Abstract
Rationale
Previous studies in rats showed that postnatal day (P)11–20 exposure to ±3,4-methylenedioxymethamphetamine (MDMA, ecstasy) causes learning and memory deficits in adulthood. The emergence and permanence of these learning deficits are currently unknown.
Objective
This study was designed to investigate learning and memory deficits in adolescent (P30 or P40) and older (P180 or P360) rats exposed to MDMA from P11–20.
Materials and methods
Within each litter half the animals were exposed to MDMA (20 mg/kg) and half to saline (SAL) twice a day (8 h apart) from P11–20. In experiment (exp) 1, behavioral testing began on either P30 or P40, whereas in exp 2, testing began on either P180 or P360. Offspring were tested in the Cincinnati water maze (CWM), a test of path integration learning (2 trials/day for 5 days), and the Morris water maze (MWM) (three phases, with 5 days of 4 trials/day and a probe trial on the sixth day per phase).
Results
MDMA-treated rats took longer to find the platform and traveled a greater distance to find the platform at all ages tested in all phases of the MWM. MDMA-treated animals also spent less time in the target quadrant during probe trials. In the CWM, P30 and P40 animals took longer to find the goal and committed more errors in locating the goal, while P180 and P360 MDMA-treated animals performed similarly to SAL-treated animals.
Conclusion
The data suggest that the spatial learning and memory deficits induced by MDMA are long lasting, while the path integration deficits recover over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The principal abusers of the popular club drug 3,4-methylenedioxymethamphetamine (MDMA) are young adults. In the United States in 2004, 2.2–2.4% of high school seniors and college age adults reported annual MDMA use and half of these were women (Johnston et al. 2005a,b). A predictable percentage of women who use MDMA during these peak reproductive ages will become pregnant and some of these will use MDMA during some portion or throughout pregnancy (Ho et al. 2001). Maternal use of substituted amphetamines, including MDMA and methamphetamine result in exposure to the fetus, as these substances are lipophilic and therefore easily cross the placental (Burchfield et al. 1991) and the blood brain barriers. To date, there have been few clinical studies of the effects of prenatal exposure to MDMA. MDMA exposure may increase the incidence of congenital malformations, namely, cardiac malformation and clubfoot (McElhatton et al. 1997, 1999). No studies have specifically investigated the cognitive and behavioral alterations of children prenatally exposed to MDMA.
The neonatal rat can be used as a model of second and third trimester human development because brain development in some regions is comparable between the two organisms at this stage. For example, the development of the granule cells of the dentate gyrus continues to postnatal day (P)19 in rats and until birth in humans (Bayer et al. 1993). This cross-species comparison is supported by a newer brain comparative algorithm that can be found via the Internet (Clancy et al. 2006). Exposure to MDMA from P11–20 in rats induces spatial and path integration learning and memory deficits when the animals are tested during adulthood; however exposure from P1–10 induces no deficits in either learning paradigm (Broening et al. 2001). In the Morris water maze (MWM), MDMA-treated animals show increased latencies, path lengths, and cumulative distances from the platform during acquisition, reversal (where the platform is moved to the opposite quadrant), and reduced platform (the platform is reduced from 10 × 10 cm to 5 × 5 cm and moved to the original quadrant) phases of hidden platform training (Broening et al. 2001; Williams et al. 2003; Vorhees et al. 2004; Cohen et al. 2005). During probe (memory) trials, MDMA-treated animals had a greater average distance from the platform than saline (SAL)-treated animals. The learning and memory deficits in the MWM appear to be independent of sensorimotor changes, as MDMA-treated animals performed similarly to SAL-treated animals in the visible platform phase of the MWM (Broening et al. 2001; Williams et al. 2003). Unlike spatial learning in which the animal uses external cues to navigate the maze, path integration learning requires an animal to use its own locomotion cues in reference to the start while exploring the environment to locate a goal (Etienne and Jeffery 2004). Path integration learning after developmental MDMA exposure was assessed using the Cincinnati water maze (CWM; described in “Materials and methods” and Vorhees 1987b). MDMA-treated animals took longer to find the escape and committed more errors compared to their SAL-treated counterparts in the CWM (Broening et al. 2001; Williams et al. 2003; Cohen et al. 2005). The learning deficits are independent of undernutrition because MDMA-treated animals showed a decrease in performance in the MWM and the CWM compared to animals raised in a large litter that mimicked the undernutrition produced by MDMA exposure (Williams et al. 2003). Maternal behavior could be an issue because neonates exposed to MDMA from P1–4 exhibit increased activity and ultrasonic vocalizations (Winslow and Insel 1990), behaviors that are known to affect dam/pup interactions. However, the effects of MDMA from P11–20 are independent of litter composition or maternal behavior (Williams et al. 2003) because the use of either a between- or within-litter design for MDMA treatment produced the same deficits on MWM performance (Broening et al. 2001; Williams et al. 2003).
The purpose of this experiment was to examine the emergence and relative permanence of MWM and CWM deficits seen in neonatally MDMA-treated animals. Two experiments were performed. The first experiment examined spatial and path integration learning and memory when tested early, during adolescence (P30 and P40), while the second experiment examined learning and memory when tested much later than in previous studies, during later adulthood (P180 and P360).
