Abstract
Objective
To study the mesolimbic dopamine system during expectation and receipt or omission of rewards in partially remitted patients with schizophrenia treated with the atypical antipsychotic olanzapine.
Methods
We studied 16 patients with a current episode of schizophrenia, all treated with the atypical drug olanzapine, and 16 healthy subjects using functional magnetic resonance imaging. Subjects performed a delayed incentive paradigm with monetary rewards.
Results
During reward expectation, both, patients with schizophrenia and healthy control subjects, showed activation of the ventral striatum and midbrain in the vicinity of the ventral tegmental area. Significant categorical group differences emerged in the anterior cingulate cortex with only healthy controls showing increasing activation with increasing reward. In the patients, activation of this region was inversely correlated with positive symptoms. During outcome, both, patients with schizophrenia and healthy controls, showed activation of the ventral striatum and the mesial prefrontal cortex. Significant categorical group differences emerged in the right ventrolateral prefrontal cortex for the salience contrast with healthy controls showing a U-shaped activation curve, i.e., higher activation for either omission or receipt of reward compared to no reward.
Conclusions
Our findings partially support the current concept of dopaminergic dysfunction in schizophrenia, suggesting a rather hyperactive mesolimbic dopamine system and reduced prefrontal activation, at least in partially remitted patients treated with atypical antipsychotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a major psychiatric disease characterized by symptoms like delusions and hallucinations (positive symptoms), anhedonia and apathy (negative symptoms), and impairments in cognitive function, e.g., in working memory. The dopamine hypothesis of schizophrenia (Carlsson and Lindqvist 1963; Carlsson et al. 2000) postulates a central role for dopamine (DA) for schizophrenic symptoms based on the mechanisms of antipsychotic drug action as well as on recent neuroimaging findings (Abi-Dargham 2003; Alves Fda et al. 2008). In its current state, a subcortical/cortical dysbalance is hypothesized: subcortical mesolimbic DA projections might be hyperactive, resulting in hyperstimulation of D2 receptors and positive symptoms, whereas mesocortical DA projections to the prefrontal cortex might be hypoactive, resulting in hypostimulation of D1 receptors related to negative symptoms and cognitive impairment. Several other neurotransmitters have been implicated in the pathophysiology of schizophrenia, in particular glutamate (Goff and Coyle 2001), and it is not clear at present whether dopamine transmission is the primary deficit in schizophrenia. However, most of these neuronal circuits, for example the fronto-striatal glutamatergic system, are involved in the regulation of mesolimbic neurotransmission, so that the dopamine system is still one of the most important targets for today’s neurobiological schizophrenia research. With relation to psychopathology, both negative and positive symptoms have been discussed with reference to the mesolimbic dopamine system. Hypoactivation of this system might explain negative symptoms of schizophrenia (Juckel et al. 2006a), a notion based on the anhedonia hypothesis of dopamine function (Wise et al. 1978). Dopaminergic hyperactivation is commonly suggested to explain positive symptoms like delusions, a view that has recently been reframed as the aberrant salience hypothesis (Kapur 2003; Kapur et al. 2006; Spitzer 1997), which is in accordance with the idea that the mediation of incentive salience is the central function of mesolimbic dopamine (Berridge and Robinson 2003).
Investigating activation of the reward system by means of functional resonance magnetic imaging (fMRI) is a non-invasive and robust way to indirectly measure the function of the mesolimbic dopamine system, i.e., the ventral tegmental area, the ventral striatum, and the mesial prefrontal cortex (Abler et al. 2006, 2007; Knutson and Cooper 2005; Knutson et al. 2001; Yacubian et al. 2006). To date, there have been only a few studies investigating patients with schizophrenia with tasks tapping the reward system (Abler et al. 2008; Jensen et al. 2008; Juckel et al. 2006a, b; Murray et al. 2008). Consistent with the anhedonia hypothesis, two studies found hypoactivation of the ventral striatum in anticipation of a possible monetary gain in unmedicated patients (Juckel et al. 2006b) as well as in patients treated with typical antipsychotics (Juckel et al. 2006a), and an inverse relationship between activation of the ventral striatum and negative symptoms. In these studies, patients on atypical neuroleptics showed normal reward-related activation of the mesolimbic system (see also Abler et al. 2008). Another study found hyperactivation of the ventral striatum to non-conditioned stimuli in an aversive conditioning paradigm which was interpreted as evidence for the aberrant salience hypothesis (Jensen et al. 2008).
