Abstract
Rationale
Central fatigue, such as that found in chronic fatigue syndrome, is a state in which cognition and action require increasing effort and performance is impaired without evidence for reduced peripheral motor responsiveness. Previous studies identified functional changes in subcortical regions in patients who experience central fatigue but did not address neural correlates of the subjective experience of fatigue.
Objectives
This study investigated responses to acute tryptophan feeding (after administration of 30 mg/kg body mass) using functional magnetic resonance imaging to investigate neural correlates of central fatigue during a cognitively demanding exercise, the counting Stroop task.
Materials and methods
In a double-blind, cross-over study, eight subjects ingested l-tryptophan (Trp) or placebo (Plac) on two separate test days. Neutral (N) and interference (I) Stroop tasks were carried out.
Results
Plasma-free tryptophan (p[FT]) increased tenfold after l-Trp administration (P < 0.01). Although reaction times were longer after Trp (mean±SD, Plac-Neut 669 ± 163 ms, I 715 ± 174 ms, P < 0.01; Trp-Neut 712 ± 193 ms, I 761 ± 198 ms, P < 0.05), the Stroop effect was not significantly different between Plac and Trp. l-Trp administration was associated with relatively decreased activation in regions, including the left postcentral, angular, inferior frontal, and the lateral orbital gyri and the inferior frontal sulcus relative to Plac. Relatively increased activation was found after Trp in the left precuneus and in the posterior cingulate gyrus.
Conclusions
Thus, Trp administration before the Stroop task caused distributed functional changes in primary sensory and in multimodal neocortex, including changes in a brain region, the activity of which has been shown previously to vary with conscious awareness (precuneus). Previous reports suggest that primary mechanisms of central fatigue may be predominantly subcortical. The present results demonstrate that neocortical activity changes are also found. Whether this activity contributes to the primary mechanisms underlying central fatigue or not, the neocortical activity changes may provide an index of the conscious experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic systemic stress (e.g., from exercise in elite athletes or pathological inflammation states) can produce a sense of mental and physical tiredness described as “central fatigue”, which, if sustained, may lead to chronic fatigue syndrome. This central fatigue, a state in which cognition and action require increasing effort (Sharpe et al. 1991; Budgett et al. 1998), is associated with impaired physical performance without evidence for altered peripheral sensory or motor responsiveness. In a recent review, Nybo and Secher (2004) discuss exercise-induced perturbations of brain functions. Recent work has suggested that functional changes in the thalamus and basal ganglia are trait markers for central fatigue associated with inflammatory diseases (Filippi et al. 2002). However, these observations do not address the question as to which specific physiological substrate is involved when central fatigue is experienced.
To approach the problem of determining the neural correlates of central fatigue, we have employed the pharmacological model of increasing plasma tryptophan, which allows the same individuals to be studied in both normal and fatigued states. Tryptophan (Trp) is the precursor for 5-hydroxytryptamine (5-HT or serotonin), a neurotransmitter involved in sleep and mood changes (Newsholme and Leech 1983; Wurtman 1988). Drowsiness and a sense of mental “slowing” occurs after oral administration of Trp (Lieberman et al. 1982; Thorleifsdottir et al. 1989). These subjective feelings are correlated with objective measures of fatigue (Cunliffe et al. 1998). The administration of l-Trp has been shown to increase the synthesis of hypothalamic 5-HT in the rat (Curzon and Marsden 1975) and, with pretreatment intra-peritoneally, to enhance exercise-induced release in the rat hippocampus (Meeusen et al. 1996).
It has been hypothesised that there is an association between the central fatigue accompanying intensive exercise or physical stress and increases in the plasma tryptophan concentration (p[TT]; Newsholme and Leech 1983). There is a surge in plasma-free tryptophan (p[FT]; i.e., that which is not bound to albumin) after prolonged exercise in rats (Blomstrand et al. 1989) and in humans (Blomstrand et al. 1997). Elevated (p[FT]) in patients after surgery also correlates with fatigue (McGuire et al. 2003).
