Abstract
Rationale
Haloperidol is a representative of typical antipsychotics that are still in clinical use and which can lead to abnormal motor activity following repeated administration. The mechanisms underlying antipsychotic-induced dyskinesias are not well understood but are widely held to be related to excessive loss of dopamine function. In several models of dopamine hypofunction, serotonin 5-HT2C receptors have been shown to mediate vacuous chewing movements (VCM), a form of abnormal motor activity. It is well established that repeated haloperidol administration also elicits VCM, but there is no information on how repeated haloperidol administration affects 5-HT2C receptor signaling.
Objectives
In the present study, we tested the hypothesis that repeated daily administration of haloperidol leads to enhanced serotonin 5-HT2C receptor signaling that is associated with increased 5-HT2C-mediated VCM.
Methods
Rats were treated by subcutaneous injection once daily for 21 days with either vehicle, a low dose of haloperidol (0.1 mg kg−1 day−1), or a high dose of haloperidol (1.0 mg kg−1 day−1). Following 1-day withdrawal, rats were either used for behavioral scoring of VCM or sacrificed for biochemical assessment of 5-HT2 receptor-mediated phospholipase C activity and radioligand binding. VCM were scored following two successive “drug” challenges. The first challenge was an injection of vehicle (0.9% saline), and the second challenge was an injection of the 5-HT2C agonist meta-chlorophenylpiperazine (1.0 mg/kg). In this manner, a measure of “spontaneous” and “5-HT2C-elicited” orofacial activity could be made while minimizing animal use.
Results
Following 21-day haloperidol treatment at either dose, there was an increase in expression of meta-chlorophenylpiperazine-induced VCM. In a separate experiment, meta-chlorophenylpiperazine-induced VCM were shown to be mediated through 5-HT2C receptors. Striatal 5-HT2C receptor-mediated phospholipase C (PLC) activity and high-affinity agonist-labeled 5-HT2C receptors were also increased following either dose of haloperidol as compared to vehicle treatment. GTP-stimulated PLC activity and striatal Gq proteins were unchanged by haloperidol suggesting that enhanced signaling could be accounted for by alterations at the level of the receptor and not at downstream mechanisms.
Conclusions
Repeated daily administration of haloperidol leads to an adaptive increase in 5-HT2C signaling which may contribute to abnormal motor function associated with antipsychotic use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antipsychotic drugs have found widespread use not only for psychosis, but in the treatment of agitation and affective disorders among adult, pediatric, and elderly patient populations as well (Cooper et al. 2004; Kasckow et al. 2004; Masan 2004; McIntyre et al. 2004; Tariot et al. 2004; Worrel et al. 2000). Typical antipsychotics such as haloperidol have a propensity to cause early onset motor side effects such as dyskinesias, dystonia, parkinsonism (often referred to as extrapyramidal side effects or EPS), and late-emerging tardive dyskinesias (Janno et al. 2004; Kane 2004; Muscettola et al. 1999; Tarsy et al. 2002). Despite the advent of newer antipsychotic drugs with reduced liability to inducing EPS, such side effects remain a clinical concern, especially in vulnerable patient populations such as the elderly. Considerable effort has been devoted to understanding the mechanisms that contribute to EPS.
There are numerous reports demonstrating that a variety of haloperidol treatment paradigms induce vacuous chewing movements (VCM) in rats (Rupniak et al. 1985; Marchese et al. 2004a; Turrone et al. 2003b; Egan et al. 1996; Steinpreis et al. 1993; Waddington 1990). The emergence of VCM within days to weeks of initiating haloperidol treatment has been referred to as “early onset” VCM and appears to be associated with mechanisms related to EPS (Egan et al. 1996; Steinpreis et al. 1993; Marchese et al. 2004a). Late-emerging VCM that are associated with weeks to months of treatment can persist for months after drug is withdrawn and have been taken to model tardive dyskinesias (Egan et al. 1995, 1996; Waddington 1990). The precise mechanisms underlying early-onset or late-emerging VCM are still unclear. However, a primary mechanism of action of all antipsychotic drugs is the attenuation of the actions of dopamine (DA) at the D2 receptor and haloperidol exhibits high-potency D2 blockade (Wadenberg et al. 2001). Since DA is also intimately involved in control of motor function, it is widely considered that the repeated blockade of D2 receptors caused by drugs such as haloperidol is a critical factor in causing motor side effects such as VCM. In support of this argument, it has been shown that DA hypofunction caused by selective destruction of DA neurons increases the sensitivity of rats to exhibiting VCM when challenged with dopaminergic drugs (Gong et al. 1992). Interestingly, DA neuronal loss also increases the sensitivity of rats to exhibiting VCM elicited by meta-chlorophenylpiperazine, a serotonin 5-hydroxytryptamine (5-HT) agonist with 5-HT2C-stimulating properties (De Deurwaerdere and Chesselet 2000; Gong and Kostrzewa 1992; Gong et al. 1993).
Clinical and preclinical observations also support a role for 5-HT in mediating motor side effects of antipsychotic drugs. Specifically, newer generation “atypical” antipsychotics that possess high-potency 5-HT2 receptor antagonist properties in addition to D2 antagonist properties are observed to have a lower propensity for eliciting EPS (Worrel et al. 2000; Tamminga 2003). Thus, enhanced activity at 5-HT2 receptors is believed to contribute to EPS caused by antipsychotics like haloperidol (Stockmeier et al. 1993; Meltzer et al. 1989; Kapur 1996; Casey 2004). Accordingly, one study using VCM in rats to model EPS has demonstrated that the 5-HT2A/2C antagonist ritanserin can ameliorate haloperidol-induced dyskinesia (Marchese et al. 2004b). Despite these data, surprisingly, little is known about how antipsychotic drugs actually affect 5-HT2 receptor signaling.
In the brain, the predominant 5-HT2 receptor subtypes are the 5-HT2A and 5-HT2C receptor. Both subtypes are positively coupled to phosphoinositide-specific phospholipase C (PLC) through members of the Gq alpha subunit family of G proteins (Sanders-Bush et al. 1990; Hartig et al. 1990; Wolf and Schutz 1997). While there has been much focus on a role for 5-HT2A antagonism in mediating the reduced EPS liability of atypical antipsychotics (Leysen et al. 1994; Meltzer et al. 1989), atypical antipsychotics that exhibit high affinity at the 5-HT2A receptor also possess high affinity at the 5-HT2C receptor where they have been shown to act as inverse agonists (Herrick-Davis et al. 2000).
Our focus has been on understanding the role of 5-HT2C receptors in control of motor function and in mediating dyskinetic activity associated with antipsychotic drug administration. Neuroanatomical, biochemical, and behavioral evidences support such a role for 5-HT2C receptors. 5-HT2C receptors are distributed in basal ganglia regions such as striatum, substantia nigra pars reticulata (SNr), and subthalamic nucleus (Eberle-Wang et al. 1997; Clemett et al. 2000). Using an assay for measuring 5-HT2A- and 5-HT2C-linked PLC activity in membranes of rat brain, we have previously shown that the 5-HT2C receptor regulates a substantial fraction of 5-HT-mediated PLC activity in striatum (Wolf and Schutz 1997). 5-HT2C receptors also appear to contribute to haloperidol-induced acute catalepsy as well as VCM (Eberle-Wang et al. 1996; Reavill et al. 1999).