In the treatment of schizophrenia, antipsychotics can relieve positive symptoms, which are thought to be mainly related to the mesolimbic dopamine system, in many cases within a relatively short time period. In contrast, negative symptoms and cognitive impairment which have been related rather to cortical dysfunction resolve considerably slower or persist after successful treatment of positive symptoms, resulting in impaired coping with everyday life situations, reduced motivation, and deficits in learning and decision making. There is some evidence suggesting that atypical antipsychotics ameliorate negative symptoms, but treatment is still challenging in a considerably large proportion of patients.
To study the postulated dysfunction of the dopaminergic system in schizophrenia, the use of reward tasks in fMRI is promising. Based on studies in healthy subjects (Abler et al. 2005, 2006), we recently characterized the effects of olanzapine on the healthy dopamine system suggesting dampening of ventral striatal activation in reward tasks (Abler et al. 2007). Here, we investigated partially remitted patients with schizophrenia with only mild positive but still present negative symptoms treated with the same neuroleptic drug as in our study with healthy controls to minimize confounding effects due to unknown medication interaction. Based on the current state of the dopamine hypothesis of schizophrenia, we assumed that originally increased activation of the ventral striatum in patients will tend to normalize under medication, i.e., that we should find normal or still slightly increased activation in the mesolimbic system, particularly the ventral striatum which was considered as a region of interest, due to still mild positive symptoms, but no hypoactivation. In contrast, for mesocortical brain regions, we expected to find decreased reward-related reactivity in patients as a potential explanation for persisting negative symptoms.
Methods
Subjects
Sixteen patients with a diagnosis of schizophrenia (SZ) were included and completed the study (Table 1). The patients were current or former (two patients) inpatients of the Departments of Psychiatry II or III of the University of Ulm at Ulm or Günzburg. They were compared with 16 healthy controls (C). Two additional control subjects volunteered for participation but were not included as they displayed symptoms of depression in the psychiatric screening concerning psychiatric symptoms currently and in the past. Patients and controls were matched for age, gender, and handedness. Diagnoses were assessed with a psychiatric interview by a specialist psychiatrist involved in the treatment of the patient but not in conducting the study and were confirmed before enrollment. Interviews comprised a general part concerning the patient’s history and a structured part to confirm the diagnosis. All patients matched DSM-IV criteria for the diagnosis of schizophrenia. None of the subjects had a history of major medical or neurological illness, and none of the included healthy subjects was identified to have a history of psychiatric illness. All patients were taking a stable dose of olanzapine for at least two full weeks. Two patients additionally took up to 1 mg lorazepam per day, one a birth control pill. Other than that, patients did not take any medication on a regular basis or acutely before scanning. Olanzapine was considered an effective antipsychotic in all patients by their physician and had effectuated mainly partial responses, in some patients even close to remission of symptoms. None of the subjects met ICD-10 criteria for alcohol or substance abuse within the previous 6 months or gave a history of alcohol or substance dependence. Six of the patients and four of the control subjects were smokers; seven patients and seven control subjects were non-smokers. Smoking status of the remaining participants remained unclear. However, from the numbers obtained, it can be estimated that the distribution of smokers and non-smokers in both groups did not differ to a very large degree. None of the participants suffered from current depression or had a diagnosis of another concomitant psychiatric illness. Patients were scanned about 6.5 weeks (0.5–20 weeks) after initial admission to the hospital. Two patients were current outpatients with their last hospitalization within the past year.
All participants, patients and controls, gave written informed consent after complete description of the study. The study was carried out in accordance with the latest version of the Declaration of Helsinki and approved by the ethics committee of the University of Ulm, Germany.
Ratings and psychological testing
Current scores on German Versions of the Positive and Negative Syndrome Scale (PANSS), Brief Psychiatric Rating Scale (BPRS), and the Hamilton Depression Rating Scale (HAMD) of the patients were assessed. Additionally, patients and healthy subjects were asked to fill in two questionnaires: the Beck Depression Inventory (German Version, 21 items; Hautzinger et al. 1995) and the Physical and Social Anhedonia Scale (German Version, Burgdörfer and Hautzinger 1987).