The rate of production of 5-HT in the rat brain increases with higher concentrations of serum tryptophan (Fernstrom and Wurtman 1972; Fernstrom 1990). Increases in p[FT] are also accompanied by significant increases in 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) in striatal synaptosomes (Yamamoto and Newsholme 2000). Similarly, Blomstrand et al. (1989) observed that, after exercise, 5-HT only increased in the rat brain stem and hypothalamus, whereas 5-HIAA was also elevated in the hippocampus and striatum as well. However, the increase in the concentration of brain 5-HT is less than linearly proportional to an acute increase in p[FT] (Fernstrom 1990; Bloxam et al. 1980).
The specific aim of the present study was to contrast (using a cross-over design) the effects of oral administration of either l-Trp or Plac on brain activation patterns measured using functional magnetic resonance imaging (fMRI) while performing a modified Stroop task to define brain activity patterns associated specifically with central fatigue.
Materials and methods
Subjects
Eight subjects (two women, six men, age range: 24–33 years) were studied. Subjects had no history of neurological, major medical, or psychiatric disorders and were not taking medication. All subjects were right-handed with English as a native language and had a visual acuity of 6/6 (Snellen chart) with correction if necessary. Subjects gave informed and written consent to the procedure, which was approved by the Oxford Psychiatric Research Ethics Committee.
At each of two visits, subjects either performed the Stroop task after ingesting either (Trp) or a placebo (Plac) using a randomised, double-blind cross-over design. Test days were at least 2 weeks apart. Both l-Trp and Plac contained the same flavourings and sweeteners (Scientific Hospital Supplies International, Liverpool, UK). The women performed each test at the same stage in their menstrual cycle.
The measurement of plasma concentrations of FT and total Trp
Pre- and post-experimental blood samples were taken by venepuncture into lithium heparin vacutainers, while the subject was seated. Post-fMRI blood was collected approximately 1.5 h after the initial baseline sample. Samples were placed on ice, centrifuged, and the plasma aliquotted and stored at −80°C until assayed for p[FT] and p[TT] concentration (Bloxam and Warren 1974).
Administration of l-Trp or Plac
Immediately after the baseline blood sample, the subject drank either l-Trp or Plac (30 mg/kg body mass) dissolved in 200 ml of water, in less than 1 min.
Scanning for fMRI during performance of the Stroop task started 50–60 min after the subject had ingested either l-Trp or Plac. This time interval was chosen following pilot trials demonstrating that peak p[FT] and p[TT] occurred 50–60 min post-ingestion.
Paradigm
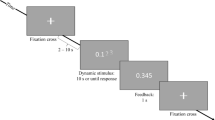
The paradigm used was a modified version of the counting Stroop (CS) test (Bush et al. 1998; Parry et al. 2003). A 9-min block-design with eight alternating blocks of neutral (N; 30 s) and interference (I) stimuli (30 s) was used. The paradigm started and ended with a 30-s rest period. The N stimuli consisted of animal words (e.g., cat, dog, mouse, bird), whereas the I stimuli consisted of number words (e.g., one, two, three, four). Stimuli were presented at 1.5-s intervals. Subjects were instructed to press the button corresponding to the number of words seen on the screen, to answer as quickly as possible, but not to forsake accuracy for speed.
Stimulus presentation
Subjects were positioned supine in the MRI scanner with prism glasses to enable them to see the vertical screen (In Focus LP1000: National Projector, Dallas, TX, USA), 2.5 m from the head of the patient. Responses were relayed to a computer in the console room. Reaction time was averaged across each block and across all presentation blocks in both the N and I tasks, together with the total number of errors.
Image acquisition
Acquisition of fMRI
A 3.0 Tesla Varian/Siemens MRI system was used to acquire images of the brain. A 9-min echo-planar imaging sequence was acquired during the CS (21 × 6 mm axial slices, echo time (TE) = 30 ms, repetition time (TR) = 3,000 ms, field of view \({\left( {{\text{FOV}}} \right)} = 256 \times 256\), \({\text{matrix}} = 64 \times 64\)).
Acquisition of structural image
A T1-weighted anatomical scan was also acquired for each subject (IR 3D Turbo Flash, 64 × 3 mm axial slices, TR = 30 ms, TE = 5 ms, inversion time (TI) = 500 ms, flip angle = 15, \({\text{FOV = }}256 \times 256\), \({\text{matrix = }}256 \times 256\)). The high-resolution T1-weighted images from the subjects were co-registered into standard space and averaged to produce a mean structural image on which the thresholded Z statistical images were overlaid. This allowed assessment of activation areas in terms of anatomical landmarks (Duvernoy 1995), as well as reporting the co-ordinates of the peak activations based on the Montreal Neurological Institute Standard brain template (http://www.fmrib.ox.ac.uk/fsl).