Materials and methods
Subjects and treatments
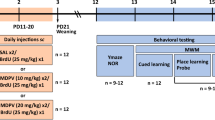
Nulliparous female Sprague–Dawley CD, IGS rats were obtained from Charles Rivers Laboratories (Raleigh, NC, USA) and were allowed to acclimate to the temperature (21 ± 1°C) and light cycle (14:10 h light–dark cycle with lights on at 0600 h) of the vivarium for a minimum of 1 week before breeding with males of the same strain. Food and water were available ad libitum throughout the experiment. Breeding occurred in hanging wire cages and the day a sperm plug was detected was considered embryonic day (E) 0. On E14 females were singly housed in polycarbonate cages (46 × 24 × 20 cm) and left undisturbed until parturition. Date of birth was considered P0, and on P1 litters were culled to eight with equal numbers of males and females. Animals were randomly assigned to one of two treatment groups within each litter. Half of the litter, divided equally by sex, received ± MDMA HCl (20 mg/kg, expressed as freebase; > 95% pure and obtained from the National Institute on Drug Abuse) twice a day delivered 8 h apart while the other half received SAL. MDMA or SAL was administered subcutaneously in the dorsum at a dosing volume of 3 ml/kg. Injection sites were varied and no visible signs of skin lesions were observed. Using interspecies scaling (Mordenti and Chappell 1989), the doses administered to the offspring in this study are, by extrapolation, consistent with what are seen in chronic MDMA users (Green et al. 2003). These doses are also consistent with previous studies from our lab that show that this dose and regimen of MDMA are effective in inducing learning and memory deficits in neonatally treated animals. This dose of MDMA did not result in any mortality or hyperthermia. It was previously shown that MDMA does not induce hyperthermia in preweaning rats (Broening et al. 1994). In addition, despite initial weight loss and evidence of elevated gait in older pups (∼P15–20) while the drug is on board, MDMA-treated offspring had milk in their stomachs and did not exhibit observable alterations during interactions with their dam.
The vivarium is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and all protocols were approved by the Cincinnati Children’s Research Foundation’s Animal Use and Care Committee. For each experiment, 20 litters were used. For experiment (exp) 1, litters were divided equally so that half of the litter with one treatment pair per sex began behavioral testing at P30 while the other half of the litter began testing at P40. Eight groups were created per litter with this design split by age tested, treatment, and sex. Exp 2 followed the same design with the exception that testing began on either P180 or P360.
Straight channel swimming
Beginning on the appropriate day, animals were tested in a 15 × 244-cm straight channel filled to 35 cm with 21 ± 1°C water and with a wire escape ladder mounted on one end. Animals were placed in the channel facing away from the ladder (i.e., facing the back wall of the tank). Latency to escape was measured on four consecutive trials with a maximum time of 2 min/trial with no intertrial interval (ITI).
Cincinnati water maze
Three days after straight channel, animals were tested in the CWM for 6 days (exp 1) or 5 days (exp 2). The CWM, described in detail previously (Vorhees 1987b; Fig. 1), is a 9-unit multiple T-maze constructed of black acrylic and positioned in a larger square tank. The cul-de-sacs and main channel are 15 cm wide and the walls are 51 cm high. Water depth is 25 cm and maintained at a temperature of 21 ± 1°C. Testing occurred under a single red 25-W light bulb to minimize the use of distal cues. Animals were started facing the wall in position B as described in Vorhees (1987b) and were allowed 5 min to attempt to escape the maze at position A where a wire ladder was placed for escape. Animals were administered two trials per day for 5 days with an ITI of 5 min. Latency to escape the maze and errors of commission were counted for each trial. An error was defined as a whole body entry into a dead-end arm. Repetitive arm entries within a dead-end T of the maze were each counted as multiple errors; one for entering the first arm of the T and one for each subsequent crossing into an opposite arm.
The CWM is a multiple T-maze that is used to assess path integration learning. The maze is filled with water to a depth of 25 cm and the animal is started in position “B.” Latency to reach a wire ladder at position A is recorded as are errors of commission. An error is considered an entry or perseveration in a dead end “T.” Animals were tested under red light conditions (single 25-W bulb) for two trials per day for 5 or 6 days
Morris water maze
The MWM is 210 cm in diameter, constructed of stainless steel, and painted black. On three walls (arbitrarily representing the N, E, and W points) large geometric figures were mounted. White curtains, which can be opened or closed to reveal or obscure room cues, surround the maze. Each phase consisted of 4 trials/day for 5 days, and on the sixth day a probe trial (30 s) was performed. The time limit for each learning trial was 2 min; animals that failed to find the platform were placed on it. The ITI was 15 s on the platform. The platforms were either 10 × 10 or 5 × 5 cm and made of acrylic with a thin nylon mesh attached. The platforms were submerged 1.5–2.0 cm below the water. Water temperature was 21 ± 1°C.