Reward task
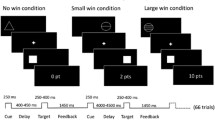
Subjects were presented with a validated paradigm (Abler et al. 2005), a monetary incentive task with a parametric variation of possible wins (1€, 20¢, no win). Each of the two sessions consisted of 60 trials (5,750 ms each; 10 no-win trials, 25 trials with potential gain of 1€, and 25 trials with potential gain of 20¢). Each trial started with one of three symbols (cue, 750 ms) indicating the possible amount of money to win. After an expectation period (delay, 3,000 ms), subjects had to correctly react with a left or right button press to one of two symbols (a square or a triangle; target) within a fixed interval of 1,000 ms with the index or middle finger of their dominant (left or right) hand. Subjects were informed that they did not need to react faster and that their chances to win were independent of their reaction times. In reacting correctly, they preserved themselves a 60% chance to win the announced amount of money (1€ or 20¢; win trial). In 40% of the trials, subjects were not rewarded despite pressing the correct button (omission trial). Incorrect button presses resulted in a feedback of zero Euro at any rate. Win and omission trials as well as the three trial types (1€, 20¢, no win) appeared in a random order. In the control trials (no win), no money was announced; subjects only had to press an arbitrary button and could not win any money. To make sure that all trials included a button press of any kind, subjects were informed that they would lose 1€ if no button press occurred. Feedback (outcome, 1,500 ms) followed the target’s disappearance and notified subjects the amount of money they won in the trial. Reaction times and errors were registered. Median reaction times were calculated across trials for each single subject; means were calculated to average over subjects.
fMRI acquisition
A 3 T Siemens ALLEGRA Scanner (Siemens AG, Germany) equipped with a head coil was used to acquire T1 anatomical volume images (1 × 1 × 1 mm voxels) and functional MR images. Axial slices (23) were acquired with an image size of 64 × 64 pixels and a FoV of 192 mm. Slice thickness was 3 mm with 0.75 mm gap resulting in a voxel size of 3 × 3 × 3.75 mm. Images were angled along a line connecting basal forebrain and basal cerebellum. Images were centered on basal structures of the brain including subcortical regions of interest (basal ganglia, orbitofrontal and ventral frontal regions). Functional images were recorded using a T2*-sensitive gradient echo sequence measuring changes in BOLD contrast. Volumes (401) were obtained during each of the two reward sessions at a TR of 1,500 ms (TE 35 ms, flip 90°).
fMRI analysis
Image processing and statistical analysis were carried out using Statistical Parametric Mapping (SPM2, Wellcome Department, London, UK).
Preprocessing
Images were pre-processed including slice timing, realignment to correct for motion artifacts, and spatial normalization to a standard template (Montreal Neurological Institute, MNI) with a resampled voxel size of 3 × 3 × 3 mm. Smoothing was applied with an 8 mm Gaussian kernel. Intrinsic autocorrelations were accounted for by AR(1) and low frequency drifts were removed via high pass filter.
First level analysis
After preprocessing, first level analysis was performed on each subject estimating the variance of voxels according to a general linear model: the three expectation periods (high, low, no; including presentation of the cue), the button press, and the five different outcome events (receipt of high, receipt of low, receipt of no, omission of low, omission of high reward) were each modeled as a boxcar function and convolved with the hemodynamic response function resulting in nine orthogonal regressors. The six realignment parameters were included in the model hence resulting in 15 regressors. Contrast images of contrasts between regressors of interest were calculated on the single subject level.
Second level analyses
The contrast images of parameter estimates from the first level analysis were then included in a second level group analysis (random effects model), treating inter-subject variability as a random effect to account for interindividual variance. We computed analyses separately for expectation and outcome trials: one (analysis 1) on the three different expectations and a second (analysis 2) on the five outcome events (receipt/omission of reward). Effects were tested separately for each group (patients/control subjects) and by interaction analyses of the two groups with either expectation or outcome.
-
Analysis 1 (expectation): The conditions in analysis 1 were weighted with a linear contrast (−1 0 1) modeling increasing reward (high, low, or no reward expected). According to the findings of our previous studies (Abler et al. 2005, 2007, 2008), we expected to detect ventral striatal activation using this contrast.
-
Analysis 2 (outcome): The conditions in analysis 2 were weighted in two different ways (Table 2): First (analysis 2a), with a linear contrast (−2 −1 0 1 2) modeling the error in the reward predictions according to the findings of our previous study (Abler et al. 2006) (receipt of high, receipt of low, receipt of no, omission of low, omission of high reward; high positive, low positive, no, low negative, high negative prediction error). Second (analysis 2b), we built a regular U-shaped contrast (4 −1 −6 −1 4) modeling the salience of outcome events. Receipt and omission of high rewards were weighted with the highest contrast values, receipt and omission of low rewards were weighted with medium values, and the no reward events with the lowest contrast values.