Image analysis
The fMRI data was analysed using a fMRI Expert Analysis Tool, version 4 (FEAT; http://www.fmrib.ox.ac.uk/fsl) and MEDx (Sensor Systems, VA, USA). The following pre-statistics processing was applied: motion correction using fMRIB’s motion corrected linear image registration tool (Jenkinson and Smith 2001); spatial smoothing using a Gaussian kernel of full width at half maximum, 5 mm; mean-based intensity normalisation of all volumes by the same factor; nonlinear highpass temporal filtering (Gaussian-weighted linear fitting, with sigma = 45.0 s). Statistical analysis was carried out using fMRIB’s improved linear model, with local autocorrelation correction (Woolrich et al. 2000). Z (Gaussianised T or F) statistical images were thresholded using clusters determined by Z > 3.0 and a cluster significance threshold of P = 0.01 (Worsley et al. 1992; Friston et al. 1992; Forman et al. 1995). Registration of functional to high-resolution and standard space images was carried out using fMRIB’s linear image registration tool (Jenkinson and Smith 2000). Mixed effect group analysis were performed using fMRIB’s local analysis of mixed effects (http://www.fmrib.ox.ac.uk).
Within-group mixed effects analyses of the Trp and Plac conditions were performed using an I–N contrast to determine relative changes in activation after Plac or Trp administration. Between-group analyses were then performed to determine further whether there were differences in the patterns of brain activation with the I–N contrast between the two conditions, Trp and Plac. It was decided to confine our hypothesis testing for effects of Trp relative to Plac and for effects of Plac relative to Trp to brain regions identified as being significantly activated in the within group analyses of Trp and Plac, respectively. In addition, the hypothesis testing was confined to brain regions that were “positively activated” with respect to rest in both the N and I condition.
Statistical analysis
A two-factor within-subjects repeated measures analysis of variance was used to test for significant differences between trial (Trp and Plac) and task (N and I) performance with CS and between trial (Trp and Plac) and time (pre- and post-ingestion) of p[FT] and total Trp (p[TT]). In cases where Student’s paired t-test was used to test for specific differences, significance levels for all comparisons were set at P < 0.05, means are displayed ±SD. All statistics were performed using SPSS for Windows (version 11.0).
Results
Plasma concentrations of free and total Trp
Increases in p[FT] (pre, 4.3 ± 1.6 μmol l−1; post, 43.9 ± 14.8 μmol l−1; mean increase, 920%; P < 0.01) and p[TT] (pre, 86.3 ± 28.0 μmol l−1; post, 388.2 ± 65.6 μmol l−1; mean increase, 770%; P < 0.05) were observed after oral administration of l-Trp. No significant changes in either p[FT] (pre, 3.7 ± 1.2 μmol l−1; post, 2.8 ± 0.8 μmol l−1) or p[TT] (pre, 74.0 ± 14.3 μmol l−1; post, 68.9 ± 17.5 μmol l−1) were observed after administration of Plac.
Counting Stroop task performance
Subjects showed a significant CS effect: the reaction time was greater (P < 0.05) during the I task than during the N task after administration both of Trp and Plac (Table 1). The mean number of errors during I was also significantly greater than during N for both Trp and Plac (mean±SD: Plac N, 3 ± 2; I, 5 ± 2 [P < 0.05]; Trp N, 3 ± 2; I, 7 ± 4 [P < 0.05]). There was a trend for slower N and I responses with Trp (six out of eight subjects showed a slower reaction time after Trp intake), but differences did not reach statistical significance. The magnitude of the CS effect was similar after administration of either Plac or Trp.
Between-group analysis of fMRI changes with the Stroop task
To test for effects of Trp administration on the pattern of CS-associated brain fMRI activation, I–N responses after Trp were contrasted with those after Plac administration. Significant changes in the pattern of brain activation after Trp relative to Plac were found in brain regions, including the left precuneus and posterior cingulate gyrus (Table 2; Fig. 1a). The left postcentral, angular, inferior frontal, and the lateral orbital gyri and the inferior frontal sulcus were more active during the CS after administration of Plac than after Trp (Table 3; Fig. 1b).
