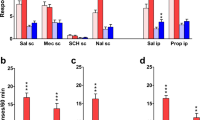
One rat from each group (i.e., light and white noise, n = 11–12) was identified as an outlier (active lever responding, studentized residuals >15) and hence, excluded from analysis. A comparison of active vs. inactive lever presses from the five drug-free control sessions revealed that neither the light nor the white noise was detectably reinforcing (paired t tests, respectively: t(10)=0.03, p > 0.05, and t(9)=0.77, p > 0.05; Fig. 3a and b). In neither the light nor white noise group was there a significant main effect of drug or drug x lever interaction (p > 0.05 for all).
-
Experiment 3:
testing for secondary reinforcement
Responding for the white light (a) or white noise (b) stimuli in rats tested acutely with nicotine or mecamylamine (Experiment 2). Data represent the mean (+SEM) number of active (black bars) and inactive (white bars) lever responses made on a FR1 schedule during the 120-min sessions (n = 10–11). The Before Test condition represents the control data pooled from the five nondrug days each occurring immediately prior to one of the test days. NIC pulse, 60 × 0.003 mg/kg nicotine (each over 30 s, every 120 s); NIC cont, 0.18 mg/kg by continuous infusion; NIC acute, 0.1 mg/kg, SC; MEC cont, mecamylamine 1 mg/kg by continuous infusion; MEC acute, 1 mg/kg, SC The inset represents the mean (+SEM) number of infusions and/or stimulus presentations corresponding to the conditions shown on the x-axis
As shown in Fig. 4, the saline/white noise combination was not reinforcing on its own (Control group), whereas the combination of the white noise and nicotine was clearly self-administered by the three remaining groups. The latter all showed a sharp decline in nicotine intake following the switch in reinforcement contingencies (t tests, p < 0.02 to 0.001) suggesting that the combination of nicotine and white noise was significantly more reinforcing than either component alone. Following the contingency switch, none of the groups responded preferentially on the active lever (Fig. 1). Hence, the white noise had acquired little or no secondary reinforcing characteristics, and nicotine alone was not detectably reinforcing. In the brief retraining phase, some reinstatement of responding was apparent, although this was significant only in the SAL Alone switch group.
-
Experiment 4:
testing for aversive properties of the sensory stimuli
The effect of changing reinforcement contingencies on operant responding in Experiment 3 for the training (black bars), switch (white bars), and retraining (gray bars) phases. The table (below c) displays group contingencies. The y-axes show mean (+SEM) number of a infusions, b active lever presses, and c inactive lever presses in the last 2 days of each phase (n = 7-8/group). Switching the lever contingencies resulted in a significant reduction in active lever pressing in all the groups (with the exception of the control group). *p < 0.05, ***p < 0.001, unprotected paired t tests
On each reinforcement schedule, individual rats turned off the light or white noise only a few times within each 120-min session (Fig. 5). Since each stimulus termination was brief (i.e., 2 min), the light or white noise was on for the great majority of the session. Rats made between 5 and 15 active lever responses per session with no clear evidence of discrimination between active and inactive levers. In particular, although the light group tended to press more on the active lever than the inactive lever, this was not confirmed statistically (main effect of lever, lever x schedule interaction both p > 0.05).