Animals were tested in three phases of the MWM: acquisition, reversal, and reduced. The acquisition phase began 1 (exp 1) or 2 (exp 2) days after the cessation of the CWM and subsequent phases began the day after the probe trail of the previous phase. In each phase, platform position was counterbalanced so that during acquisition, half the litters were trained to the platform in the NE quadrant, while the second half was trained to the SW quadrant. For the reversal phase, the platform was moved to the opposite quadrant of the acquisition phase (i.e., those trained to the NE were now trained to the SW). For the reduced phase, the platform was moved back to the quadrant used for acquisition. The 10 × 10 cm platform was used in the acquisition and reversal phases, while the 5 × 5 cm platform was used in the reduced phase. Four start positions were used randomly with the exception that no start position was used twice on a given day and the pattern of start positions was varied on consecutive days. The start positions used for the SW were N, E, SE, and NW. The start positions used for the NE were S, W, NW, and SE. On probe trials, the platform was removed and the animals were given one 30-s trial beginning from a novel start location (NE for SW platform; SW for NE platform).
Performance was recorded using a Polytrack video tracking system (San Diego Instruments, San Diego, CA, USA). Latency, path length, cumulative distance, and first bearing were measured for learning trials. For probe trials, average distance, first bearing, platform crossings, time in target quadrant, and distance in the target quadrant were measured. Cumulative distance is the sum of the distances the animal was from the platform measured in 55-ms intervals. First bearing was calculated by measuring the heading after the first 13 cm of travel relative to a straight line from the start position to the platform.
Statistical methods
Because each experiment used a split litter design, offspring were matched on multiple factors by virtue of being littermates (Kirk 1993). To ensure that litter effects were controlled, litter was treated as a random factor (block) in a complete randomized block model analysis of variance (ANOVA). In this model, treatment, sex, and age at testing were between factors within each block and litter was the blocking factor. Measures taken repetitively on the same animal were treated as repeated measure factors. For maze testing, the ANOVA was a 3-between randomized and 1-within randomized block model. In this model, treatment had two levels (MDMA or SAL), sex had two levels, and age had two levels (P30/P40 for exp 1 or P180/P360 for exp 2), while test interval had five levels (day). Data were analyzed using SAS Proc Mixed (SAS Institute, Carey, NC, USA). Each model was checked for best fit against covariance matrix models provided by Proc Mixed. Autoregressive [AR(1)] covariance structure was optimal in most cases, however in a few cases compound symmetry was the better fit. Proc Mixed provides adjusted degrees of freedom and do not match those used in standard ANOVAs and therefore can be fractional or different even among multiple dependent variables within a given behavioral test (i.e., latency, path length, and cumulative distance in the MWM). Significant interactions were analyzed using one-way simple-effect slice ANOVAs at each level of the repeated measure factor. For the sake of clarity, F values will only be shown for effects involving treatment. Significance was considered at P≤0.05 and trends at P ≤ 0.1. Data are presented as the least square means ± SE derived from the ANOVA from Proc Mixed.
Results
Body weights
For exp 1, a main effect of treatment [F(1, 149) = 208.54, P < 0.0001; Fig. 2a] was observed during the dosing period with MDMA-treated animals weighing less than SAL-treated animals. A treatment × day interaction was also observed [F(9, 1,382) = 54,25, P < 0.0001] and showed that beginning on P12, MDMA-treated animals weighed significantly less than SAL-treated counterparts. After weaning, a main effect of treatment was observed for body weight [F(1, 85.6) = 7.05, P = 0.0095; Fig. 2b] with MDMA-treated animals weighing significantly less than SAL-treated animals. There were significant sex (males weighed more than females) and day (all animals gained weight over time) effects observed both in dosing and postweaning weights.
Mean (± SEM) body weights during dosing (a, c) and testing (b, d) for exp 1 (a, b) and exp 2 (c, d). Rats were treated with 20 mg/kg of MDMA or SAL twice daily from P11–20. MDMA administration produced significant decreases in weight gain from P11–20; however, rats quickly return to control levels. *P < 0.05 compared to SAL
For exp 2, a main effect of treatment [F(1, 137) = 320.06, P < 0.0001; Fig. 2c] was observed during dosing with MDMA-treated animals weighing less than SAL-treated animals. A treatment × day interaction [F(9, 1,398) = 13.84, P < 0.0001] was observed with MDMA-treated animals having significantly lower body weights beginning on P11 than SAL-treated animals. For adult weights, no significant main effect or interaction with treatment was observed (Fig. 2d). Main effects of sex and day were observed during dosing and adulthood with effects similar to those in exp 1.
Experiment 1: postnatal day 30 and 40 testing
Straight channel swimming
No main effect for treatment was observed in latency to escape the 244-cm straight channel.
Cincinnati water maze
MDMA-treated animals took longer to reach the escape ladder [F(1, 131) = 11.55, P < 0.001] and committed more errors [F(1, 133) = 8.45, P < 0.01; Fig. 3] than SAL-treated animals. No treatment × age effect was observed. A trend for a treatment × day interaction was observed for latency [F(5, 551) = 2.07, P < 0.1] but not for errors. Post hoc analysis on each day showed that MDMA-treated animals took longer to reach the escape ladder on days 1–4 compared to SAL-treated animals, and asymptotic levels were achieved by day 5 (not shown).