Discussion
Trp administration provides a pharmacological model of central fatigue (Cunliffe et al. 1998). The main finding of the present study is that central fatigue in this model is associated with neocortical activation changes in several brain regions. These activation changes occurred without a significant change in the magnitude of the behavioural Stroop effect, although there was a trend towards slowing of the motor response to both the N and I stimuli after Trp administration. This suggests that, in central fatigue, although subcortical functional changes (Chaudhuri and Behan 2000; Filippi et al. 2002) may be involved in the mechanisms responsible for central fatigue, an index of the actual experience of central fatigue is associated with changes in neocortical activity.
Trp administration modulates central serotoninergic systems. Serotoninergic neurons in the raphe nuclei project diffuse through the medial forebrain (Baynes and Dominiczak 1999) to the cerebral cortex, striatum (namely, the caudate nucleus and the putamen), limbic structures (including the cingulate gyrus), olfactory tubercle, hippocampus, and the diencephalon (Kandel et al. 1995). Serotonin released in the diencephalon (consisting largely of the thalamus) and cerebrum plays an essential inhibitory role to help generate normal sleep (Guyton and Hall 1996). However, we found Trp administration decreased brain activation during the CS only in specific regions in frontal and temporal–parietal cortex; changes were not seen diffusely in regions of task-associated activation (see Parry et al. 2003). This suggests an interaction between Trp-mediated changes and local neuronal activity in mediating central fatigue. Interestingly, in Trp depletion studies, anterior cingulate activity was linked to administration of the Stroop colour-word test.
There were reduced blood oxygenation level-dependent signal increments after Trp administration. Some of the regions in which differential activation was defined have activity associated specifically with performance on the CS (e.g., the left inferior frontal gyrus). Other regions show activation changes that can be associated with components of the task (e.g., button presses in responses) that are behaviourally “matched” in the task (e.g., the left post-central gyrus). This interaction between Trp administration and task suggests a depression of central responsiveness to somatosensory input. This suggests the possibility that a similar modulation could contribute to central fatigue after exercise, during which substantial increases in p[FT] may occur. An alternative interpretation is that the reaction times are slowed by action elsewhere in the motor planning and control network, and that the changes in the sensory cortex are secondary to performance differences. However, the average frequency of movements was unchanged, and the degree of reaction time slowing was modest. The authors are not aware of previous reports suggesting changes in primary somatosensory cortex activation on the basis of simple reaction time for an overlearned movement.
Significant increases in relative task-related activity in the left precuneus and posterior cingulate cortex were observed with the Trp–Plac contrast. These regions normally have a high resting metabolic rate. Anatomical connections demonstrate that they are integrative regions with input from wide regions, including the mesial temporal lobe (Greicius et al. 2003). Functional studies have associated activity in the precuneus with general vigilance; activity is increased during resting periods in cognitive tasks, when subjects are alert, but attention is not directed (Gusnard and Raichle 2001). Activity is decreased in sleep (Maquet 2004). Observations of patients in vegetative states argue that precuneus connectivity and function is associated with conscious awareness (Laureys et al. 1999).
The interpretation of the relative increase in precuneus activity with the Stroop task in subjects after Trp cannot be unequivocal, as absolute measures of activity are not provided by fMRI. One possible interpretation is that task-associated activity is simply is greater after Trp than Plac. This would suggest that there is an enhancement of activity that is at least partially selective for this region. Alternatively, the results could suggest that the baseline (“resting”) activity of the precuneus is reduced by Trp administration to a greater extent than is the task-associated activity. This interpretation seems consistent with well-established behavioural observations that Trp ingestion induces drowsiness, as well as the more widespread reduction in responsiveness observed. In the pilot trials used to establish timing and dose of tryptophan for the present experiment, subjects reported a feeling of sleepiness and/or lethargy at about 30 min after ingestion. Thus, to the extent that Trp administration is a model for central fatigue, the changes we have observed therefore suggest that central fatigue is associated with depression of a “default” state of brain activity (Raichle et al. 2001).