Active and inactive lever pressing in rats permitted to turn off the white light (WL) or the white noise (WN) in Experiment 4 on FR1, FR5, and PR schedules of reinforcement. Shown are the mean (+SEM) number of lever presses on the last 5 days of FR1, the 3 days of FR5, and the 5 days on the PR schedule (n = 8). The inset represents the corresponding mean (+SEM) number of stimulus terminations for rats in the white light (hatched bar) or white noise (stippled bar) groups during the FR1, FR2, and PR schedules
Discussion
Main findings
The present study yielded three main sets of observations. First, slow (30-s) nicotine infusions were mildly reinforcing when presented alone, and the white noise stimulus alone appeared neither reinforcing nor aversive. Nevertheless, the combination of nicotine infusions and the auditory stimulus produced behavioral synergy. Second, the white noise stimulus gained no detectable secondary reinforcing capacity after repeated pairing with nicotine. Third, noncontingent nicotine did not significantly increase responding for either the white light or white noise. Taken together, these results indicate that the white noise stimulus was motivationally neutral and was not rendered reinforcing by acute noncontingent nicotine or repeated pairing with nicotine; nevertheless, this stimulus facilitated nicotine self-administration. Sensory stimuli have been repeatedly shown to enhance nicotine self-administration (Caggiula et al. 2009; Goldberg et al. 1981); but to our knowledge, the present study provides the first clear evidence that a motivationally neutral stimulus has this ability.
Nicotine infusions alone were at best only weakly reinforcing
We recently observed that adult rats would reliably work for slow (i.e., 30-s) infusions of nicotine (0.003–0.060 mg/kg) that are coupled with a light cue (Sorge and Clarke 2009). In the present study, 30-s infusions of nicotine (0.015 mg/kg/inf) were tested for the first time in the absence of sensory cues and found to be only weakly reinforcing (Experiment 1). Specifically, rats tended to respond more for nicotine than for saline infusions (on FR1 and FR2 schedules), but a statistically significant preference for the active lever occurred only on the FR1 schedule. Interestingly, the rats self-administering nicotine alone in Experiment 3 during the Switch phase did not show this same preference for the active lever suggesting little reinforcing effect of nicotine. However, only in Experiment 1 were rats initially trained with this nicotine-alone contingency, and thus the lack of reinforcement in Experiment 3 may have been due to a contrast effect following the switch.
In the absence of sensory cues, bolus (i.e., 1-s) infusions of nicotine usually exert only marginally reinforcing effects; the present report generalizes this finding to slow infusions. With bolus delivery, the primary reinforcing effect of nicotine only becomes robust at high (i.e., 0.06 or 0.09 mg/kg/inf) doses in rats (Chaudhri et al. 2005, 2007). An intermediate dose of 0.03 mg/kg, standard in many intravenous self-administration studies, has been reported to produce undetectable (Chaudhri et al. 2005, 2007; Donny et al. 2003, Expt. 1), weak (Caggiula et al. 2002; Palmatier et al. 2006), or moderately strong primary reinforcing effects (Donny et al. 2003, Expt. 5), all within the same laboratory. Although it is possible that slow (30-s) infusions of nicotine would become robustly reinforcing at doses higher than the 0.015 mg/kg used in the present study, even a dose as "low" as 0.015 mg/kg/infusion is roughly equivalent, in terms of mg/kg delivered, to the nicotine yield from an entire cigarette (Matta et al. 2007).
Motivational valence of sensory stimuli
The auditory (white noise) stimulus, when presented alone, was neither detectably reinforcing (Experiments 1–3) nor detectably aversive (Experiment 4), suggesting neutrality. The cue light, in contrast, tended to engender preferential responding on the active lever in Experiment 1 but not in Experiment 2, indicating that it may have been marginally reinforcing. Since this visual stimulus was also intended to be motivationally neutral, it was presented alone rather than combined with house light extinction as in other studies (Caggiula et al. 2002; Donny et al. 2003).
Possible mechanisms underlying synergy between sensory stimuli and nicotine
Both the white noise and light cue significantly increased intake of nicotine and active lever responding (Experiment 1), especially on FR2 and PR schedules of reinforcement. These super-additive effects could conceivably have arisen via several mechanisms: (1) nicotine might have enhanced primary reinforcing effects of sensory stimuli (Chaudhri et al. 2006b), (2) the drug might have acutely rendered neutral sensory stimulus reinforcing, (3) through association with nicotine, sensory stimuli might have developed secondary reinforcing properties (Cohen et al. 2005; Palmatier et al. 2007a), and (4) the latter might, in turn, have been augmented by nicotine (Olausson et al. 2004b; Palmatier et al. 2007a). These possibilities are now discussed.
