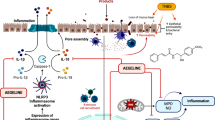
Abstract
Ulcerative colitis (UC) is an enduring and complex inflammatory bowel disease that is clinically prevalent, progressive, and debilitating. As of now, the few effective medical treatments for UC have unacceptably high side effects. It is crucial to find safer and more effective UC treatments. Nodakenin possesses anti-inflammatory and antioxidant activity by suppressing several pro-inflammatory mediators. In the present study, we aimed to evaluate the colonoprotective effect of nodakenin in combating colitis through the NFƙB-mediated NLRP3 inflammasome pathway. In mice, UC was induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS). Nodakenin (10, 20, and 40 mg/kg) was introduced intragastrically, and disease activity index (DAI) score was calculated. Malondialdehyde (MDA), myeloperoxidase (MPO), superoxide dismutase (SOD), nitric oxide (NO) levels, tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) concentration were evaluated in colon homogenate. Colon samples were used for histopathological investigation and mRNA expression studies involving nuclear factor kappa B (NFƙB), cyclooxygenase-2 (COX-2), inducible nitric oxide (iNOS), nucleotide-binding receptor domain 3 (NLRP3), interleukin-1β (IL-1β), and interleukin-18 (IL-18). Nodakenin treatment was found effective in lowering the DAI score, histological score, MPO, MDA, and NO levels while elevating SOD levels as compared to the model control group, showcasing its anti-inflammatory and antioxidant properties. Nodakenin (40 mg/kg) significantly downregulated the expression of TNF-α, IL-6, NFƙB (1.24-fold), iNOS (1.2-fold), COX-2 (1.98-fold), NLRP3 (1.78-fold), IL-1β (1.29-fold), and IL-18 (1.17-fold) conferring its great anti-inflammatory potential in combating colitis. Taking together, nodakenin presumably alleviated TNBS-induced colitis by NFƙB-mediated NLRP3 inflammasome pathway and reduced colon damage by downregulating various transcriptional genes and pro-inflammatory mediators.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel diseases (IBD) are complex and recurrent inflammatory conditions characterized by ulcerative colitis (UC) and Crohn’s disease. IBD is becoming more common, with India leading the way among Southeast Asian nations. The complicated interactions between the environment, food, some drugs, hereditary factors, and a strong immunological reaction against healthy bacteria in the gut are thought to be the cause of inflammatory bowel disease (IBD) (Gandhi et al. 2021; Abhirami et al 2022). The etiology of IBD is unclear and has multiple contributing factors. It includes abnormalities in the adaptive immune system, dysregulation of the innate immunological response to gut microbiota, and disruption of the epithelial barrier (Alatab et al. 2020; Kobayashi et al. 2020). IBD necessitates lifetime medication and ongoing observation; it represents a substantial healthcare burden. The current assessment focuses on the challenges that are relevant to a nation like India, specifically mentioning risk attribution and evolving IBD trends and financial concerns (Jain and Venkataraman 2021; Snell et al. 2021).
The nuclear factor kappa B (NFƙB) plays a critical role in the development and progression of UC (Tong et al. 2023). Researchers stated that it triggers multiple transcriptional enzymes, such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), which might cause inflammatory disturbances in the colon (Singh et al. 2020). Nuclear NFƙB triggers the release of many transcriptional genes, including pro-interleukin-1β (pro-IL-1β) and NOD-like receptor protein-3 (NLRP3). Interleukin-18 (IL-18) and interleukin-1β (IL-1β) are two pro-inflammatory mediators that are released by the signaling, which additionally fosters neutrophil infiltration and produces reactive oxygen species (Natarajan et al. 2018; Wang et al. 2018; Mao et al. 2020; Song et al. 2021). Thus, the novel therapeutics that trigger the NLRP3 inflammasome pathway, which is derived from NFƙB, would be favorable in battling against this complicated illness.
There are new developments in treating UC to combat IBD by targeting various pathways. However, there are various side effects of allopathic medications and several new medications account for the high rate of resistance in patients with UC. The lack of satisfaction with existing treatments has led to a rise in interest in novel pharmacological strategies involving natural materials (Witaicenis et al. 2014; Di Stasi 2021; Haftcheshmeh et al. 2022). Phytoconstituents from natural sources play a crucial role in developing new drug discovery processes. Coumarins are naturally derived from many natural sources, and it has been proven that coumarin and its derivatives are anti-inflammatory and antioxidant compounds that may be beneficial as adjuvant therapy for individuals with UC (Kirsch et al. 2016; Sharifi-Rad et al. 2021; Carneiro et al. 2021).