Morris water maze: acquisition phase
Path length is shown as the representative measure (Fig. 4a). MDMA-treated animals took longer to find the hidden platform [F(1, 125) = 17.21, P < 0.0001], traveled a greater path [F(1, 124) = 23.77, P < 0.0001], had greater cumulative distances from the platform [F(1, 126) = 27.58, P < 0.0001], and started at a greater angle of first bearing to the platform [F(1, 125) = 24.12, P < 0.0001] compared to SAL-treated animals. A treatment × day interaction for cumulative distance [F(4, 415) = 2.35, P ≤ 0.05] was the only interaction seen during the acquisition phase. This interaction was not followed up because it was not supported by comparable effects on any other measure. For all measures during all three phases of the MWM in this experiment, there was a main effect of day, with animals performing better over consecutive days of training.
Mean path length for hidden platform (a, c, and e) or percent time in target quadrant during probe trials (b, d, and f) in P30 and P40 animals. Rats treated with MDMA from P11–20 show a main effect of treatment with increased path length during acquisition (a), reversal (c), and reduced platform (e) phases of the MWM and decreased time in the target quadrant during probe trials (b, d, and f, respectively). † P<0.10,*P < 0.05 and *** P < 0.001 compared to SAL
During probe trials, MDMA-treated animals had a higher angle of first bearing [F(1, 104), P < 0.01], crossed the platform site less [F(1, 104) = 8.24, P < 0.01], and spent less time [F(1, 104) = 4.27, P = 0.0413] and traveled less distance in the target quadrant [F(1, 104) = 5.24, P < 0.05] than SAL-treated animals. Percent time in target quadrant is shown in Fig. 4b. For time in the target quadrant, there was a treatment × sex interaction [F(1, 104) = 4.33, P < 0.05] and a treatment × age interaction [F(1, 104) = 3.13, P < 0.10]. For the treatment × age interaction, effect slice ANOVAs revealed treatment effects at P30 (P < 0.01), however no effect on P40. Treatment × sex analysis revealed that females showed a treatment effect (SAL = 37.2 ± 2.4% and MDMA = 27.8 ± 2.4%; P < 0.01), while males (SAL = 36.8 ± 2.4% and MDMA = 36.8 ± 2.5%) did not. For distance in the target quadrant, a trend for a treatment × age interaction was observed [F(1, 104) = 3.33, P < 0.10], suggesting that P30 animals were more affected by MDMA treatment (P < 0.01) than P40 animals.
Morris water maze: reversal phase
As seen during the acquisition phase, MDMA-treated animals had longer latencies [F(1, 137) = 21.55, P < 0.0001], greater path lengths [F(1, 135) = 22.06, P < 0.0001; Fig. 4c] and cumulative distance from the platform [F(1, 135) = 18.83, P < 0.0001], and had a larger angle of first bearing to the platform [F(1, 117) = 16.94, P < 0.0001] than SAL-treated animals. Treatment × age effects were seen for latency [F(1, 134) = 4.53, P < 0.05], path length [F(1, 135) = 7.18, P < 0.01], and cumulative distance [F(1, 135) = 6.73, P < 0.01]. For latency, slice-effect ANOVA revealed significant treatment effects at P30 (P < 0.001) and P40 (P < 0.05). For path length, MDMA treatment increased path length on P30 (P < 0.0001) and showed a trend toward increased path length at P40 (P < 0.10). MDMA increased the cumulative distance from the platform in P30 animals (P < 0.0001) but not in P40.
During reversal phase probe trials, MDMA treatment increased first bearing [F(1, 112) = 7.78, P < 0.01], decreased platform crossings [F(1, 112) = 5.26, P < 0.05], and decreased time [F(1, 112) = 4.57, P < 0.05; Fig. 4d] and distance [F(1, 112) = 4.91, P < 0.05] in the target quadrant. There was also a trend toward a treatment effect in average distance from the platform [F(1, 112) = 3.38, P < 0.10]. Treatment × age interactions were observed for first bearing [F(1, 112) = 10.59, P < 0.001], platform crossings [F(1, 112) = 7.25, P = 0.01], time [F(1, 112) = 6.48, P < 0.01], and distance [F(1, 112) = 7.65, P < 0.01] in the target quadrant. MDMA treatment increased the angle of first bearing (P < 0.001), and decreased platform crossings (P < 0.001), time (P < 0.001), and distance (P < 0.001) in the target quadrant on P30; however, no effects of treatment were seen in P40.
Morris water maze: reduced platform trials
Treatment effects were seen for latency [F(1, 136) = 26.77, P < 0.0001], path length [F(1, 135) = 24.47, P < 0.0001; Fig. 4e], cumulative distance [F(1, 136) = 22.74, P < 0.0001], and angle of first bearing [F(1, 117) = 14.37, P < 0.001]. Treatment × age interactions were observed for latency [F(1, 136) = 6.45, P < 0.01], path length [F(1, 135) = 7.27, P = 0.01], and cumulative distance [F(1, 136) = 7.59, P = 0.01]. For latency, MDMA treatment increased time to reach the platform at P30 (P < 0.0001) and P40 (P < 0.05). For path length, MDMA treatment increased the distance to reach the platform on P30 (P < 0.0001) and showed a trend toward increased path length on P40 (P < 0.10). Similar effects were seen for cumulative distance (P30, P < 0.0001; P40, P < 0.10).
For probe trials during the reduced phase, MDMA treatment increased the angle of first bearing [F(1, 109) = 9.83, P < 0.01], reduced platform crossings [F(1, 109) = 6.33, P < 0.01], time [F(1, 109) = 4.42, P < 0.05; Fig. 4f], and distance [F(1, 109) = 5.03, P < 0.05] in the target quadrant. No treatment × age interactions were observed for any parameter.