Despite the observations discussed above, there has been no coordinated study into the effects of haloperidol on 5-HT2C receptor signaling and its association with abnormal motor function. The present study was undertaken to determine how repeated haloperidol affects 5-HT2C receptor function. To accomplish this, 5-HT2C receptor signaling was assessed biochemically using PLC and radioligand binding assays in rats that received vehicle or haloperidol for 21 days. Behavioral assessment of 5-HT2C function was carried out through observations of 5-HT2C-mediated VCM.
Materials and methods
Materials
3H-Phosphatidylinositol (11 Ci/mmol) and 125I-DOI (2,200 Ci/mmol) were obtained from NEN Research Products (Boston, MA). Guanosine 5′-O-[3-thiotriphosphate] (GTPγS) was purchased from Boehringer Mannheim. Unlabeled phosphatidylinositol, 5-HT, meta-chlorophenylpiperazine (mCPP), SB 206553, and ketanserin were purchased from Sigma Chemical Co. (St. Louis, MO). SDZ SER082 was purchased from Tocris Cookson (St. Louis, MO). All other buffers and reagents were purchased from Fisher (Itasca, IL) and were of the highest grade possible.
Animals
Male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN) 3–4 months old were used throughout. Animals were maintained on a 12-h light/dark cycle, and food and water were available ad libitum. All animal care and experimentation was carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee.
Behavioral assessment of dyskinesias
Vacuous chewing movements were assessed essentially as described in the literature (Gong et al. 1992; Waddington 1990). Rats were placed individually in empty Plexiglas cages (25×43 cm) that resembled their home cages and were allowed to acclimate for 15 min prior to drug injection. An observer blinded to drug treatment scored behavioral activity by counting vacuous chewing movements. This activity was defined as small to moderate amplitude jaw movements resembling chewing behavior, but “not directed onto any evident physical material” (Waddington 1990). Behavioral assessment began 5 min after drug injection and consisted of four 2-min assessment periods carried out t=5, 11, 17, and 23 min postinjection. Data are presented as the sum total of vacuous chewing movements observed in the four assessment periods. Preliminary data and published reports indicate that drug effects peak within 10 min and slowly decline thereafter (Gong et al. 1992). All chronically pretreated animals were scored twice in succession on the indicated day. The first assessment period followed an injection of vehicle (0.9% saline), and the second assessment period followed the challenge injection of the 5-HT2C agonist mCPP. In this manner, a measure of “spontaneous” and “5-HT2C-elicited” orofacial activity following pretreatment could be assessed while minimizing animal use.
Brain dissection
For biochemical analyses, animals were killed by decapitation without anesthetic 1 day after cessation of haloperidol or vehicle pretreatment. Brains were quickly removed from the skull and placed ventral side up in a chilled metal brain block. To obtain striatum, a 3-mm coronal slice extending rostrally from just anterior to the optic chiasm (approximately −0.3 mm from bregma) was removed and placed, rostral side up on a chilled alumina plate. The striatum was dissected free from adjacent structures (cortex, anterior globus pallidus), and a cut was made to separate ventral striatum/nucleus accumbens. The remaining dorsal striatum was frozen on dry ice. Frozen tissue was weighed at the time of dissection and stored at −80°C until assayed.
Brain membrane preparation for phospholipase C assay
Tissue was prepared essentially as described previously (Wolf and Schutz 1997). Briefly, tissues were homogenized in 20 vol of 25 mM HEPES–Tris, pH 7.4, containing 1 mM EGTA (homogenization buffer). Homogenates were centrifuged at 20,000×g for 10 min at 4°C, and the pellet was washed by resuspension and centrifugation four times. Membranes was then washed once more using 25 mM HEPES–Tris, pH 7.4, containing 3 mM EGTA and 10 mM LiCl, and pellets were frozen and stored at −80°C until assayed.
Phospholipase C assay
Phospholipase C activity was assayed essentially as described previously (Wolf and Schutz 1997). Briefly, 30 μg membrane protein was incubated in a total volume of 100 μl. Final assay components were 25 mM HEPES–Tris, pH 7.4, 3 mM EGTA, 6 mM MgCl2, 1 mM sodium deoxycholate, and 100 μM 3H-phosphatidylinositol (3H-PI; 104 dpm/nmol final specific activity). GTPγS (a nonhydrolyzable analogue of GTP; 1 μM), Ca2+ (300 nM free Ca2+), and drugs were present where indicated. When spiperone was used to occlude 5-HT2A sites, tissue was preincubated with spiperone in assay buffer for 15 min on ice prior to adding Ca2+, GTPγS, 5-HT, and 3H-phosphatidylinositol. The final concentration of spiperone in the assay was 100 nM. Incubations were carried out for 20 min at 37°C and terminated by adding 0.9 ml of chloroform/methanol (1:2). Next, 0.3 ml chloroform and 0.3 ml of 0.25 M HCl were added, and tubes were mixed vigorously for 90 s. Tubes were then centrifuged at 8,000×g for 2 min to separate the phases. A 0.5-ml aliquot of the aqueous (upper) phase [containing the product, 3H-inositol phosphate (3H-IP)] was mixed with 8 ml of scintillation medium, and the sample was counted by liquid scintillation spectrometry. All assays were performed in duplicate. Blank values, obtained by counting extracts from incubations carried out in the absence of tissue, were 5–10% of total counts and were subtracted from raw data. Incubations carried out with tissue, but in an ice-water bath, were identical to blank values (data not shown). PLC activity was normalized to protein content.
5-HT2C radioligand binding
Tissue was homogenized and washed as described for PLC assays (see above). Quantitation of the high-affinity (i.e., coupled) state of the 5-HT2C receptor was carried out using 125I-DOI by incubating approximately 100 μg membrane protein in a final incubation volume of 250 μl consisting of 50 mM HEPES–Tris, pH 7.4, 5 mM MgCl2, and routinely 0.3 nM 125I-DOI. Incubations were stopped after 45 min at 30°C by rapid filtration over glass fiber filters (Whatman GF/B) soaked in 0.3% polyethyleneimine followed by two 4-ml washes with ice-cold buffer. Filters were placed in vials and were counted on a gamma counter. Assays were conducted in triplicate. To allow for selective labeling of 5-HT2C sites by 125I-DOI, it was necessary to occlude 5-HT2A sites, which outnumber 5-HT2C sites in rat striatum approximately three- or fourfold (Wolf and Schutz 1997), with the 5-HT2A antagonist MDL 100907 (30 nM). This concentration of MDL 100907 was chosen based on initial competition studies using striatal tissue from drug-naive rats. MDL 100907 inhibited approximately 80% of specific 125I-DOI binding monophasically with a calculated K i of 0.39±0.04 nM (n=4). These data are consistent with previous reports on the affinity and selectivity of MDL 100907 and the preponderance of 5-HT2A sites over 5-HT2C sites in rat striatum (Johnson et al. 1996; Lopez-Gimenez et al. 1998; Wolf and Schutz 1997). The 5-HT2C antagonist SB 206553 (300 nM) was used to define nonspecific binding. At 0.3 nM, 125I-DOI specific binding represented approximately 65% of total binding. Binding data were normalized to protein content.