Nodakenin is a coumarin compound initially isolated from the roots of Angelica gigas. It has neuroprotective activity against glutamate-induced toxicity anti-allergic activity and anti-inflammatory activity (Kang and Kim 2007). A study revealed that nodakenin inhibits IgE/Ag-induced degranulation in mast cells. It has been stated that nodakenin has several pharmacological properties such as anti-inflammatory, antimicrobial, anti-aggregatory, neuroprotective, and memory-boosting properties (Kim et al. 2011; Rim et al. 2012). Nodakenin prevents the release of inflammatory cytokines in mast cells, including TNF-α and IL-4, and suppresses anaphylactic shock in an animal model. Nodakenin inhibited the growth of skin lesions resembling atopic dermatitis in mice (Park et al. 2014).
Nodakenin ameliorated scopolamine-induced memory disruption in mice depicting its cognitive enhancement activity (Kim et al. 2007). Nodakenin at the dose of 10 mg/kg and 30 mg/kg was found effective against LPS-induced liver injury in mice by suppressing pro-inflammatory cytokines such as TNF-α, IL-6, IL-1β, and other transcriptional factors such as iNOS and COX-2. It has also been proven to inhibit NFƙB overexpression and depicted anti-inflammatory activity and anti-oxidant activity against liver injury in mice (Lim et al. 2021). It also suppresses LPS-induced inflammatory response in RAW 264.7 murine macrophage cells and mice peritoneal macrophages by acting through the NFƙB pathway (Rim et al. 2012). It has also been proven that nodakenin at 20 mg/kg dose inhibits NLRP3 inflammasome activation in chronic kidney disease along with downregulation of several pro-inflammatory mediators and also inhibits NFƙB activation (Liao et al. 2021a). However, the protective role of nodakenin in IBD has not been reported, and given the aforementioned findings, the current investigation focuses on nodakenin’s ability to mitigate inflammatory bowel disease in TNBS-induced colitis in mice through the NFƙB-mediated NLRP3 inflammasome pathway.
Materials and methods
Animals
Eight to 10-week-old female BALB/c mice weighing 18–25 gm were procured from Zydus Research Centre in Ahmedabad, Gujarat, India. Mice were housed in facilities that averaged 25 °C with 0.5 °C variability. The lighting schedule was 12 h of light and 12 h of darkness, with a 5% deviation. The relative humidity was kept at roughly 50%. The Institutional Animal Ethics Committee approved the experimental protocol (RPCP/IAEC/2022–2023/R6).
Induction of colitis
Mice (n = 8) were randomly divided into six groups: NC (normal control, 0.5% CMC suspension), MC (model control, TNBS), STD (sulfasalazine 100 mg/kg + TNBS), NO1 (nodakenin 10 mg/kg + TNBS), NO2 (nodakenin 20 mg/kg + TNBS), and NO3 (nodakenin 40 mg/kg + TNBS). Previous research served as the basis for the nodakenin dosage for the study. Nodakenin (97.2% purity, Clearsynth Labs Ltd., Mumbai, India; CAS NO.: 415–31-8) and sulfasalazine (Sigma-Aldrich) were suspended in 0.5% CMC and given orally once a day. The start of the treatment plan was 24 h prior to the administration of TNBS (Fig. 1A). Morris et al. (1989) claimed that TNBS (Sigma-Aldrich) was used to produce colitis in female BALB/c animals. Colitis was induced by TNBS (Sigma-Aldrich) in female BALB/c as described by Morris’s method (Morris et al.). To summarize, the mice that had been fasted over a full night were put to sleep, and then, using an 18 gauge lavage inserted 3.5–4 cm proximal to the anus, 100 µL of TNBS (2.5% TNBS in 50% ethanol) was gradually injected into the descending colon. Only 50% ethanol was given intracolonically to normal control animals. For 1 min, mice were kept in the Trendelenburg position to avoid TNBS spillage (Luo et al. 2017; Wang et al. 2018; Zhang et al. 2020).