Experiment 2: postnatal day 180 and 360 testing
Straight channel swimming
No effects of treatment were seen for straight channel swimming.
Cincinnati water maze
No effects of treatment were observed for latency and errors in the CWM (data not shown). An effect of day was observed for both measures, showing that the animals, regardless of treatment, learned the task. A main effect of age was observed for latency. Further investigation showed that P360 animals took longer to reach the platform than P180 animals.
Morris water maze: acquisition
Similar to the effects observed in the young animals, MDMA-treated older animals took longer to reach the platform [F(1, 130) = 9.08, P < 0.01], traveled a greater path to the platform [F(1, 130) = 9.22, P < 0.01; Fig. 5a], remained farther away from the platform [F(1, 131) = 11.86, P < 0.001], and started with a larger angle of first bearing to the platform [F(1, 129) = 38.19, P < 0.0001] compared to SAL-treated animals. A main effect of age was observed for latency, path length, and cumulative distance that demonstrated that P180 animals found the platform faster and with a shorter distance traveled than P360 animals. A main effect of day was observed for this and all phases of the MWM in exp 2 showing that all animals learned the task over days. No treatment × age interaction was observed for the acquisition phase of the MWM.
Mean path length for hidden platform (a, c, and e) or percent time in target quadrant during probe trials (b, d, and f) in P30 and P40 animals. Rats treated with MDMA from P11–20 show a main effect of treatment with increased path length during acquisition (a), reversal (c), and reduced platform (e) phases of the MWM and decreased time in the target quadrant during probe trials (b, d, and f, respectively). *P < 0.05 and *** P < 0.001 compared to SAL
For the probe trial, neonatal MDMA treatment increased average distance from the platform [F(1, 121) = 7.11, P < 0.01], angle of first bearing [F(1, 121) = 5.85, P < 0.05], and decreased platform crossings [F(1, 121) = 6.96, P < 0.01], but percent time in the target quadrant was not significant (Fig. 5b). For platform crossings, an interaction of treatment × age [F(1, 121) = 4.46, P < 0.05] was observed and showed that MDMA-treated animals crossed the platform fewer times when tested at P180 compared to SAL-treated animals; however, there was no treatment effect at P360. A main effect of sex was observed for average distance, first bearing, and platform crossings with males being closer to the platform, having a smaller first bearing, and crossing the platform more often than females.
Morris water maze: reversal phase
Consistent with the acquisition phase of this experiment and the previous experiment, MDMA treatment increased latency [F(1, 129) = 15.53, P < 0.0001] to reach the platform, increased the path taken to the platform [F(1, 130) = 6.47, P < 0.01; Fig. 5c], and the distance from the platform [F(1, 131) = 10.12, P < 0.001]. MDMA-treated animals also started with a greater angle of first bearing to the platform [F(1, 129) = 50.91, P < 0.0001]. For all measures, a sex effect was observed with males performing better in the maze compared to females. An age effect was observed for latency, path length, and cumulative distance with younger animals finding the platform more efficiently than older animals. For path length, a treatment × sex × age interaction was observed [F(1, 130) = 6.47, P < 0.05]. This interaction was minor and did not materially change the interpretation of the findings based on the main effects noted above.
In the probe trial, a main effect of treatment was observed for first bearing [F(1, 128) = 19.85, P < 0.0001], platform crossings [F(1, 128) = 3.95, P < 0.05], time [F(1, 28) = 4.29, P < 0.05; Fig. 5d], and distance [F(1, 128) = 5.41, P < 0.05] in the target quadrant. MDMA-treated animals were farther off course, spent less time, traveled less in the target quadrant, and crossed the platform fewer times than SAL-treated animals. A treatment × sex × age interaction was observed for distance [F(1, 128) = 4.13, P < 0.05] and time in the target quadrant [F(1, 128) = 4.47, P < 0.05]. Subsequent analyses showed that MDMA treatment decreased time (P < 0.01) and distance (P < 0.01) in the target quadrant for males at P180 (means for percent time: SAL = 39.8 ± 3.3% and MDMA = 31.9 ± 3.3%, P < 0.01) but not for females (percent time: SAL = 25.6 ± 4.2% and MDMA = 33.0 ± 4.2%, P > 0.05) and females at P360 (P < 0.01 and P < 0.05, respectively; means for percent time: SAL = 36.6 ± 3.37% and MDMA = 27.3 ± 3.37%, P < 0.01, but not for males: SAL = 30.6 ± 4.5% and MDMA = 23.4 ± 4.4%, P > 0.05).
Morris water maze: reduced phase
Main effects of treatment were observed with MDMA-treated animals having a longer latency [F(1, 130) = 25.30, P < 0.0001], path length [F(1, 128) = 17.23, P < 0.0001; Fig. 5e], cumulative distance [F(1, 129) = 23.31, P < 0.0001], and angle of first bearing [F(1, 129), P < 0.0001]. Cumulative distance [F(1, 129) = 4.93, P < 0.05] and path length [F(1, 128) = 8.21, P < 0.01] showed a treatment × sex × age interaction that indicated that MDMA-treated females at P180 and MDMA-treated males at P360 had greater path lengths and were further from the platform than SAL-treated counterparts. Main effects of sex and day were observed on all measures, with males performing better than females and all animals had improved performance over days.