Table 2 fMRI activations
Whole brain random-effects statistical maps (analyses 1 and 2) were thresholded at p < 0.001 uncorrected for multiple comparisons. Only activations with a minimum cluster size of 15 voxels are reported. FWE corrections for multiple comparisons were performed on all results and are reported when significant. Concerning the ventral striatum, FWE corrections were applied in the form of small volume corrections that are considered appropriate for small structures like the ventral striatum where a relatively small number of voxels per cluster is to be expected. To ensure that the results of the interaction contrasts were clearly driven by actually elevated fMRI signal in the respective group, we used an inclusive masking procedure: This means that contrasts showing a significant result for controls > patients were inclusively masked with the linear reward contrasts of the control group; an interaction contrast showing a significant result for patients > controls was inclusively masked with the linear reward contrast of the patient group. Masking was thresholded at p < 0.001 at the voxel level.
Testing of linear and quadratic trends with extracted beta values within our regions of interest
As outlined in the Introduction, we had clear a priori hypotheses for the ventral striatum as well as for mesocortical regions. In addition to our results obtained within our imaging software, we performed further analyses within those regions in order to test linear and quadratic trends. The beta values were extracted from the voxel displaying maximal group differences. Statistics on the beta values (different regression lines and their characteristics) were calculated externally using the software packages Microsoft Excel and Statistica 6.0. As the analyses were done to either investigate effects in a brain region with a strong a priori hypothesis, i.e., the ventral striatum, or to further characterize the signal in regions where we found group differences in the whole brain analysis before, the external statistics on second level results were thresholded at p < 0.05. We considered this post hoc approach necessary, because a simple SPM model of three or five regressors does average all regressors above zero (multiplied by the contrast weight) and all regressors below zero (multiplied by the contrast weight), which means that a significant result for the contrast might result from an extraordinary high or low value in just one of the conditions and not from the fact that the regressors are in a good linear or parabolic relationship which is what we intended to investigate.
Correlational analyses
To assess relations between psychopathology and brain activation, we calculated planned correlations between our main results concerning brain activation and the main psychopathology scores, PANSS positive and negative. Social anhedonia was chosen as the third parameter for calculating correlations as patients did differ from controls in this scale. These correlations were only calculated for those brain regions where group differences were found, in order to allow interpreting the results in the light of our theoretical background.
Results
Behavioral results
Subjects responded in 97.2% of the trials in the reward task correctly, i.e., they pressed the correct button within the required time. Patients made more errors than controls (Table 1). Concerning reaction times, we found a significant main effect of group (F(15,1) = 6.376; p = 0.023) as patients were slower in the reward task (613 ms, SD 66) than controls (540 ms, SD 75).
Like in our previous studies (Abler et al. 2005, 2006), we found a main effect of reward (F(30,2) = 10.918; p = 0.0003) as reaction times in the controls (no/low/high reward=559/540/522 ms) and likewise SZ patients (no/low/high reward=625/621/595 ms) were significantly faster in the high reward trials than in the low (SZ—p = 0.002; C—p = 0.008) or the no reward trials (SZ—p = 0.05; C—p = 0.000004). The interaction ‘group × reward’ was not significant (F(30,2) = 0.656; p = 0.53). However, significant reaction time differences between low and no reward trials were only found in the controls (p = 0.03), but not in the patients (p = 0.80). Thus, an acceleration of reaction times in the patients was only found for the high reward trials.
Concerning questionnaires and rating scales (see Table 1), we found higher scores for social but not physical anhedonia and higher scores on the Beck Depression Inventory (BDI) in SZ patients compared to controls. Social anhedonia was correlated with subjective ratings of depression (BDI—r = 0.72; p = 0.006) but not Hamilton depression scores (r = 0.44; p = 0.13) in the patients consistent with findings of some overlap of the concepts of depression and anhedonia in schizophrenia (Kollias et al. 2008). No correlation was found between BDI and social anhedonia scores in healthy subjects (r = 0.08; p = 0.77).
fMRI results during the reward task
We investigated simple (SZ, Controls) and interaction (SZ vs. Controls) effects for the two regression analyses for expectation (analysis 1) and outcome phases (analysis 2).
Analysis 1 (expectation)
Analyses of the expectation phase revealed an effect of reward amount in brain regions that are part of the mesolimbic–mesocortical dopaminergic system (Table 2): the ventral striatum (Fig. 1, upper part) and midbrain regions in the vicinity of the ventral tegmental area/substantia nigra were active in both patients and controls. As suggested by our previous studies (Abler et al. 2005, 2006), a linear contrast models these activations best. The dorsal anterior cingulate and the ventrolateral prefrontal cortex and adjacent anterior insula were only active in controls, but not in patients. Analyses of group interactions revealed that activation in the dorsal anterior cingulate was significantly higher in the controls when comparing patients and controls (Fig. 2). No brain region was found significantly more active in the patients than in the controls for this analysis.