Effect of nodakenin against TNBS-induced colitis in mice. A Effect of nodakenin on body weight loss. B DAI score post-TNBS induction. Values are expressed as the mean ± SEM (n = 8). **p < 0.05, ***p < 0.001 vs model control group; #p < 0.01, ##p < 0.05, ###p < 0.001 vs normal control group. NC (normal control), MC (model control, only TNBS), STD (sulfasalazine, 100 mg/kg), NO1, NO2, NO3 (nodakenin 10, 20, 40 mg/kg), respectively
Daily records of the body loss of weight, stool consistency, and gastrointestinal bleeding were recorded after the administration of TNBS. The three features were summed up, and the disease activity index (DAI) was calculated using the scoring system (Table 1). Following the outcome of the examination, mice were euthanized on day 7, colon samples were collected, and colon weight and length were measured. The colon homogenate was prepared using cold phosphate-buffered saline (PBS), it was centrifuged, and the supernatant was used in the measurement of antioxidant markers such as MPO, MDA, NO, and SOD levels. A small colon segment was employed for quantitative real-time PCR and various oxidative stress, while the distal part of the colon was stored in a 4% formaldehyde solution and was examined for histopathological changes.
Oxidative stress and antioxidant marker measurement
The level of malondialdehyde (MDA) was measured in colon homogenate at 532 nm as per the method earlier described by Ohkawa et al., and the results were expressed as mol/g of tissue (Ohkawa et al. 1979). Myeloperoxidase (MPO) levels were measured by the method described by Krawisz et al., and the data were presented as U/gm of tissue (Krawisz et al. 1984). Nitric oxide (NO) levels were determined using Griess reagent according to the previously mentioned method by Green et al. The results were stated as µM/gm of protein (Green et al. 1982). The superoxide dismutase (SOD) was analyzed by the method described by Misra et al., and the data were analyzed at 450 nm and expressed as U/g of tissue (Misra and Fridovich 1972).
Evaluation of TNF-α and IL-6 levels
Colon tissue was homogenized using PBS solution and was centrifuged for 10 min at 12,000 g at 4 °C. The supernatant was then moved to fresh tubes and kept at − 80 °C until analysis. TNF-α and IL-6 levels were measured by using a standard protocol of enzyme-linked immunosorbent assay (ELISA) by using Mouse ELISA kits by Elabscience, USA, and the data were expressed as pg/mL.
Quantitative real-time PCR
The colon samples were utilized for total RNA extraction using TRIzol reagent and NucleoSpin RNA isolation kit (Takara Bio, USA) after being kept in an RNA protector (Takara Bio, USA) and kept at − 80 °C. Using a QIAxpert nanodrop spectrophotometer, the amount of RNA was measured. Using a 2 µg RNA sample and the PrimeScript 1st strand cDNA synthesis kit (Takara Bio, USA), cDNA was synthesized. The TB Green Premix Ex Taq II (Tli RNase H Plus, Takara Bio, USA) master mix kit was used for quantitative RT-PCR. Table 2 contains a list of the primers’ forward and reverse sequences. Using GAPDH as a reference gene, the cycle threshold (Ct) for the particular gene of interest was recorded, and the relative quantification was calculated using the 2-△△CT method (Livak and Schmittgen 2001).
Histopathological analysis
The distal portion of the isolated colon samples were preserved in a 10% formalin solution and embedded in paraffin. Five-micrometer-thick sections were cut and stained using hematoxylin and eosin (H&E). The sections were observed under a microscope, and the colonic damage was evaluated in the samples by scoring as described in Table 3 (Dieleman et al. 1998).
Statistical analysis
GraphPad Prism software (version 8.4.2, Trial Version) was used to analyze the data. The disease activity index (DAI) and histological colitis score were analyzed by the Kruskal-Wallis with Dunn’s multiple comparison tests, whereas all the other parameters were analyzed by one-way analysis of variance (ANOVA) test followed by Tukey’s multiple comparison test, and the data are expressed as mean ± standard error of the mean (SEM). The significance level was p < 0.05.
Results
Effect of nodakenin on body weight loss against TNBS-induced colitis
Following the TNBS administration, the DAI score and body weight loss in the MC group were significantly higher (p < 0.0001) than in the NC group. Nodakenin and sulfasalazine protected the TNBS-induced body weight loss in a dose-dependent manner, and there was a significant difference (p < 0.001) when compared to the MC group. When compared to the MC group, the DAI scores for STD (100 mg/kg), NO2, and NO3 (20 and 40 mg/kg) considerably decreased (p < 0.001) (Fig. 1A, B).
Effect of nodakenin on gross colonic changes against TNBS-induced colitis
Inflammation of the colon is characterized by a reduction in colon length and an increase in colon weight/length ratio. The MC group showed significant (p < 0.0001) elevation in colon weight/length ratio and shorter colon length as compared to the NC group. Nevertheless, the STD (sulfasalazine, 100 mg/kg), NO2 (20 mg/kg), and NO3 (40 mg/kg) treatment groups provided considerable protection (p < 0.0001) against the decrease in colon length and increase in colon weight/length ratio (Fig. 2A–C).