For probe trials, main effects of treatment were observed with MDMA-treated animals having greater average distances from the platform [F(1, 123) = 23.85, P < 0.0001], decreased platform crossings [F(1, 123) = 4.57, P < 0.05], larger angles of first bearings [F(1, 123) = 13.57, P < 0.001], and decreases in time [F(1, 123) = 15.15, P < 0.001; Fig. 5f] and distance [F(1, 123) = 23.85, P < 0.0001] in the target quadrant. A treatment × age interaction was observed only for distance traveled in the target quadrant [F(1, 123) = 11.77, P < 0.01]. A main effect of sex was observed with males performing better than females during the probe trials.
Discussion
We have previously shown that MDMA treatment from P11–20 causes spatial and path integration learning and memory deficits when the animals are tested at P60 (Broening et al. 2001; Williams et al. 2003; Vorhees et al. 2004) and at approximately P90 (Cohen et al. 2005). The purpose of this study was to further elucidate the behavioral effects of MDMA by testing younger (P30 and P40) and older (P180 and P360) rats in the MWM and CWM. We found that MDMA treatment caused deficits in the hidden platform and probe trial phases of the MWM at all ages tested. It is interesting to note that path integration deficits, examined by the CWM, were seen in the animals tested at P30 or P40, but not in animals tested at P180 or P360. The path integration deficits do not appear to be the result of the SAL-treated animals performing more poorly in this task as they age because control performance was similar at all ages. Instead, the data suggest that the effects of MDMA on path integration learning are transient and persist only into early adulthood. Path integration is thought to be one of the most basic types of learning found in organisms from ants to humans (Etienne and Jeffery 2004). Currently, the brain region or regions responsible for path integration learning are not well defined, although several regions were implicated (see Etienne and Jeffery 2004). Path integration and spatial mapping share overlapping neural networks (Rondi-Reig et al. 2006; Sargolini et al. 2006). It is possible that spatial learning is more sensitive to neuronal insult during early development than path integration circuits or that path integration circuits feed into to spatial circuits redundantly such that path integration may recover even as spatial mapping is disrupted. This is further supported by the type of learning required to complete the MWM compared to the CWM. In the MWM, the animal learns to navigate to the goal using extramaze cues, while the CWM requires the animal to use self-movement cues to locate its position relative to the start and goal. In the MWM, an experienced animal has no trouble finding the goal if a novel start position is used, however, in the CWM, even an experienced animal’s performance is disrupted if the start or goal is moved because the trajectory of the path is rearranged. In the CWM, as run here with overhead lighting, it is likely that animals use some combination of both place and path cues. To fully dissociate these contributions to CWM performance, newer data indicate animals must be tested under infrared conditions that prevent the animal from using distal cues (unpublished observations). While treatment effects were still significant in the MWM, age was also a factor with P180 animals performing better than P360 animals in hidden platform training.
This is the first study to examine path integration learning in younger rats (i.e., P30) using the CWM. As previously stated, the younger animals treated with SAL performed similarly to their older counterparts. The differences seen in the MDMA-treated animals at P30 compared to SAL-treated animals at this age show that the CWM is effective in detecting learning deficits in younger animals. The CWM may also be more effective in detecting learning deficits caused by amphetamine analogs. For example, adult animals treated with the potent serotonin (5-HT)-depleting agent fenfluramine fail to show deficits in the MWM; however, they show deficits in CWM learning (Williams et al. 2002; Skelton et al. 2004) and developmental exposure to fenfluramine induces both CWM and MWM deficits (Morford et al. 2002). Administration of MDMA to adult animals produces CWM deficits while producing only memory deficits in the MWM (Sprague et al. 2003; Cohen et al. 2005; Able et al. 2006).
While MDMA-treated animals do not perform as well in the MWM as SAL-treated animals, the MDMA-treated animals do learn the task. Learning curves for treatments by age are shown in Fig. S1. Similar learning curves were seen for the CWM. It is interesting to note that there are no effects of sex in the younger animals in the MWM while the older animals show sex-related learning differences: Males performed better than the females in the MWM. This sex difference is likely due to the activational effects of gonadal steroids because the P30 and P40 animals were tested before puberty or during the transition from puberty to adulthood. Therefore, the incomplete maturation of the gonadal hormones may prevent the sexually dimorphic effects seen in young-adult and older animals (tested after P60) (Broening et al. 2001; Williams et al. 2003; Vorhees et al. 2004). Slight weight differences were seen in the younger animals; however, these did not appear to affect the motor performance of MDMA-treated animals. For example, all animals performed similarly in straight channel swimming, a measure that was previously shown to uncover motor deficits in offspring exposed to valproic acid (Vorhees 1987a). Offspring exposed to MDMA were also shown not to be affected in the visible platform phase of the MWM (Broening et al. 2001; Vorhees et al. 2004; Williams et al. 2005), which further supports that MDMA does not induce deficits to sensorimotor systems. The weight differences seen in the pups during dosing do not appear to be due to the inability of the animals to nurse, as large litter control groups that simulate comparable degrees of undernutrition to that induced by MDMA do not show learning and memory deficits as do the MDMA-treated offspring (Williams et al. 2003). Further, treating animals using split- or between-litter designs that could create theoretically different maternal–pup interactions produce essentially identical effects after developmental MDMA treatment on later learning (Broening et al. 2001; Williams et al. 2003).