Immunoblotting for Gq protein
An aliquot of the initial homogenate used for PLC assays was immediately mixed 1:1 with gel buffer (25 mM HEPES–Tris, pH 7.4 at 25°C, containing 1 mM EGTA, 1 mM EDTA, and 100 μM PMSF). This mixture was centrifuged for 8 min at 15,000×g, and the pellets were resuspended in fresh gel buffer. A small aliquot of suspension was assayed for membrane protein as described below. Membrane suspensions were then solubilized at a concentration of 2 mg/ml in NuPage LDS sample buffer with reducing agent (Invitrogen, Carlsbad, CA) and heated for 10 min at 70°C. An aliquot of 10 μg protein/lane was loaded onto 4–12% Bis–Tris Novex gels (1 mm thick; Invitrogen) and electrophoresed in a MES buffer system. Proteins were electrophoretically transferred to 0.45 μm PVDF membranes using NuPage transfer buffer (Invitrogen). Membranes were blocked for 90 min at room temperature in blocking buffer (25 mM Tris–HCl, pH 7.5, containing 140 mM NaCl, 0.1% Tween 20, 5% nonfat dry milk, and 1% Hammerstein casein). Following a brief wash in TTBS (25 mM Tris–HCl, pH 7.5, containing 140 mM NaCl and 0.1% Tween 20), membranes were incubated overnight at 4°C in blocking buffer containing primary antibody (rabbit anti-Gq/11, Cat. no. sc-392, Santa Cruz Biotechnology, Santa Cruz, CA, 0.1 μg/ml). Following four 10-min washes in TTBS, membranes were incubated in blocking buffer containing secondary antibody (alkaline phosphatase-conjugated goat antirabbit, Cat. no. s373B, Promega Corp., Milwaukee, WI, 1:12,000 dilution) for 1 h at room temperature. Following four 10-min washes in TTBS, membranes were incubated in ImmunStar chemiluminescence substrate (BioRad, Hercules, CA) for 5 min and then exposed to Kodak film (BioMax ML, Kodak, Rochester, NY).
Films were scanned and analyzed densitometrically using Un-SCAN-IT v5.1 digitizing software (Silk Scientific, Orem, UT). On each gel, three samples of control (i.e., vehicle pretreatment) and three of each experimental group (0.1 and 1 mg kg−1 day−1 haloperidol) were randomly represented. Each sample was run on two independent gels. To account for intergel variability, a reference value for each gel was obtained by averaging the mean optical density of the three control samples on that gel. The optical density in each individual lane of a given gel was divided by the reference value of that gel to determine the relative amount of Gq for each sample. The data are expressed as “% of control” and represent the average of the two replicates for each animal.
Protein determination and data analysis
Membrane protein was determined by the method of Bradford (1976) using gamma globulin as standard. Behavioral data were analyzed by two-way ANOVA as indicated in the text. Following a significant interaction effect, post hoc comparisons were made using Student–Newman–Keuls test for comparison among individual groups, as indicated in the text. Biochemical data were analyzed by one-way ANOVA (for more than two groups) or Student's t-test. Post hoc comparisons among individual groups were carried out using Student–Newman–Keuls test.
Results
Pharmacological analysis of mCPP-elicited vacuous chewing movements
To establish that the orofacial activity elicited by mCPP drug challenge was mediated by 5-HT2C receptors, an acute pharmacological antagonism study was carried out. Animals that had not received any chronic drug pretreatment were used. Figure 1 depicts the results obtained when animals were challenged with either vehicle (0.9% saline) or mCPP (1 mg/kg) 15 min after receiving either vehicle, the 5-HT2C-selective antagonists SB 206553 (2 mg/kg; Forbes et al. 1995) or SDZ SER082 (1 mg/kg; Nozulak et al. 1995), or the 5-HT2A-selective antagonist ketanserin (1 mg/kg). Acute challenge with mCPP in vehicle-pretreated rats (“VEH+mCPP”) produced a fivefold increase in vacuous chewing movements (VCM) during the 25-min observation period as compared to vehicle challenge in vehicle-pretreated rats (“VEH+VEH”). The statistical analysis (two-way ANOVA) indicated that there was a significant effect of drug challenge (F 1,40=166.45, P<0.001), pretreatment (F 3,40=28.29, P<0.001), and pretreatment×challenge interaction (F 3,40=18.34, P<0.001). Post hoc comparisons with Student–Newman–Keuls established that no significant differences in VCM existed among pretreatment groups receiving subsequent vehicle challenge. In contrast, VCM elicited by mCPP challenge were significantly attenuated by SB 206553 pretreatment (P<0.001) and SDZ SER082 pretreatment (P<0.001), but not by ketanserin pretreatment (P=0.369), when compared to vehicle-pretreated animals receiving mCPP challenge.
meta-Chlorophenylpiperazine (mCPP)-induced VCM are mediated by 5-HT2C receptors. Drug-naive rats received an acute administration of either vehicle (VEH, 1 ml/kg s.c.), the 5-HT2C-selective antagonists SB 206553 (2 mg/kg s.c.) or SDZ SER082 (1 mg/kg, s.c.), or the 5-HT2A-selective antagonist ketanserin (KET, 1 mg/kg s.c.). Fifteen minutes later, animals were acutely challenged with an injection of either vehicle or mCPP (1 mg/kg, s.c.) and were scored for VCM as described in Materials and methods. Data represent the mean±SEM of six animals per group and were analyzed by two-way ANOVA to assess antagonist×challenge interactions. Post hoc comparisons were made using Student–Newman–Keuls to determine individual group differences. *Significantly different from VEH+mCPP, P<0.001
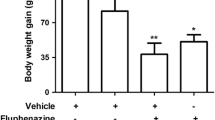
Effect of 21-day treatment with haloperidol on “spontaneous” and mCPP-elicited vacuous chewing
Animals receiving vehicle or haloperidol pretreatment, at either 0.1 mg kg−1 day−1 (“low dose”) or 1.0 mg kg−1 day−1 (“high dose”), were challenged 1 day after withdrawal with either vehicle or mCPP (1 mg/kg). These results are shown in Fig. 2. The statistical analysis (two-way ANOVA) established a significant effect of pretreatment (F 2,45=54.48, P<0.001), drug challenge (F 1,45=437.94, P<0.001), and pretreatment×challenge interaction (F 2,45=19.30, P<0.001). Post hoc comparisons with Student–Newman–Keuls established that rats that received high-dose haloperidol once daily for 21 days exhibited a small twofold increase in “spontaneous” VCM (i.e., VCM elicited by saline challenge on day 22) that was significantly greater than “spontaneous” VCM in vehicle-pretreated or low-dose haloperidol-pretreated animals (Fig. 2; P<0.05). In contrast, 21-day pretreatment with either dose of haloperidol significantly increased mCPP-elicited VCM as compared to 21-day vehicle pretreatment (Fig. 2; P<0.05). Post hoc comparison further established that mCPP-elicited VCM were significantly higher in rats pretreated with high-dose haloperidol as compared to low-dose haloperidol (P<0.05).