Nodakenin improved the clinical features and macroscopic damage to the colon against TNBS-induced colitis in mice. A Representative images of the colon. B Colon length of all experimental groups. C Colon weight/length ratio (mg/cm). D Histological score. Values are expressed as the mean ± SEM (n = 8). NC (normal control), MC (model control, only TNBS), STD (sulfasalazine, 100 mg/kg), NO1, NO2, NO3 (nodakenin 10, 20, 40 mg/kg), respectively
Effect of nodakenin on oxidative stress parameters in colon tissues
Higher MDA levels are an indicative of oxidative damage and are a potent marker for free radical-induced lipid peroxidation. MDA levels in colon tissues of the MC group were significantly (p < 0.0001) elevated as compared to the NC group. Nonetheless, nodakenin (NO) and sulfasalazine suppressed the MDA levels rise as compared to the MC group (Fig. 3A). MPO, a lysosomal protein found in neutrophils, serves as a biomarker for assessing the colic state. MC group depicted a significant elevation (p < 0.001) in MPO level than the NC group showcasing the well-established colitis in mice. However, STD and nodakenin treatment significantly (p < 0.001) averted the rise in MPO levels as compared to the MC group, depicting a decrease in neutrophil infiltration in colonic tissues (Fig. 3B). The substantial rise in MPO level and NO level suggests a persistent phagocyte-dependent nitro-oxidative stress throughout the colon in TNBS-induced colitis. The NO level of the MC group was significantly (p < 0.0001) raised than the NC group. Pretreatment with nodakenin (NO3, 40 mg/kg nodakenin) and sulfasalazine was effective in controlling oxidative stress and significantly (p < 0.0001) lowered the NO level depicting its antioxidant activity (Fig. 3C). One important antioxidant enzyme that helps prevent tissue against oxidative damage is SOD, its levels are reduced in the TNBS model, and similar results were seen in the present study. The SOD level of the MC group was significantly (p < 0.0001) reduced as compared to the NC group. However, nodakenin (20 mg/kg and 40 mg/kg) and sulfasalazine prevented the decline in SOD level illustrating its protective effect against oxidative damage due to TNBS (Fig. 3D).
Effect of nodakenin on oxidative stress parameters and inflammatory mediators. A Malondialdehyde (MDA) levels. B Myeloperoxidase (MPO) levels. C Nitric oxide (NO) levels. D Superoxide dismutase (SOD) levels. E TNF-α concentration. F IL-6 concentration. Values are expressed as the mean ± SEM (n = 8). NC (normal control), MC (model control, only TNBS), STD (sulfasalazine, 100 mg/kg), NO1, NO2, NO3 (nodakenin 10, 20, 40 mg/kg), respectively
Effect of nodakenin on pro-inflammatory cytokines, TNF-α, and IL-6 levels in colon
TNF-α and IL-6 levels in the MC group were significantly (p < 0.0001) elevated than the NC group. Nonetheless, all the doses of nodakenin and sulfasalazine significantly (p < 0.0001) decreased the TNF-α and IL-6 levels than the MC group demonstrating the anti-inflammatory activity of nodakenin (Fig. 3E, F).
Effect of nodakenin on TNBS-induced histopathological changes in colon
The colonic architecture of the NC group was normal, as seen in Fig. 4A. Increased mucosal thickening, goblet cell loss, inflammatory cell infiltration, and crypt abscess loss are the characteristics of TNBS-induced colitis. The MC group’s H&E stain (Fig. 4B) exhibited all the attributes of TNBS colitis. The NO1 group had anomalies such as minor damage to goblet cells, inflammatory cell infiltration, and mucosal thickening (Fig. 4D). However, the STD, NO2, and NO3 groups restored the colonic architecture, and intact goblet cells are seen in histopathological analysis (Fig. 4C, E, F). The histological score of the MC group was significantly elevated (p < 0.0001) as compared to the NC group. Compared with the MC group, the nodakenin and sulfasalazine groups showed a considerably higher histological score (p < 0.0001) (Fig. 2D).