The dose of MDMA used in this study was 20 mg/kg. Typically, the recreational user of MDMA consumes 100–150 mg of MDMA per use, or approximately 2 mg/kg for a 60-kg human (Green et al. 2003). However, using the interspecies scaling formula, DoseHuman = DoseAnimal[WTHuman/WTAnimal]0.7, the dose to a 25-g pup on P11 is equivalent of a 116-mg dose to a 60-kg human (Mordenti and Chappell 1989). On P20, with the average weight of a pup increasing to approximately 40 g, the equivalent human dose is equal to a 133-g dose to the same 60-kg human. Both of these doses are well within the range of human exposure, and may actually be below doses used by chronic abusers (Scholey et al. 2004; Kouimtsidis et al. 2006). Furthermore, it was shown that administration of 10 mg/kg to rats creates similar concentrations in plasma of MDMA to a human who consumes a 150-mg tablet (Green et al. 2003).
The mechanisms related to MDMA-induced learning and memory deficits are still unclear. P11–20 MDMA exposure slightly decreases hippocampal 5-HT levels when examined as adults; however, these differences appear to be unrelated to the learning and memory deficits because animals that were treated from P1–10 showed similar 5-HT reductions but no learning and memory deficits (Broening et al. 2001). A possible mechanism involving 5-HT signaling, however, does exist. First, recent data showed that after MDMA exposure on P11, hippocampal 5-HT levels drop sharply (Williams et al. 2005; Schaefer et al. 2006). Second, MDMA treatment from P11–20 increases 5-HT1A receptor activity in the hippocampus (Crawford et al. 2006). Third, depletion of 5-HT with the tryptophan hydroxylase inhibitor p-chlorophenylalanine from P10–20 was shown to cause deficits in the radial arm maze in adult animals (Mazer et al. 1997). Similar depletions were shown to alter synaptic architecture and decrease long-term potentiation in brain slice preparations (for review, see Sodhi and Sanders-Bush 2004). Therefore, early 5-HT perturbation may permanently increase 5-HT signaling, such that 5-HT reductions per se are not as important as changes in receptors (Williams et al. 2002; Skelton et al. 2004). Alterations in brain-derived neurotrophic factor (BDNF) levels may be another influence for the learning deficits after MDMA administration because it was reported that P11–20 MDMA exposure increases BDNF levels in the hippocampus and striatum on P21 (Koprich et al. 2003). BDNF is known to play a role in learning and memory, neuronal plasticity, and survival (Figurov et al. 1996; Linnarsson et al. 1997). Another possible mechanism for the long-term learning and memory deficits may involve the hypothalamic-pituitary-adrenal axis. In rodents, the stress hyporesponsive period (SHRP) begins at approximately P2 and extends to P14 (Sapolsky and Meaney 1986). This is during a time of significant hippocampal neurogenesis, and the SHRP is thought to protect developing neurons from the aversive effects of high levels of glucocorticoids. MDMA administration on P11 produces marked increases in corticosterone (CORT) levels (Williams et al. 2005), which could lead to alterations in neural development. Increases in CORT were shown to alter learning and memory in adult animals, and blocking CORT was shown to alleviate the path integration deficits in fenfluramine-treated animals (de Quervain et al. 1998; Williams et al. 2002; Skelton et al. 2004). Finally, preliminary data suggest that the NMDA receptor may play a role in P11–20 MDMA-induced learning and memory deficits (Skelton et al. 2005).