It is unlikely that nicotine enhanced primary reinforcing effects of the sensory stimuli for two reasons. First, the white noise was not reinforcing in our experiments, and the light cue appeared only marginally so at best. Second, noncontingent nicotine administration failed to increase lever pressing detectably for either the white noise or the light (Experiment 2). This finding also suggests that acute nicotine administration did not somehow render a neutral sensory stimulus reinforcing. Experiment 2 tested the effects of three nicotine challenges: continuous infusion, multiple low-dose pulses, and subcutaneous injection. The infusion dose (0.18 mg/kg IV) was chosen to approximate the amount self-administered per session in Experiment 1 (NIC + WL group). The nicotine pulses collectively provided the same total dose, each pulse approximating two cigarette puffs on the basis of milligrams per kilogram delivered (Matta et al. 2007). The acute subcutaneous dose was lower than that previously used to show reinforcement enhancement (Palmatier et al. 2007b) but was chosen to provide plasma nicotine levels within the range of cigarette smokers (Pratt et al. 1983).
Experiment 2 tested the effects of passive nicotine rather than self-administered nicotine on responding for the sensory stimuli. However, this difference was probably not critical since the reinforcement enhancement effect of nicotine generalizes across several modes and routes of response-independent or contingent administration (Chaudhri et al. 2006a, 2007; Donny et al. 2003; Palmatier et al. 2007b).
Synergy might also have occurred in Experiment 1 if the visual or auditory stimuli had developed conditioned reinforcing properties through repeated association with nicotine. However, in Experiment 3, the sensory stimulus (white noise) alone was found incapable of maintaining responding when nicotine was withdrawn after the training period. In the present experiment, stimulus–nicotine pairings extended over a single week of training, a period sufficient to reveal an enhancement of nicotine self-administration in Experiment 1. Presumably, more extensive Pavlovian pairing would have been necessary for significant conditioned reinforcement to occur (Palmatier et al. 2007a).
In summary, none of the above mechanisms readily account for the behavioral synergy observed in the present study. This is especially the case with the auditory stimulus, which was clearly motivationally neutral. Instead, we propose two additional mechanisms by which a neutral sensory stimulus may potentiate nicotine self-administration. First, the auditory cue may provide a "marker", making the immediately preceding instrumental response more salient. Evidence suggests that response marking can enhance acquisition of instrumental conditioning tasks (Williams 1994), but whether it would acutely improve performance (Experiment 3) is not clear.
The second proposed mechanism is that a motivationally neutral stimulus may serve to bridge the temporal gap between operant response and the pharmacological actions of the drug. In the present study, the use of slow (30-s) infusions may have delayed the pharmacological actions of nicotine sufficiently to make temporal bridging important especially on noncontinuous schedules of reinforcement (i.e., FR2 and PR). Temporal bridging has been characterized mainly in the context of Pavlovian conditioning (autoshaping; Rescorla 1982; Williams 1994). In instrumental learning procedures, response marking, temporal bridging, and secondary reinforcement can clearly all play a role, but their relative importance appears task-dependent (Williams 1994).
Methodological issues
In comparing our findings to other published work, some methodological issues should also be noted. First, rats used in intravenous self-administration experiments are commonly trained to lever press for food pellets while food restricted and subsequently permitted to respond on the same active lever for nicotine infusions. This reinforcement history potentially confounds the interpretation of discriminated (i.e., active vs. inactive lever) responding for drug, at least in initial nicotine self-administration sessions (Liu et al. 2006). Our animals were neither trained to lever press for food nor were they food-deprived.
In the present study, we used 30-s infusions in order to approximate the lung-to-blood transfer time seen in cigarette smokers (Rose et al. 1999), and we tested only one dose of nicotine. Whether the kind of nicotine-cue synergy noted here would generalize to the commonly-used bolus (e.g., 1-s) infusions or to other doses of nicotine remain questions for the future.