As reviewed briefly in the introduction, animal studies have directly related p[FT] and p[TT] to brain 5-HT synthesis. This suggests that the substantial increase in p[FT] in the present study must have been associated with an increase in the rate of 5-HT synthesis in the brains of subjects studied after Trp ingestion. It is not possible to estimate reliably the extent of this increase, which may be expected to vary with brain region (and probably also between individuals). However, there are limitations to the neuropharmacological specificity possible in the interpretation of results. All of the effects need not be attributed directly to changes in serotoninergic transmission alone. The enzyme that converts Trp to 5-HT is not saturated under normal physiological conditions, and the transport of tryptophan into the brain is the rate-limiting step in 5-HT synthesis. The uptake of 5-HTP (metabolite of Trp and precursor to 5-HT) is not limited to 5-HT neurones (Fuxe et al. 1971; Lichtensteiger et al. 1967). As a consequence, 5-HTP may be converted to 5-HT in catecholamine-containing neurones, causing the release of catecholamines by displacement (Ng et al. 1972). Serotoninergic effects also may be mediated by the noradrenergic system, e.g., through changes in tonic activity of neurones of the dorsal noradrenaline (catecholamine) bundle modulating activity of the raphe system (Petitjean et al. 1975). However, lack of a complete understanding of the neuropharmacological changes does not preclude using Trp effects to induce a form of central fatigue and drawing inferences regarding the neural correlates of this fatigue.
Conclusions
Increasing plasma Trp and consequently p[FT] and brain Trp to a concentration that should substantially increase brain 5-HT is associated with activation changes in a region of the medial parietal cortex, the activity of which is associated with arousal state and awareness. An increase was also observed in specific task-associated regions including the primary somatosensory cortex. Previous behavioural studies suggest that Trp ingestion provides a model for central fatigue. To the extent that this is valid, our results argue that this state is associated with distributed changes in both primary sensory systems and in multimodal integration areas. Although subcortical changes described previously may contribute to mechanisms responsible for central fatigue, neocortical activity changes are associated with the experience of fatigue.
References
Baynes J, Dominiczak M (1999) Medical Biochemistry. Eds. Mosby
Blomstrand E, Perrett D, Parry-Billings M, Newsholme EA (1989) Effect of sustained exercise on plasma amino acid concentrations and on 5-hydroxytryptamine metabolism in six different brain regions in the rat. Acta Physiol Scand 136:473–481
Blomstrand E, Hassmen P, Ek S, Ekblom B, Newsholme EA (1997) Influence of ingesting a solution of branched chain amino acids on perceived exertion during exercise. Acta Physiol Scand 159:41–49
Bloxam DL, Warren WH (1974) Error in the determination of tryptophan by the method of Denckla and Dewey. A revised procedure. Anal Biochem 60:621–625
Bloxam DL, Tricklebank MD, Patel AJ, Curzon G (1980) Effects of albumin, amino acids, and clofibrate on the uptake of tryptophan by the rat brain. J Neurochem 34:43–49
Budgett R, Castell LM, Newsholme EA (1998) Overtraining and immunosuppression. In: Harries M (ed) Oxford textbook of sports medicine. Oxford University Press, Oxford
Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998) The counting Stroop: An interference task specialized for functional neuroimaging-validation study with functional MRI. Hum Brain Mapp 6:270–282
Chaudhuri A, Behan PO (2000) Fatigue and basic ganglia. J Neurol Sci 179:34–42
Cunliffe A, Obeid OA, Powell-Tuck J (1998) A placebo controlled investigation of the effects of tryptophan or placebo on subjective and objective measures of fatigue. Eur J Clin Nutr 52:425–430
Curzon G, Marsden CA (1975) Metabolism of a tryptophan load in the hypothalamus and other brain regions. J Neurochem 25:251–256
Duvernoy HM (1995) In: The human brain stem and cerebellum. Springer, Berlin Heidelberg New York
Fernstrom JD (1990) Aromatic amino acids and monoamine synthesis in the central nervous system: influence of the diet. J Nutr Biochem 1:508–517
Fernstrom JD, Wurtman RJ (1972) Brain serotonin