References
Able JA, Gudelsky GA, Vorhees CV, Williams MT (2006) 3,4-Methylenedioxymethamphetamine in adult rats produces deficits in path integration and spatial reference memory. Biol Psychiatry 59:1219–1226
Bayer SA, Altman J, Russo RJ, Zhang X (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14:83–144
Broening HW, Bacon L, Slikker W Jr (1994) Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 271:285–293
Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV (2001) 3,4-Methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci 21:3228–3235
Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL (1991) Disposition and pharmacodynamics of methamphetamine in pregnant sheep. J Am Med Assoc 265:1968–1973
Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJS, Finlay BL (2006) Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics (in press)
Cohen MA, Skelton MR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT (2005) Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (ecstasy) in rats: interaction with exposure in adulthood. Synapse 57:148–159
Crawford CA, Williams MT, Kohutek JL, Choi FY, Yoshida ST, McDougall SA, Vorhees CV (2006) Neonatal 3,4-methylenedioxymethamphetamine (MDMA) exposure alters neuronal protein kinase A activity, serotonin and dopamine content, and [(35)S]GTPgammaS binding in adult rats. Brain Res 1077:178–186
de Quervain DJF, Roozendaal B, McGaugh JL (1998) Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394:787–790
Etienne AS, Jeffery KJ (2004) Path integration in mammals. Hippocampus 14:180–192
Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B (1996) Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381:706–709
Green AR, Mechan AO, Elliott JM, O’shea E, Colado MI (2003) The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 55:463–508
Ho E, Karimi-Tabesh L, Koren G (2001) Characteristics of pregnant women who use ecstasy (3, 4-methylenedioxymethamphetamine). Neurotoxicol Teratol 23:561–567
Johnston LD, O’Malley PM, Bahman JG, Schulenberg JE (2005a) Monitoring the future national survey results on drug use, 1975–2004. Volume I: secondary school students. National Institute on Drug Abuse, Bethesda, MD
Johnston LD, O’Malley PM, Bahman JG, Schulenberg JE (2005b) Monitoring the Future national survey results on drug use, 1975–2004. Volume II: college students and adults ages 19–45. National Institute on Drug Abuse, Bethesda, MD
Kirk RE (1993) Experimental design. Brooks/Cole Publishing Company, Pacific Grove, California
Koprich JB, Campbell NG, Lipton JW (2003) Neonatal 3,4-methylenedioxymethamphetamine (ecstasy) alters dopamine and serotonin neurochemistry and increases brain derived neurotrophic factor in the forebrain and brainstem of the rat. Brain Res Dev Brain Res 147:177–182
Kouimtsidis C, Schifano F, Sharp T, Ford L, Robinson J, Magee C (2006) Neurological and psychopathological sequelae associated with a lifetime intake of 40,000 ecstasy tablets. Psychosomatics 47:86–87
Linnarsson S, Bjorklund A, Ernfors P (1997) Learning deficit in BDNF mutant mice. Eur J Neurosci 9:2581–2587
Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P (1997) Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res 760:68–73
McElhatton PR, Bateman DN, Evans C, Pughe KR, Worsley AJ (1997) Does prenatal exposure to ecstasy cause congenital malformations? A prospective follow-up of 92 pregnancies. Br J Clin Pharmacol 45:184
McElhatton PR, Bateman DN, Evans C, Pughe KR, Thomas SH (1999) Congenital anomalies after prenatal ecstasy exposure. Lancet 354:1441–1442
Mordenti J, Chappell W (1989) The use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V (eds) Toxicokinetics in new drug development. Pergamon, New York pp 42–96
Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV (2002) Impaired spatial and sequential learning in rats treated neonatally with D-fenfluramine. Eur J Neurosci 16:491–500
Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A (2006) Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci 26:4071–4081
Sapolsky RM, Meaney MJ (1986) Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res 396:64–76
Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI (2006) Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312:758–762
Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT (2006) A comparison of monoamine and corticosterone levels 24 hours following (+) methamphetamine, (±)3,4-methelynedioxymethamphetamine, cocaine, (+) fenfluramine, or (±)methylphenidate administration in the neonatal rat. J Neurochem 98(5):1369–1378
Scholey AB, Parrott AC, Buchanan T, Heffernan TM, Ling J, Rodgers J (2004) Increased intensity of Ecstasy and polydrug usage in the more experienced recreational ecstasy/MDMA users: a WWW study. Addict Behav 29:743–752
Skelton MR, Blankenmeyer TL, Gudelsky GA, Brown-Strittholt CA, Vorhees CV, Williams MT (2004) Metyrapone attenuates the sequential learning deficits but not monoamine depletions following d,l-fenfluramine administration to adult rats. Synapse 54:214–222
Skelton MR, Williams MT, Vorhees CV (2005) Increases in NMDA receptor subunit 1, nitric oxide synthase, and postsynaptic density 95 in adult rats following neonatal MDMA exposure. Society for Neuroscience Abstracts
Sodhi MS, Sanders-Bush E (2004) Serotonin and brain development. Int Rev Neurobiol 59:111–174
Sprague JE, Preston AS, Leifheit M, Woodside B (2003) Hippocampal serotonergic damage induced by MDMA (ecstasy): effects on spatial learning. Physiol Behav 79:281–287
Vorhees CV (1987a) Behavioral teratogenicity of valproic acid: selective effects on behavior after prenatal exposure to rats. Psychopharmacology (Berl) 92:173–179
Vorhees CV (1987b) Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol 9:235–241
Vorhees CV, Reed TM, Skelton MR, Williams MT (2004) Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci 22:247–259
Williams MT, Morford LL, McCrea AE, Wood SL, Vorhees CV (2002) Administration of D,L-fenfluramine to rats produces learning deficits in the Cincinnati water maze but not the Morris water maze: relationship to adrenal cortical output. Neurotoxicol Teratol 24:783–796
Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV (2003) Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res 968:89–101
Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees CV (2005) 3,4-Methylenedioxymethamphetamine administration on postnatal day 11 in rats increases pituitary-adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res 1039:97–107
Winslow JT, Insel TR (1990) Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther 254:212–220
Acknowledgements
This research was supported by NIH grants: DA006733 (CVV) and DA014269 (MTW) and a training grant ES07051 (MRS).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
MWM acquisition phase learning curves for P30 (a), P40 (b), P180 (c), and P360 (d) animals. Path length analysis by day show that SAL- and MDMA-treated animals improve their performance each day, although MDMA-treated animals did not learn the task as well (DOC 137 kb)
Rights and permissions
About this article
Cite this article
Skelton, M.R., Williams, M.T. & Vorhees, C.V. Treatment with MDMA from P11–20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology 189, 307–318 (2006). https://doi.org/10.1007/s00213-006-0563-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0563-4