Conclusions
Sensory stimuli that are paired with nicotine infusions can enhance self-administration behavior through several mechanisms. Parsing the relative contribution of each mechanism is not straightforward. The present findings demonstrate for the first time that even a motivationally neutral stimulus can significantly enhance nicotine intravenous self-administration. This effect is independent of the well-established reinforcement enhancing effects of nicotine. Instead, neutral stimuli likely act as response markers and/or serve as a temporal bridge between the operant response and nicotine infusion. Thus, the use of motivationally neutral stimuli may help to isolate the weak primary reinforcing effects of nicotine.
Abbreviations
- DA:
-
dopamine
- FR:
-
fixed ratio
- PR:
-
progressive ratio
References
Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR (2006) Beta2-subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 184:328–338
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 163:230–237
Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009) The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv 55:91–109
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA (2005) Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 180:258–266
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF (2006a) Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 189:27–36
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006b) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 184:353–366
Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF (2006c) Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 184:353–366
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF (2007) Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 190:353–362
Clarke PB, Kumar R (1984) Effects of nicotine and d-amphetamine on intracranial self-stimulation in a shuttle box test in rats. Psychopharmacology (Berl) 84:109–114
Cohen C, Perrault G, Griebel G, Soubrie P (2005) Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30:145–155
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 169:68–76
Goldberg SR, Spealman RD, Goldberg DM (1981) Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science 214:573–575
Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN (2006) Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology (Berl) 184:417–425
Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF (2007) Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology (Berl) 194:463–473
Manzardo AM, Stein L, Belluzzi JD (2002) Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res 924:10–19
Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190:269–319
Olausson P, Jentsch JD, Taylor JR (2004a) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171:173–178
Olausson P, Jentsch JD, Taylor JR (2004b) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 171:173–178
Olausson P, Jentsch JD, Taylor JR (2004c) Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 173:98–104
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF (2006) Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 184:391–400
Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF (2007a) Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 195:235–243
Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF (2007b) The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend 89:52–59
Picciotto MR, Addy NA, Mineur YS, Brunzell DH (2008) It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84:329–342
Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C (1983) Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology (Berl) 81:54–60
Raiff BR, Dallery J (2008) The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology (Berl) 201:305–314
Rescorla RA (1982) Effect of a stimulus intervening between CS and US in autoshaping. J Exp Psychol Anim Behav Processes 8:131–141
Rose JE, Behm FM, Westman EC, Coleman RE (1999) Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend 56:99–107
Sorge RE, Clarke PB (2009) Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists. J Pharmacol Exp Ther 330:633–640
Stewart J (1960) Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol 53:187–193
Stewart J, Hurwitz HMB (1958) Studies in light-reinforced behaviour III. The effect of continuous, zero and fixed-ratio reinforcement. J Comp Physiol Psychol 10:56–61
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117:2–10 discussion 14-20
United States Department of Health and Human Services U (1988) Nicotine addiction: a report of the surgeon general. Office of the Assistant Secretary for Health, Office on Smoking and Health, Rockville, MD
Williams BA (1994) Conditioned reinforcement: neglected or outmoded explanatory construct? Psychon Bull Rev 1:457–475
Acknowledgements
This study is supported by a Natural Science and Engineering Research Council of Canada Post-Doctoral Fellowship (R.E.S.), a Canadian Institutes of Health Research of Canada operating grant (MOP-10516, to P.B.SC), and a Canadian Tobacco Control Research Initiative ICE Team Grant (to P.B.SC). Declaration: all experiments reported here comply with the current laws of Canada, and the authors have no financial relationship with the organizations that sponsored this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorge, R.E., Pierre, V.J. & Clarke, P.B.S. Facilitation of intravenous nicotine self-administration in rats by a motivationally neutral sensory stimulus. Psychopharmacology 207, 191–200 (2009). https://doi.org/10.1007/s00213-009-1647-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1647-8