-
Testing of linear trends: We calculated the slopes of the regression lines for the beta values from the peak voxel in the anterior cingulate for the conditions for no, low, and high reward expectation for each patient and control subject. The slopes of the regressors (increasing from no to high reward expectation, Fig. 2) were significantly higher (i.e., steeper) in the control subjects (slope = 0.23) than in the patients (slope = −0.06, T(27) = 4.38, p = 0.00008).
-
Correlational analyses: We found a significant negative correlation (r = −0.58, p = 0.009) of the PANSS positive score in the patients and the individual slopes of the regressors, i.e., patients with lower psychopathology ratings display steeper curves, more similar to controls. No significant correlation was found with the PANSS negative scores and social anhedonia.
Brain activation and beta weights in ventral striatum as found for patients and controls in the contrasts modeling expectation of increasing rewards (analysis 1 = lowest at expectation of no reward, highest at expectation of high reward, upper part of figure) and prediction error (analysis 2a = highest at receipt of high reward, lowest at omission of high reward, lower part of figure). To obtain the beta values of modeled effects, conditions in both groups were weighted with the same contrast. The beta values were then extracted from the maximum voxel (x/y/z—expectation = −12/6/−6, outcome = −12/10/−4) in both groups taken together in a single analysis. Maps were thresholded at p < 0.001. Only activations with 15 or more voxels are displayed. The slopes of the regression lines on the beta values of the outcome contrast (decreasing from receipt of high to omission of high reward) in the left ventral striatum were significantly (*) higher (i.e., steeper) in the patients than in the control subjects (p = 0.04)
Brain activation and beta weights in the anterior cingulate as found for the interaction analysis comparing expectation of increasing rewards (analysis 1, see Table 2) in patients and controls (see upper part of Fig. 1 for the contrast separately in each of the two groups). The beta values were extracted from the maximum voxel of the demonstrated activation (x/y/z = −6/12/30). The map was thresholded at p < 0.001. Only activations with 15 or more voxels are displayed. The interaction contrast is masked by the contrast in the controls’ group alone thresholded at p < 0.001 at the voxel level. The slopes of the regression lines on the beta values of expectation contrast (increasing from expectation of no to high reward) in the anterior cingulate were significantly (*) higher (i.e., steeper) in the control subjects than in the patients (p = 0.00008). Further, the individual slopes of the regressor in the patients were negatively correlated with their PANSS positive scores, i.e., patients with lower psychopathology ratings displayed steeper curves, more similar to controls
Analysis 2a (outcome)
Analyses of the outcome phase revealed an effect of reward prediction error again in the mesolimbic–mesocortical dopamine system (Fig. 1, lower part): the medial orbitofrontal cortex and ventral striatum were active in both patients and controls. As suggested by our previous studies (Abler et al. 2005, 2006), a linear contrast models these activations best. The SPM analysis of the interaction effect of prediction error between groups revealed a significant interaction in the left ventral striatum (patients > controls) at p = 0.035 FWE corrected for small volume.
-
Testing of linear trends: We calculated the slopes of the regression lines for the beta values from the peak voxel in both groups in the left and right ventral striatum for each the conditions for receipt of high, receipt of low, receipt of no, omission of low, and omission of high reward for each patient and control subject. The slopes of the regression lines (decreasing from receipt of high to omission of high reward, Fig. 1) in the left, but not right ventral, striatum were significantly higher (i.e., steeper) in the patients (left = −0.43, right = −0.27) than in the control subjects (left = −0.23, T(27) = 1.80, p = 0.04; right = −0.28, T(27) = 0.057, p = 0.48).
Analysis 2b (outcome)
An effect of salience (Table 2), was found in the right ventrolateral prefrontal cortex and adjacent anterior insula and anterior cingulate in controls, but not in patients. Interaction contrasts of the between groups analysis showed differences in the ventrolateral prefrontal cortex/anterior insula (Fig. 3).