Repeated haloperidol (21 days) increases “spontaneous” (i.e., saline-induced) and mCPP-induced VCM. Rats were administered vehicle (saline 1 ml kg−1 day−1) or haloperidol once daily s.c. for 21 days at a dose of either 0.1 or 1.0 mg kg−1 day−1. One day following the last haloperidol administration, rats were given an initial challenge with saline and scored for VCM (SALINE). Following this test, rats received a second challenge with mCPP (1.0 mg/kg s.c.) and were scored again for VCM (mCPP). Data represent the mean±SEM of eight animals per group and were analyzed for pretreatment×challenge interaction by two-way ANOVA followed by post hoc comparisons with Student–Newman–Keuls. *Significantly different from vehicle-pretreated group, P<0.05; #significantly different from 0.1 mg/kg haloperidol, P<0.05
Effect of 21-day haloperidol treatment on 5-HT-mediated phospholipase C activity in rat striatum
The striatum has been implicated in mediating mCPP-elicited VCM (Plech et al. 1995). In order to determine whether repeated haloperidol altered 5-HT2C receptor-mediated signal transduction in the striatum, phospholipase C activity assays were carried out. Figure 3a demonstrates that G-protein-activated phospholipase C activity was unchanged by low-dose or high-dose haloperidol treatment as compared to control. However, 5-HT-stimulated PLC activity was significantly greater in striatal tissue from animals receiving either low-dose or high-dose haloperidol as compared to vehicle-pretreated animals (Fig. 3b). Statistical analysis of the data (one-way ANOVA) indicated a significant treatment effect (F 2,21=10.10, P<0.001). The elevation in 5-HT-stimulated PLC activity was similar for both dose regimens of haloperidol.
Repeated haloperidol (21 days) increases 5-HT-stimulated phospholipase C activity in rat striatum. Rats were administered vehicle (saline 1 ml kg−1 day−1) or haloperidol once daily s.c. for 21 days at a dose of either 0.1 or 1.0 mg kg−1 day−1. One day following the last haloperidol administration, rats were sacrificed and striatal tissue was dissected and frozen for PLC assay as described in Materials and methods. a GTP-stimulated activity, which is represented by PLC activity in the presence of 300 nM free Ca2+ plus 1 μM GTPγS (i.e., without 5-HT); b 5-HT-stimulated activity, which is represented by PLC activity in the presence of 300 nM free Ca2+, 1 μM GTPγS, and 300 nM 5-HT. Data for each parameter were analyzed by one-way ANOVA followed by post hoc comparisons with Student–Newman–Keuls and represent the mean±SEM of eight animals per group. *Significantly different from vehicle-pretreated group, P<0.05
In a separate experiment, striatal tissue from animals treated with high-dose haloperidol or vehicle was also run in assays to specifically assess the contribution of 5-HT2C receptors to striatal PLC activity. These results are shown in Fig. 4. In these assays, parallel incubations were carried out for each tissue sample in which spiperone (100 nM) was included to occlude 5-HT2A sites as previously described (Wolf and Schutz 1997) or vehicle was included to estimate total (5-HT2C+5-HT2A-mediated activity). Figure 4a depicts PLC activity obtained in the presence of spiperone (i.e., 5-HT2C-stimulated activity). Figure 4b depicts 5-HT2A-stimulated activity as estimated by the difference between 5-HT-stimulated PLC activity in the absence of spiperone (i.e., total activity) and 5-HT-stimulated PLC activity in the presence of spiperone (i.e., 5-HT2C-stimulated). Under these conditions, 5-HT2C-mediated PLC activity was significantly higher in striatal tissue from haloperidol-pretreated animals as compared to vehicle-pretreated animals (t-test, t=−2.53, P<0.05), but 5-HT2A-mediated activity was not different between the two groups.
Repeated haloperidol (21 days) increases 5-HT2C-mediated phospholipase C activity in rat striatum. Rats were administered with vehicle (saline 1 ml kg−1 day−1) or 1 mg/kg haloperidol once daily s.c. for 21 days. One day following the last haloperidol administration, rats were sacrificed and striatal tissue was dissected and frozen for PLC assay as described in Materials and methods. For each tissue, parallel assays were carried out (each in duplicate) in which either 100 nM spiperone was added to occlude 5-HT2A sites or vehicle was added to determine “total” 5-HT-stimulated PLC activity. a 5-HT2C-stimulated activity, which is represented by PLC activity in the presence of 300 nM free Ca2+, 1 μM GTPγS, 300 nM 5-HT, and 100 nM spiperone; b 5-HT2A-stimulated activity, which is represented by the difference in PLC activity obtained in assays in the absence of spiperone (5-HT2A+5-HT2C-stimulated activity) and PLC activity obtained in assays in the presence of spiperone (5-HT2C-stimulated activity). Data for each parameter were analyzed by Student's t-test and represent the mean±SEM of six animals per group. *Significantly different from vehicle-pretreated group, P<0.05
Effect of 21-day haloperidol treatment on agonist-labeled 5-HT2C receptors in striatum
The radiolabeled, nonselective 5-HT2A/2C agonist 125I-DOI was used to assess 5-HT2C receptor sites. Appropriate conditions for selectively labeling 5-HT2C sites were established (see Materials and methods), and binding studies were conducted in tissues from haloperidol- or vehicle-treated animals. These results are depicted in Fig. 5. A significant treatment effect was found (one-way ANOVA, F 2,21=5.11, P<0.05), and post hoc comparison established that 21-day pretreatment with either dose of haloperidol led to a similar, significant increase in agonist-labeled 5-HT2C sites as compared to vehicle pretreatment (P<0.05).
Repeated haloperidol (21 days) increases high affinity agonist-labeled 5-HT2C receptors in rat striatum. Rats were administered with vehicle (saline 1 ml kg−1 day−1) or haloperidol once daily s.c. for 21 days at a dose of either 0.1 or 1.0 mg kg−1 day−1. One day following the last haloperidol administration, rats were sacrificed and striatal tissue was dissected and frozen for radioligand binding using 125I-DOI as described in Materials and methods. Data represent the mean±SEM of eight animals per group and were analyzed by one-way ANOVA followed by post hoc comparisons with Student–Newman–Keuls. *Significantly different from vehicle pretreatment, P<0.05
Effects of 21-day haloperidol treatment on membrane-associated Gq levels in striatum
Immunoblotting of striatal membranes was carried out to determine whether an increase in Gq/11 levels was associated with the up-regulation in high-affinity agonist receptor sites. The effects of haloperidol on membrane-associated Gq levels can be seen in Fig. 6. Neither dose of haloperidol led to a measurable change in membrane-associated Gq levels in striatum.