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, Comi G (2002) Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. NeuroImage 15:559–567
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–647
Friston KJ, Worsley KJ, Frakowiak RSJ, Mazziotta JC, Evans AC (1992) Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1:214–220
Fuxe K, Butcher LL, Engel J (1971) d l-5-hydroxytryptophan-induced changes in central monoamine neurons after peripheral decarboxylase inhibition. J Pharm Pharmacol 23:420–424
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci 100:253–258
Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 10:685–694
Guyton AC, Hall JE (1996) Textbook of Medical Physiology. WB Saunders Co, Philadelphia
Jenkinson M, Smith S (2000) Global optimisation for robust affine registration. In: Sixth International Conference on Functional Mapping of the Human Brain. Texas, USA, p 479, June
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration. Med Image Anal 5:143–156
Kandel ER, Schwartz JH, Jessell TM (1995) In: Essentials of neural science and behavior. Appleton and Lange, New York
Laureys S, Goldman S, Phillips C, Van Bogaert P, Aerts J, Luxen A, Franck G, Maquet P (1999) Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. NeuroImage 9:377–382
Lichtensteiger W, Mutzner U, Langemann H (1967) Uptake of 5-hydroxytryptamine and 5-hydroxytryptophan by neurons of the central nervous system normally containing catecholamines. J Neurochem 14:489–497
Lieberman HR, Corrin S, Spring BJ, Growdon JH, Wurtman RJ (1982) Mood performance and pain sensitivity: changes induced by food constituents. J Psychiatr Res 17:135–145
Maquet P (2004) A role for sleep in the processing of memory traces. Contribution of functional neuroimaging in humans. Bull Mem Acad R Med Belg 159:167–170
McGuire J, Ross GL, Price H, Mortensen N, Evans J, Castell LM (2003) Biochemical markers for post-operative fatigue after major surgery. Brain Res Bull 60:125–130
Meeusen R, Thorre K, Chaouloff F, Sarre S, De Meirleir K, Ebinger G, Michotte Y (1996) Effects of tryptophan and/or acute running on extracellular 5-HT and 5-HIAA levels in the hippocampus of food-deprived rats. Brain Res Bull 740:245–252
Newsholme EA, Leech AR (1983) In: Biochemistry for the medical sciences. Wiley, Chichester p 802
Ng LK, Chase TN, Colburn RW, Kopin IJ (1972) Release of (3 H) dopamine by l-5-hydroxytryptophan. Brain Res 45:499–505
Nybo L, Secher NH (2004) Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol 72:223–261
Parry A, Corkill R, Blamire AM, Palace J, Narayanan S, Arnold D, Styles P, Matthews PM (2003) Beta-interferon treatment does not always slow the progression of axonal injury in multiple sclerosis. J Neurol 250:171–178
Petitjean F, Sakai K, Blondaux C, Jouvet M (1975) Hypersomnia by isthmic lesion in cat. II. Neurophysiological and pharmacological study. Brain Res 88:439–453
Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682
Sharpe M, Archard LC, Banatvala JE et al (1991) A report-chronic fatigue syndrome: guidelines for research. J R Soc Med 84:118–121
Thorleifsdottir B, Bjornsson JK, Kjeld M, Kristbjarnarson H (1989) Effects of l-tryptophan on daytime arousal. Neuropsychobiology 21:170–176
Woolrich M, Ripley B, Brady J, Smith S (2000) Nonparametric estimation of temporal autocorrelation in FMRI. In: Sixth International Conference on Functional Mapping of the Human Brain. Texas, USA, p 610, June
Worsley KJ, Evans AC, Marrett S, Neelin P (1992) A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12:900–918
Wurtman JJ (1988) Carbohydrate craving, mood changes, and obesity. J Clin Psychiatry 49:37–39
Yamamoto T, Newsholme EA (2000) Diminished central fatigue by inhibition of the l-system transporter for the uptake of tryptophan. Brain Res Bull 52:35–38
Acknowledgement
We are grateful to the subjects for their cheerful participation, to Scientific Hospital Supplies for providing the tryptophan and placebo, to Professor Peter Jezzard for assistance in scanning, and to Professor Phil Cowen for pharmacological advice. RMA was in receipt of a grant from the Brazilian Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morgan, R.M., Parry, A.M.M., Arida, R.M. et al. Effects of elevated plasma tryptophan on brain activation associated with the Stroop task. Psychopharmacology 190, 383–389 (2007). https://doi.org/10.1007/s00213-006-0609-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0609-7