Brain activation and beta weights in the right ventrolateral prefrontal cortex/anterior insula as found for the interaction analysis comparing the contrasts modeling salience (analysis 2b, see Table 2) in patients and controls. The beta values were extracted from the maximum voxel of the demonstrated activation (39/21/−51). The map was thresholded at p < 0.001. Only activations with 15 or more voxels are displayed. The interaction contrast is masked by the contrast in the controls’ group alone thresholded at p < 0.001 at the voxel level. The coefficient ‘a’ taken as a measure of the shape of a U-shaped regression curve indicates a more shallow curve the lower the levels and an inverse U shape with negative values. The values in control subjects were significantly higher than in the patients. r/high, r/low, no, o/low, o/high receipt of high reward, receipt of low reward, receipt of no reward, omission of low reward, omission of high reward
Plots of the beta values within this activation cluster revealed that this interaction contrast was driven by comparatively low activation for the condition ‘omission of high reward’ in the patients. A planned T test of interaction contrasts confirmed this finding at a significance level close to the predefined threshold: T test (Controls > Patients [Receipt of no < omission of high reward] significant at p < 0.001, T = 3.79, 13 voxels, x/y/z = 36/24/−12).
-
Testing of quadratic trends: The regression curve for a U-shaped contrast is best described with a parabolic line that can be characterized by a second order equation (ax 2 + bx + c = y). The coefficient “a” can be taken as a measure of the shape of the U with lower values indicating a more shallow curve and negative values an inverse U shape. The values for the coefficient “a” in the control subjects (a = 0.37) were significantly higher than in the patients (a = −0.006, T(27) = 2.63, p = 0.007).
Discussion
Investigating partially remitted patients with schizophrenia, all treated successfully with the same single neuroleptic olanzapine, we could add to the dopamine hypothesis of schizophrenia predicting a differential affection of subcortical, i.e., mesolimbic and cortical dopaminergic pathways in a reward task using fMRI: Whereas the ventral striatum showed normal or even exaggerated responses, we found cortical hypoactivation in the dorsal anterior cingulate and right ventrolateral prefrontal cortex correlating with current psychopathology.
Behavioral results
Patients were generally slower and made more errors than healthy controls. However, they showed the same pattern of acceleration, i.e., decreasing reaction times decreased with increasing magnitude of anticipated reward, although the difference to the not rewarded control trials in reaction times was significant only for the high, not for the low reward in the patients. In line with similar results of our previous studies (Abler et al. 2005, 2007, 2008), we take this acceleration in reaction times as evidence for the motivational force of the anticipated reward. This can be interpreted in a way that the reward system of the patients is reactive in the expected way similar to the healthy subjects but only under high motivational conditions. Findings from behavioral studies pointing to altered value representation of rewards and demonstrating a steeper discounting of future rewards in clinically stable, medicated patients with schizophrenia are in line with this interpretation of altered motivational processing (Gold et al. 2008).
Mesolimbic system
Paralleling the behavioral findings, during anticipation of rewards we found increasing activation of the main nodes of the mesolimbic reward system in both healthy controls and patients with schizophrenia with patients showing a somewhat decreased activation upon expectation of low rewards compared to the control condition (no reward), consistent with a reaction predominantly under high motivational conditions. However, we found no significant group differences in the two mesolimbic regions, i.e., midbrain in the vicinity of the ventral tegmental area and the ventral striatum, nor was there a significant group difference in the slope of the regression from no to high reward as depicted in Fig. 1. This finding is in accordance with the published fMRI reward studies on schizophrenia that investigated patients treated with atypical neuroleptics (Abler et al. 2008; Jensen et al. 2008; Juckel et al. 2006a). These studies either found no reduced activation in the ventral striatum in patients (Juckel et al. 2006a) or even an exaggerated response for non-conditioned cues (Jensen et al. 2008).
Similarly, activation of mesolimbic regions upon receipt or omission of rewards revealed the expected modulation according to the prediction error theory in both patients and control subjects. The prediction error, defined as the difference between the expected and the received reward, is coded by the mesolimbic dopamine system (Abler et al. 2006; Schultz 2001). In the outcome phase, a parametric activation was observed which was highest when high rewards were received and lowest when high rewards were omitted. The fact that the increase of the prediction error signal (slope) was even steeper in the patients could reflect remainders of a previous hyperactivation during the acute phase of the illness (Jensen et al. 2008). An alternative interpretation would be a reflexive increase of activation upon recovery from positive symptoms in our sample of partially remitted patients, as, in acute, untreated psychosis, a decrease of mesolimbic activation has been observed (Murray et al. 2008).