Repeated haloperidol (21 days) does not alter membrane-associated Gq levels in rat striatum. Rats were administered with vehicle (saline 1 ml kg−1 day−1) or haloperidol once daily s.c. for 21 days at a dose of either 0.1 or 1.0 mg kg−1 day−1. One day following the last haloperidol administration, rats were sacrificed and striatal tissue was dissected and frozen for immunoblotting as described in Materials and methods. Data were analyzed by one-way ANOVA and represent the mean±SEM of eight animals per group
Discussion
In the present study, 21 days of once-daily haloperidol administration at 0.1 mg kg−1 day−1 (“low dose”) or 1.0 mg kg−1 day−1 (“high dose”) increased 5-HT2C receptor signaling in striatum and enhanced 5-HT2C-mediated vacuous chewing movements (VCM) when tested at 1 day of withdrawal. We focused on VCM elicited by a challenge injection of the 5-HT2C agonist meta-chlorophenylpiperazine (mCPP) in order to probe how subchronic haloperidol specifically affects 5-HT2C mechanisms involved in dyskinetic activity. We confirmed the involvement of 5-HT2C receptors in mediating mCPP-elicited VCM by demonstrating that 5-HT2C-selective antagonists, but not the 5-HT2A-selective antagonist ketanserin, blocked the ability of mCPP to elicit VCM in otherwise drug-naive (i.e., nonpretreated) rats (Fig. 1). In addition, we used a dose of mCPP (1 mg/kg) that is submaximal for eliciting VCM (Gong et al. 1993) to improve our chances of detecting a change in the sensitivity of 5-HT2C mechanisms related to motor dyskinesias.
It is noteworthy that both the low-dose and high-dose haloperidol pretreatment regimen predisposed animals to exhibit significantly more VCM when challenged with mCPP, but only high-dose haloperidol enhanced VCM seen following saline challenge (i.e., “spontaneous” dyskinesias; Fig. 2). The significance of this observation as it relates to the issue of whether VCM model early onset extrapyramidal side effects (EPS) or tardive dyskinesia is discussed later. Nevertheless, the observation that haloperidol pretreatment increased mCPP-elicited VCM parallels the finding of increased mCPP-mediated VCM in animals which received selective DA neuronal lesions using 6-hydroxydopamine (De Deurwaerdere and Chesselet 2000; Gong and Kostrzewa 1992; Gong et al. 1993). This would suggest that blockade or reduction of DA function leads to an adaptive increase in 5-HT2C mechanisms related to dyskinesias. Future studies comparing different D2 antagonists would be a useful extension of this work to rule out the possibility of involvement of other pharmacological properties of haloperidol.
In order to biochemically assess 5-HT2C-related signaling, we assayed 5-HT-stimulated PLC activity and high-affinity agonist binding to 5-HT2C receptors. We analyzed striatal tissue because previous work has shown that intrastriatal injection of mCPP elicits VCM, and that intrastriatal injection of the 5-HT2A/2C antagonist mianserin blocks VCM elicited by systemic mCPP (Plech et al. 1995). Both low-dose and high-dose haloperidol pretreatment for 21 days significantly increased striatal 5-HT-stimulated PLC activity (Fig. 3b), which is consistent with the behavioral evidence of enhanced 5-HT2C sensitivity. In these assays, we utilized 300 nM 5-HT to stimulate PLC because our previous work has shown that this concentration elicits predominantly 5-HT2C receptor-associated PLC activity, although 5-HT2A receptor-mediated activity is evident to a small degree (Wolf and Schutz 1997). We chose this procedure to minimize tissue use and enable us to carry out radioligand binding studies and PLC assays in the same tissue sample. Nevertheless, we carried out an additional experiment in which residual 5-HT2A-mediated PLC activity was blocked by inclusion of spiperone in the biochemical assays so that 5-HT2C-mediated PLC activity could be specifically assessed. This method has been previously established in our lab (Wolf and Schutz 1997). Consistent with the findings represented in Fig. 3b, we observed that the 5-HT2C component of PLC activity was significantly increased by haloperidol pretreatment, whereas the 5-HT2A component was apparently unchanged (Fig. 4). It should be noted from our previous study that striatal 5-HT2A-mediated PLC activity is best assayed at a higher concentration of 5-HT (Wolf and Schutz 1997). Thus, from the present PLC data, we cannot draw any substantive conclusions regarding the effects of haloperidol on 5-HT2A-mediated signaling. We did not pursue the question of 5-HT2A-mediated PLC activity due to a limitation of tissue availability and because the focus of the present study was 5-HT2C signaling and 5-HT2C-mediated dyskinesias. Given the long-standing proposal that blockade of 5-HT2A receptors contributes to the reduced incidence of EPS seen with newer “atypical” antipsychotics (Meltzer et al. 1989; Casey 2004), it would be valuable to carry out additional experiments that focus on 5-HT2A-mediated signaling and 5-HT2A-mediated motor activity following antipsychotic treatment.
It is generally acknowledged that G-protein-coupled receptors exist in a state associated with or “coupled” to G proteins as well as an uncoupled state (Kenakin 2004). Coupled 5-HT2A and 5-HT2C receptors exhibit high-affinity binding to radiolabeled agonists (Glennon et al. 1988; Lyon et al. 1987; Battaglia et al. 1984; Branchek et al. 1990; Teitler et al. 1990). In order to determine whether the increase in 5-HT2C-mediated signaling was due to an increase in receptors coupled to G proteins, radioligand binding studies were conducted using the nonselective 5-HT2A/2C agonist, 125I-DOI, to label the high-affinity, G-protein-coupled state of the receptor. Using the appropriate conditions for selective labeling (see Materials and methods), we observed an increase in 125I-DOI binding to 5-HT2C sites in rat striatum following either the low-dose or high-dose haloperidol regimen (Fig. 5). These observations parallel the PLC data and suggest increased coupling of 5-HT2C receptors to G proteins as a mechanism underlying haloperidol-induced increases in 5-HT2C signaling. Interestingly, we did not observe a change in GTPγS-stimulated PLC activity or membrane-associated Gq levels following either dose of haloperidol, suggesting that Gq-related mechanisms remained largely unchanged by haloperidol. Thus, the haloperidol-induced increase in 5-HT-stimulated PLC activity appears to be a phenomenon relatively specific to 5-HT2 receptors and not a consequence of changes downstream of receptor–G-protein coupling. Downstream changes would have raised the possibility of altered transmission through nonserotonergic Gq-coupled receptor systems, although the present data do not rule this possibility out. It remains to be determined if the increased coupling of 5-HT2C receptors is the result of an increase in receptor synthesis, a decrease in degradation, or the result of posttranslational modifications (e.g., phosphorylation) leading to altered coupling or receptor trafficking.