Cortical regions
While we found average to increased mesolimbic fMRI signals, activation of cortical regions related to rewards was markedly decreased in the patients: the dorsal anterior cingulate gyrus found hypoactive upon expectation of increasing rewards is related to as the ‘cognitive’ subregion of the cingulate gyrus (Koo et al. 2008), modulating attention and executive functions. The region has been implicated in the pathophysiology of schizophrenia in various ways: imaging data (review in Fornito et al. 2008) suggest a reduction of gray matter volume preceding psychosis onset, progressing with illness duration and correlating with cognitive deficits (Szeszko et al. 2000). Further, functional hypoactivation of the dorsal anterior cingulate gyrus was found to be correlated with positive symptoms (verbal disorganization) (McGuire et al. 1998) and to resolve after successful antipsychotic treatment (Lahti et al. 2004) in neuroimaging studies using PET. Our finding of a correlation between positive symptoms as measured by the PANSS scale and altered fMRI activation of the cingulate gyrus in the patients is in line with a potential dysfunction of attentional or executive systems related to residual positive symptoms. Attentional deficits could further help to explain the behavioral effects being close to healthy subjects only under high motivational conditions when attentional resources are more readily recruited. However, it remains unclear whether the attention deficit rather results from distraction by positive symptoms or represents a symptom of its own.
Concerning the right ventrolateral prefrontal hypoactivation, a deficit in transforming the salience signals adequately monitored by mesolimbic brain regions into actions seems a possible interpretation: the right ventrolateral prefrontal cortex has been implicated in change of response strategies (Cools et al. 2002) and response suppression (Arana et al. 2003) in the context of reward paradigms and has a critical role when performing set shifts in problem-solving tasks (Goel and Vartanian 2005) and in the selection of task-relevant stimuli (Wolf et al. 2006). Adaptations of goal selection strategies are supposed to occur most likely upon events of high behavioral significance or salience such as receipt of a highly desired incentive which makes one go for more or the surprising omission of a reward commonly resulting in changes of the unsuccessful behavior. We therefore expected to find lateral prefrontal cortical activation upon the contrast modeling salience in the healthy subjects. Hypoactivation of this region may reflect the difficulties in planning, action, and goal selection frequently observed in patients with schizophrenia when positive symptoms become less prominent. The group differences in social anhedonia scores support this interpretation, as anhedonic patients not only suffer from a deficit to experience pleasure but also show decreased efforts in pursuing pleasurable events like social interaction (Blanchard et al. 1998) to actively change the situation. Interestingly, altered activation of the right ventrolateral prefrontal cortex was the most consistent finding in a meta-analysis of fMRI studies on non-psychotic relatives of patients with schizophrenia (Macdonald et al. 2008). Patients actually suffering from schizophrenia consistently show deficits in tasks mediated by ventrolateral prefrontal brain regions like reversal learning paradigms (Waltz and Gold 2007) or the Iowa Gambling Task (Ritter et al. 2004).
Limitations
The interpretation of our results is clearly limited by the fact that all patients were treated with antipsychotic medication influencing the dopaminergic reward system. However, at least our group received homogeneous medication in contrast to many other studies. Moreover, the effects described here are different from those which we found recently in a pharmaco-fMRI in which healthy controls received a single dose of olanzapine and consequently showed no differential activation to different magnitudes of reward. Furthermore, the correlations with psychopathology we found make it likely that our results cannot be due solely to medication.
Minor limitations are the fact that the patients on average were five years older than the controls. However, this difference was not significant. Furthermore, the significance of the fMRI results is limited by the fact that not all results survived corrections for multiple comparisons. To avoid false positive results, a cluster threshold was applied. The conservative approach of masking with the activation pattern in the group with the greater activation may have led to missing some brain regions with significant interactions. However, this approach allows an unequivocal interpretation of the interaction results.
Conclusion
Our investigation provides partial support for the current version of the dopamine hypothesis in partially remitted patients treated with the atypical neuroleptic olanzapine. The subcortical mesolimbic dopamine system monitoring reward processes is normal to hyperreactive in the patients. However, cortical regions like the anterior cingulate gyrus or the right ventrolateral prefrontal cortex mediating attentional processes and action selection are rather hypoactive during reward processing. Further research should aim to test subcortical and cortical activation with more than one paradigm sensitive to subcortical and cortical dopaminergic input, most preferably also in patients without medication.
References
Abi-Dargham A (2003) Probing cortical dopamine function in schizophrenia: what can D1 receptors tell us? World Psychiatry 2:166–171
Abler B, Walter H, Erk S (2005) Neural correlates of frustration. NeuroReport 16:669–672
Abler B, Erk S, Walter H (2007) Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology (Berl) 191:823–833
Abler B, Walter H, Erk S, Kammerer H, Spitzer M (2006) Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage 31:790–795
Abler B, Greenhouse I, Ongur D, Walter H, Heckers S (2008) Abnormal reward system activation in mania. Neuropsychopharmacology 33:2217–2227
Alves Fda S, Figee M, Vamelsvoort T, Veltman D, Haan L (2008) The revised dopamine hypothesis of schizophrenia: evidence from pharmacological MRI studies with atypical antipsychotic medication. Psychopharmacol Bull 41:121–132
Arana FS, Parkinson JA, Hinton E, Holland AJ, Owen AM, Roberts AC (2003) Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci 23:9632–9638
Berridge KC, Robinson TE (2003) Parsing reward. Trends Neurosci 26:507–513
Blanchard JJ, Mueser KT, Bellack AS (1998) Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull 24:413–424
Burgdörfer G, Hautzinger M (1987) Physische und soziale Anhedonie—Die Evaluation eines Forschungsinstruments zur Messung einer psychopathologischen Basisstörung. Eur Arch Psychiatry Neurological Sci 236:223–229
Carlsson A, Lindqvist M (1963) Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharm Toxicol (Copenh) 20:140–144
Carlsson A, Waters N, Waters S, Carlsson ML (2000) Network interactions in schizophrenia—therapeutic implications. Brain Res Rev 31:342–349
Cools R, Clark L, Owen AM, Robbins TW (2002) Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci 22:4563–4567
Fornito A, Yucel M, Dean B, Wood SJ, Pantelis C (2008) Anatomical Abnormalities of the Anterior Cingulate Cortex in Schizophrenia: Bridging the Gap Between Neuroimaging and Neuropathology. Schizophr Bull doi:10.1093/schbul/sbn025
Goel V, Vartanian O (2005) Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cereb Cortex 15:1170–1177
Goff DC, Coyle JT (2001) The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158:1367–1377
Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA (2008) Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull 34:835–847
Hautzinger M, Bailer M, Worall H, Keller F (1995) Beck-Depressions-Inventar (BDI); Testhandbuch. Hans Huber, Hans Huber
Jensen J, Willeit M, Zipursky RB, Savina I, Smith AJ, Menon M, Crawley AP, Kapur S (2008) The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology 33:473–479
Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A (2006a) Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 29:409–416
Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, Knutson B, Kienast T, Gallinat J, Wrase J, Heinz A (2006b) Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 187:222–228
Kapur S (2003) Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160:13–23
Kapur S, Agid O, Mizrahi R, Li M (2006) How antipsychotics work—from receptors to reality. NeuroRx 3:10–21
Knutson B, Cooper JC (2005) Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 18:411–417
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001) Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport 12:3683–3687
Kollias CT, Kontaxakis VP, Havaki-Kontaxaki BJ, Stamouli S, Margariti M, Petridou E (2008) Association of physical and social anhedonia with depression in the acute phase of schizophrenia. Psychopathology 41:365–370
Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW (2008) A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry 65:746–760
Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Frey KN, Hardin M, Tamminga CA (2004) Clozapine but not haloperidol re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology 29:171–178
Macdonald AW, 3rd, Thermenos HW, Barch DM, Seidman LJ (2008) Imaging Genetic Liability to Schizophrenia: Systematic Review of fMRI Studies of Patients’ Nonpsychotic Relatives. Schizophr Bull
McGuire PK, Quested DJ, Spence SA, Murray RM, Frith CD, Liddle PF (1998) Pathophysiology of ‘positive’ thought disorder in schizophrenia. Br J Psychiatry 173:231–235
Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC (2008) Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry 13(239):267–276
Ritter LM, Meador-Woodruff JH, Dalack GW (2004) Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophr Res 68:65–73
Schultz W (2001) Reward signaling by dopamine neurons. Neuroscientist 7:293–302
Spitzer M (1997) A cognitive neuroscience view of schizophrenic thought disorder. Schizophr Bull 23:29–50
Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, Wu H, Lieberman JA (2000) Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr Res 43:97–108
Waltz JA, Gold JM (2007) Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res 93:296–303
Wise RA, Spindler J, deWit H, Gerberg GJ (1978) Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science 201:262–264
Wolf RC, Vasic N, Walter H (2006) Retrieval processes in verbal working memory: dissociating load-effects and target-features. Neuropsychologia 44:2558–2563
Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C (2006) Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci 26:9530–9537
Acknowledgement
Thanks to Christian Wolf for the thorough expert review of the final draft of the paper.
The experiments comply with the current laws of the country in which they were performed.
Acknowledgement of funding
The study was supported in part by a personal grant to Birgit Abler from the DAAD (German Academic Exchange Service).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walter, H., Kammerer, H., Frasch, K. et al. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology 206, 121–132 (2009). https://doi.org/10.1007/s00213-009-1586-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1586-4