The concordance of biochemical data and mCPP-induced VCM suggests that striatal 5-HT2C receptors play a role in mediating abnormal motor activity following typical antipsychotics such as haloperidol. Since VCM have been widely used to model antipsychotic-induced tardive dyskinesia as well as early onset extrapyramidal side effects (EPS), it is important to consider what the present behavioral data are modeling. Based on several lines of evidence, we propose that the haloperidol-induced increase in 5-HT2C receptor function represents a mechanism more closely associated with EPS. Antipsychotic treatment lasting no more than 3–4 weeks has been shown to elicit a dose-related increase in spontaneous “early onset” VCM that rapidly subside (within hours or days) upon antipsychotic withdrawal, a property characteristic of EPS (Marchese et al. 2002, 2004a). “Early onset” VCM elicited by subchronic antipsychotic treatment are inhibited by anticholinergic antimuscarinic agents (Egan et al. 1996; Rupniak et al. 1985, 1986; Steinpreis et al. 1993; Salamone et al. 1998). This pharmacological characteristic is representative of EPS such as parkinsonism and dystonia in humans (Holloman and Marder 1997; Tonda and Guthrie 1994). Interestingly, VCM elicited by the augmentation of cholinergic activity are ameliorated by mianserin, a 5-HT2A/2C antagonist, but not by ketanserin, which is a more selective 5-HT2A antagonist (Stewart et al. 1988; Carlson et al. 2003). These observations suggest that a relationship exists between cholinergic mechanisms associated with EPS, as modeled by “early onset” VCM, and 5-HT2C receptor function. 5-HT2C antagonists have also been shown to reduce haloperidol-induced catalepsy, a model of acute EPS (Reavill et al. 1999). Our results, together with these data, support the hypothesis that augmentation of 5-HT2C receptor function contributes to acute EPS.
Given the observation that there is an increased prevalence of tardive dyskinesia in patients who exhibit EPS, it is possible that increased 5-HT2C function indirectly contributes to tardive dyskinesia. In fact, measures of spontaneous VCM during protracted antipsychotic treatment, which are used as an index of tardive dyskinesia, have shown that higher doses of haloperidol elicit a greater number of spontaneous VCM or an increased prevalence of spontaneous VCM in drug-treated groups (Waddington 1990; Egan et al. 1995, 1996; Turrone et al. 2002, 2003a). We observed that only animals receiving high-dose haloperidol exhibited a significant increase in “spontaneous” (i.e., saline-elicited) VCM (Fig. 2). This observation indicates that an increase in 5-HT2C signaling alone is not sufficient for increasing spontaneous VCM seen after higher dose (and possibly longer treatment) haloperidol, suggesting the possible involvement of additional mechanisms. Recruitment of additional mechanisms by high-dose haloperidol may also account for the dose dependency of mCPP-elicited VCM in the present study as observed in Fig. 2.
It has been proposed that the emergence of antipsychotic-induced motor dysfunction in humans and VCM in rats is a consequence of persistently high in vivo D2 receptor occupancy (≥80%; Kapur and Seeman 2001; Turrone et al. 2003a,b; Wadenberg et al. 2000). Studies assessing in vivo D2 occupancy in rats 2 h following acute administration of haloperidol demonstrate that at either dose of haloperidol used in the present study (1 or 0.1 mg/kg), in vivo D2 occupancy greater than 80% is achieved (Kapur et al. 2000). However, in vivo D2 occupancy following subcutaneous haloperidol at 0.1 mg/kg in rats is relatively transient, decreasing from 84% at 2 h to 46% at 8 h and 18% at 24 h (Kapur et al. 2000). Despite this fact, low-dose haloperidol increased 5-HT2C receptor function to a similar extent as high-dose haloperidol in the present study. Thus, enhanced 5-HT2C function and dyskinetic activity can be elicited by relatively transient D2 blockade and do not require prolonged, continuous high occupancy of the D2 receptor. These data argue against the suggestion that reduced EPS seen with atypical antipsychotics is strictly a consequence of relatively short-acting D2 receptor occupancy (Kapur and Seeman 2001) and argue for the potential significance of increased 5-HT2C function contributing to acute EPS.
A variety of atypical antipsychotics with reduced propensity to elicit EPS exhibit high affinity at 5-HT2C receptors where they may function as antagonists or inverse agonists (Herrick-Davis et al. 2000; Wood et al. 2001). Subchronic administration of clozapine, an atypical antipsychotic drug with little or no propensity to induce motor side effects, elicits a down-regulation of 5-HT2C receptors and a reduction in 5-HT-stimulated PLC activity in rat choroid plexus (Kuoppamaki et al. 1993). Functionally, clozapine acts as an inverse agonist at 5-HT2C receptors in cell systems (Berg et al. 1999; Herrick-Davis et al. 2000) and an antagonist in rat choroid plexus (Kuoppamaki et al. 1995). Whatever the mechanism, the ability to ameliorate an up-regulation in 5-HT2C function may be important for reducing motor side effects. It would be useful to know how these drugs compare with haloperidol and typical antipsychotics in affecting 5-HT2C function in brain regions involved in motor control.
In the basal ganglia, 5-HT2C receptors exist in moderate density in the subthalamic nucleus, substantia nigra pars reticulata (SNr), as well as the striatum (Pompeiano et al. 1994; Eberle-Wang et al. 1997; Clemett et al. 2000). In striatum, 5-HT2C receptor mRNA has been shown to colocalize with a variety of peptide-expressing cells intrinsic to the striatum and may be involved in regulating the outflow of striatal information (Ward and Dorsa 1996). The present findings indicate that enhanced striatal 5-HT2C signaling is associated with dyskinetic motor activity. Accordingly, Plech et al (1995) have observed that intrastriatal injection of mCPP elicits VCM and that intrastriatal injection of the 5-HT2A/2C antagonist mianserin blocks VCM elicited by systemic mCPP. 5-HT2C receptors in other basal ganglia regions may also play a role. In the subthalamic nucleus, 5-HT2C receptors are believed to reside on glutamate projection neurons that innervate internal globus pallidus and the SNr, and local injection of mCPP into the subthalamic nucleus has been shown to elicit VCM (Eberle-Wang et al. 1996). Moreover, intrasubthalamic injection of the 5-HT2C antagonist SDZ SER082 blocks VCM elicited by systemic mCPP (Eberle-Wang et al. 1996). In the SNr, 5-HT2C receptors appear to reside on GABAergic neurons (Eberle-Wang et al. 1997). GABAergic neurons in this region could serve as interneurons, providing a mechanism whereby 5-HT2C receptors mediate inhibition of DA cellular activity in the substantia nigra pars compacta (Wood et al. 2001). Accordingly, 5-HT2C antagonists increase striatal extracellular DA as measured by in vivo microdialysis (De Deurwaerdere et al. 2004; Di Giovanni et al. 1999). However, GABAergic neurons in the SNr also project to the thalamus and comprise a major efferent pathway of the basal ganglia (Wichmann and DeLong 2003). Thus, the possibility exists that 5-HT2C receptors directly control basal ganglia efferent activity. While the present biochemical data support a role for up-regulated striatal 5-HT2C receptors in mediating haloperidol-enhanced dyskinesias, they do not preclude the involvement of subthalamic or nigral 5-HT2C receptors.
In summary, our observations lend further support to the concept that an increase in 5-HT2C receptor signaling may be manifested as dyskinetic activity. Early onset motor dysfunction remains a complication of antipsychotic drug use, especially in vulnerable patient populations such as the elderly and those suffering from neurological disorders. Understanding the balance between dopaminergic and serotonergic mechanisms that leads to the clinical efficacy and side-effect profile of therapeutics is important for future drug development.
Abbreviations
- 5-HT:
-
serotonin
- DA:
-
dopamine
- PLC:
-
phosphoinositide-specific phospholipase C
- EPS:
-
extrapyramidal side effects
- mCPP:
-
meta-chlorophenylpiperazine
References
Battaglia G, Shannon M, Titeler M (1984) Guanyl nucleotide and divalent cation regulation of cortical S2 serotonin receptors. J Neurochem 43:1213–1219
Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP (1999) Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol 55:863–872
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Branchek T, Adham N, Macchi M, Kao HT, Hartig PR (1990) [3H]-DOB(4-bromo-2,5-dimethoxyphenylisopropylamine) and [3H] ketanserin label two affinity states of the cloned human 5-hydroxytryptamine2 receptor. Mol Pharmacol 38:604–609
Carlson BB, Wisniecki A, Salamone JD (2003) Local injections of the 5-hydroxytryptamine antagonist mianserin into substantia nigra pars reticulata block tremulous jaw movements in rats: studies with a putative model of parkinsonian tremor. Psychopharmacology (Berl) 165:229–237
Casey DE (2004) Pathophysiology of antipsychotic drug-induced movement disorders. J Clin Psychiatry 65(Suppl 9):25–28
Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC (2000) Immunohistochemical localisation of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology 39:123–132
Cooper WO, Hickson GB, Fuchs C, Arbogast PG, Ray WA (2004) New users of antipsychotic medications among children enrolled in TennCare. Arch Pediatr Adolesc Med 158:753–759
De Deurwaerdere P, Chesselet MF (2000) Nigrostriatal lesions alter oral dyskinesia and c-Fos expression induced by the serotonin agonist 1-(m-chlorophenyl)piperazine in adult rats. J Neurosci 20:5170–5178
De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U (2004) Constitutive activity of the serotonin2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24:3235–3241
Di Giovanni G, De Deurwaerdere P, Di Mascio M, Di M, Di Matteo V, Esposito E, Spampinato U (1999) Selective blockade of serotonin-2C/2B receptors enhances mesolimbic and mesostriatal dopaminergic function: a combined in vivo electrophysiological and microdialysis study. Neuroscience 91:587–597
Eberle-Wang K, Lucki I, Chesselet MF (1996) A role for the subthalamic nucleus in 5-HT2C-induced oral dyskinesia. Neuroscience 72:117–128
Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF (1997) Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol 384:233–247
Egan MF, Hyde TM, Kleinman JE, Wyatt RJ (1995) Neuroleptic-induced vacuous chewing movements in rodents: incidence and effects of long-term increases in haloperidol dose. Psychopharmacology (Berl) 117:74–81
Egan MF, Hurd Y, Ferguson J, Bachus SE, Hamid EH, Hyde TM (1996) Pharmacological and neurochemical differences between acute and tardive vacuous chewing movements induced by haloperidol. Psychopharmacology (Berl) 127:337–345
Forbes IT, Ham P, Booth DH, Martin RT, Thompson M, Baxter GS, Blackburn TP, Glen A, Kennett GA, Wood MD (1995) 5-Methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole: a novel 5-HT2C/5-HT2B receptor antagonist with improved affinity, selectivity, and oral activity. J Med Chem 38:2524–2530
Glennon RA, Seggel MR, Soine WH, Herrick-Davis K, Lyon RA, Titeler M (1988) [125I]-1-(2,5-dimethoxy-4-iodophenyl)-2-amino-propane: an iodinated radioligand that specifically labels the agonist high-affinity state of 5-HT2 serotonin receptors. J Med Chem 31:5–7
Gong L, Kostrzewa RM (1992) Supersensitized oral responses to a serotonin agonist in neonatal 6-OHDA-treated rats. Pharmacol Biochem Behav 41:621–623
Gong L, Kostrzewa RM, Fuller RW, Perry KW (1992) Supersensitization of the oral response to SKF 38393 in neonatal 6-OHDA-lesioned rats is mediated through a serotonin system. J Pharmacol Exp Ther 261:1000–1007
Gong L, Kostrzewa RM, Perry KW, Fuller RW (1993) Dose-related effects of a neonatal 6-OHDA lesion on SKF 38393- and m-chlorophenylpiperazine-induced oral activity responses of rats. Brain Res Dev Brain Res 76:233–238
Hartig P, Kao HT, Macchi M, Adham N, Zgombick J, Weinshank R, Branchek T (1990) The molecular biology of serotonin receptors. An overview. Neuropsychopharmacology 3:335–347
Herrick-Davis K, Grinde E, Teitler M (2000) Inverse agonist activity of atypical antipsychotic drugs at human 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther 295:226–232
Holloman LC, Marder SR (1997) Management of acute extrapyramidal effects induced by antipsychotic drugs. Am J Health-Syst Pharm 54:2461–2477
Janno S, Holi M, Tuisku K, Wahlbeck K (2004) Prevalence of neuroleptic-induced movement disorders in chronic schizophrenia inpatients. Am J Psychiatry 161:160–163
Johnson MP, Siegel BW, Carr AA (1996) [3H]MDL 100,907: a novel selective 5-HT2A receptor ligand. Naunyn-Schmiedeberg's Arch Pharmacol 354:205–209
Kane JM (2004) Tardive dyskinesia rates with atypical antipsychotics in adults: prevalence and incidence. J Clin Psychiatry 65(Suppl 9):16–20
Kapur S (1996) 5-HT2 antagonism and EPS benefits: is there a causal connection? Psychopharmacology (Berl) 124:35–39
Kapur S, Seeman P (2001) Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiatry 158:360–369
Kapur S, Wadenberg ML, Remington G (2000) Are animal studies of antipsychotics appropriately dosed? Lessons from the bedside to the bench. Can J Psychiatry 45:241–246
Kasckow JW, Mulchahey JJ, Mohamed S (2004) The use of novel antipsychotics in the older patient with neurodegenerative disorders in the long-term care setting. J Am Med Dir Assoc 5:242–248
Kenakin T (2004) Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25:186–192
Kuoppamaki M, Seppala T, Syvalahti E, Hietala J (1993) Chronic clozapine treatment decreases 5-hydroxytryptamine1C receptor density in the rat choroid plexus: comparison with haloperidol. J Pharmacol Exp Ther 264:1262–1267
Kuoppamaki M, Palvimaki EP, Hietala J, Syvalahti E (1995) Differential regulation of rat 5-HT2A and 5-HT2C receptors after chronic treatment with clozapine, chlorpromazine and three putative atypical antipsychotic drugs. Neuropsychopharmacology 13:139–150
Leysen JE, Janssen PM, Megens AA, Schotte A (1994) Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry 55(Suppl):5–12
Lopez-Gimenez JF, Vilaro MT, Palacios JM, Mengod G (1998) [3H]MDL 100,907 labels 5-HT2A serotonin receptors selectively in primate brain. Neuropharmacology 37:1147–1158
Lyon RA, Davis KH, Titeler M (1987) 3H-DOB (4-bromo-2,5-dimethoxyphenylisopropylamine) labels a guanyl nucleotide-sensitive state of cortical 5-HT2 receptors. Mol Pharmacol 31:194–199
Marchese G, Casu MA, Bartholini F, Ruiu S, Saba P, Gessa GL, Pani L (2002) Sub-chronic treatment with classical but not atypical antipsychotics produces morphological changes in rat nigro-striatal dopaminergic neurons directly related to “early onset” vacuous chewing. Eur J Neurosci 15:1187–1196
Marchese G, Bartholini F, Casu MA, Ruiu S, Casti P, Congeddu E, Tambaro S, Pani L (2004a) Haloperidol versus risperidone on rat “early onset” vacuous chewing. Behav Brain Res 149:9–16
Marchese G, Bartholini F, Ruiu S, Casti P, Casu GL, Pani L (2004b) Ritanserin counteracts both rat vacuous chewing movements and nigro-striatal tyrosine hydroxylase-immunostaining alterations induced by haloperidol. Eur J Pharmacol 483:65–69
Masan PS (2004) Atypical antipsychotics in the treatment of affective symptoms: a review. Ann Clin Psychiatry 16:3–13
McIntyre RS, Mancini DA, Lin P, Jordan J (2004) Treating bipolar disorder. Evidence-based guidelines for family medicine. Can Fam Physician 50:388–394
Meltzer HY, Matsubara S, Lee JC (1989) Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pK i values. J Pharmacol Exp Ther 251:238–246
Muscettola G, Barbato G, Pampallona S, Casiello M, Bollini P (1999) Extrapyramidal syndromes in neuroleptic-treated patients: prevalence, risk factors, and association with tardive dyskinesia. J Clin Psychopharmacol 19:203–208
Nozulak J, Kalkman HO, Floersheim P, Hoyer D, Schoeffter P, Buerki HR (1995) (+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolo[1,7- bc][2,6]-naphthyridine: a 5-HT2C/2B receptor antagonist with low 5-HT2A receptor affinity. J Med Chem 38:28–33
Plech A, Brus R, Kalbfleisch JH, Kostrzewa RM (1995) Enhanced oral activity responses to intrastriatal SKF 38393 and m-CPP are attenuated by intrastriatal mianserin in neonatal 6-OHDA-lesioned rats. Psychopharmacology (Berl) 119:466–473
Pompeiano M, Palacios JM, Mengod G (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 23:163–178
Reavill C, Kettle A, Holland V, Riley G, Blackburn TP (1999) Attenuation of haloperidol-induced catalepsy by a 5-HT2C receptor antagonist. Br J Pharmacol 126:572–574
Rupniak NM, Jenner P, Marsden CD (1985) Pharmacological characterisation of spontaneous or drug-associated purposeless chewing movements in rats. Psychopharmacology (Berl) 85:71–79
Rupniak NM, Jenner P, Marsden CD (1986) Acute dystonia induced by neuroleptic drugs. Psychopharmacology (Berl) 88:403–419
Salamone JD, Mayorga AJ, Trevitt JT, Cousins MS, Conlan A, Nawab A (1998) Tremulous jaw movements in rats: a model of parkinsonian tremor. Prog Neurobiol 56:591–611
Sanders-Bush E, Tsutsumi M, Burris KD (1990) Serotonin receptors and phosphatidylinositol turnover. Ann N Y Acad Sci 600:224–235 (discussion 235–6)
Steinpreis RE, Baskin P, Salamone JD (1993) Vacuous jaw movements induced by sub-chronic administration of haloperidol: interactions with scopolamine. Psychopharmacology (Berl) 111:99–105
Stewart BR, Jenner P, Marsden CD (1988) The pharmacological characterisation of pilocarpine-induced purposeless chewing behaviour in the rat. Psychopharmacology (Berl) 96:55–62
Stockmeier CA, DiCarlo JJ, Zhang Y, Thompson P, Meltzer HY (1993) Characterization of typical and atypical antipsychotic drugs based on in vivo occupancy of serotonin2 and dopamine2 receptors. J Pharmacol Exp Ther 266:1374–1384
Tamminga CA (2003) The science of antipsychotics: mechanistic insight. CNS Spectr 8:5–9
Tariot PN, Profenno LA, Ismail MS (2004) Efficacy of atypical antipsychotics in elderly patients with dementia. J Clin Psychiatry 65(Suppl 11):11–15
Tarsy D, Baldessarini RJ, Tarazi FI (2002) Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 16:23–45
Teitler M, Leonhardt S, Weisberg EL, Hoffman BJ (1990) 4-[125I]iodo-(2,5-dimethoxy)phenylisopropylamine and [3H]ketanserin labeling of 5-hydroxytryptamine2 (5HT2) receptors in mammalian cells transfected with a rat 5HT2 cDNA: evidence for multiple states and not multiple 5HT2 receptor subtypes. Mol Pharmacol 38:594–598
Tonda ME, Guthrie SK (1994) Treatment of acute neuroleptic-induced movement disorders. Pharmacotherapy 14:543–560
Turrone P, Remington G, Nobrega JN (2002) The vacuous chewing movement (VCM) model of tardive dyskinesia revisited: is there a relationship to dopamine D(2) receptor occupancy? Neurosci Biobehav Rev 26:361–380
Turrone P, Remington G, Kapur S, Nobrega JN (2003a) Differential effects of within-day continuous vs. transient dopamine D2 receptor occupancy in the development of vacuous chewing movements (VCMs) in rats. Neuropsychopharmacology 28:1433–1439
Turrone P, Remington G, Kapur S, Nobrega JN (2003b) The relationship between dopamine D2 receptor occupancy and the vacuous chewing movement syndrome in rats. Psychopharmacology (Berl) 165:166–171
Waddington JL (1990) Spontaneous orofacial movements induced in rodents by very long-term neuroleptic drug administration: phenomenology, pathophysiology and putative relationship to tardive dyskinesia. Psychopharmacology (Berl) 101:431–447
Wadenberg ML, Kapur S, Soliman A, Jones C, Vaccarino F (2000) Dopamine D2 receptor occupancy predicts catalepsy and the suppression of conditioned avoidance response behavior in rats. Psychopharmacology (Berl) 150:422–429
Wadenberg ML, Soliman A, VanderSpek SC, Kapur S (2001) Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology 25:633–641
Ward RP, Dorsa DM (1996) Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol 370:405–414
Wichmann T, DeLong MR (2003) Functional neuroanatomy of the basal ganglia in Parkinson's disease. Adv Neurol 91:9–18
Wolf WA, Schutz LJ (1997) The serotonin 5-HT2C receptor is a prominent serotonin receptor in basal ganglia: evidence from functional studies on serotonin-mediated phosphoinositide hydrolysis. J Neurochem 69:1449–1458
Wood MD, Heidbreder C, Reavill C, Ashby CR Jr, Middlemiss DN (2001) 5-HT2C receptor antagonists: potential in schizophrenia. Drug Dev Res 54:88–94
Worrel JA, Marken PA, Beckman SE, Ruehter VL (2000) Atypical antipsychotic agents: a critical review. Am J Health-Syst Pharm 57:238–255
Acknowledgements
This work was supported by a Merit Award (to W.A.W.) from the Department of Veteran's Affairs and by PHS grant NS 36410 (W.A.W.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wolf, W.A., Bieganski, G.J., Guillen, V. et al. Enhanced 5-HT2C receptor signaling is associated with haloperidol-induced “early onset” vacuous chewing in rats: implications for antipsychotic drug therapy. Psychopharmacology 182, 84–94 (2005). https://doi.org/10.1007/s00213-005-0033-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0033-4