The protective effect of nodakenin on the colonic damage due to TNBS-induced colitis was observed in histology stained with H&E and examined at × 4, × 10, and × 40. Black arrow: Tips of villi and goblet cells. Green arrow: Immune cell (neutrophils) infiltration. A NC (normal control): Shows intact colonic architecture, the crypts, villi, and goblet cells were of normal appearance, and the immune cell infiltration was not seen. B MC (model control, only TNBS): Loss of crypt, damaged goblet cells, loss of mucosal layer, and a massive accumulation of immune cells inside the crypts, and in the layers of the colon depicted transmural inflammation. C STD (sulfasalazine, 100 mg/kg). D–F NO1, NO2, NO3 nodakenin (nodakenin 10, 20, 40 mg/kg), respectively. Treatment groups protected the shortening of villi and infiltration of immune cells and showed much better colonic architecture and goblet cells in a dose-dependent manner
Effect of nodakenin in suppressing the inflammatory burden in TNBS-induced colitis
The NFƙB-mediated NLRP3 inflammasome pathways have major significance in colitis. The study emphasizes these pathways, their ability to interact, and various genes associated and their overexpression in TNBS-induced colitis. In the study, the mRNA expression of NFƙB, iNOS, COX-2, NLRP3, IL-1β, and IL-18 has been assessed to investigate the mechanism through which nodakenin mitigates TNBS-induced colitis. The MC group illustrated substantial (p < 0.0001) upregulation of NFƙB (4.5-fold), iNOS (3.08-fold), COX-2 (6.46-fold), NLRP3 (6.89-fold), IL-1β (4.62-fold), and IL-18 (4.91-fold) than NC group (Fig. 5A–F).
Nodakenin downregulated various pro-inflammatory cytokines and enzymes causing inflammation. The gene expression studies were performed in colon homogenate by real-time quantitative RT-PCR. A–F Relative mRNA expression of NFƙB, iNOS, COX-2, NLRP3, IL-1β, and IL-18, respectively. Values are expressed as the mean ± SEM (n = 6). NC (normal control), MC (model control, only TNBS), STD (sulfasalazine, 100 mg/kg), NO1, NO2, NO3 (nodakenin 10, 20, 40 mg/kg), respectively
The pathophysiology of TNBS-induced colitis is largely reliant on NFƙB activation. The sulfasalazine and all the doses of nodakenin groups dramatically prevented its overexpression compared to the MC group. NFƙB enhances the transcription of multiple inflammatory enzymes, including COX-2 and iNOS, and elevated levels of these enzymes have been detected in colitis. Nodakenin (20 mg/kg and 40 mg/kg) significantly (p < 0.0001) prevented the upregulation of NFƙB (1.87-fold and 1.24-fold), iNOS (1.77-fold and 1.20-fold), and COX-2 (2.61-fold and 1.98-fold) as compared to MC group (Fig. 5A–C).
NLRP3 inflammasome activation is related to the activation of NFƙB several transcriptional factors, including NLRP3, IL-1β, and IL-18. All these transcriptional factors such as NLRP3 (2.8-fold and 1.78-fold), IL-1β (1.87-fold and 1.29-fold), and IL-18 (1.80-fold and 1.17-fold) were significantly (p < 0.0001) repressed by nodakenin (20 mg/kg and 40 mg/kg) as compared to the MC group (Fig. 5D–F).
The sulfasalazine (100 mg/kg) group illustrated substantial (p < 0.0001) downregulation of NFƙB (1.36-fold), iNOS (1.29-fold), COX-2 (2.01-fold), NLRP3 (2.08-fold), IL-1β (1.33-fold), and IL-18 (1.64-fold) than MC group (Fig. 5A–F). The nodakenin treatment dose of 40 mg/kg downregulated all these transcriptional factors compared to sulfasalazine (100 mg/kg) depicting its role in combating inflammatory bowel disease through the NFƙB-mediated NLRP3 inflammasome pathway.
Discussion
UC is a colon inflammatory condition associated with ulcer development and recurrent inflammation. The precise mechanisms behind UC remain unclear, and there are currently few efficient treatments available (Segal et al. 2021). Treatment for UC aims to bring about and sustain remission depending on the severity of the disease and clinical activity. Glucocorticoids, preparations of aminosalicylic acid, and immunosuppressants are currently the three main classes of drugs used in the medical care of ulcerative colitis (Cai et al. 2021). Nevertheless, these medications’ considerable adverse effects seriously restrict their clinical effectiveness. Therefore, to cure UC, it is imperative to design a new pharmaceutical approach with greater efficacy and fewer adverse effects (Danese et al. 2022). Phytoconstituents from natural sources are an effective option for the newer drug discovery to treat UC with fewer side effects (Li et al. 2005; Saxena et al. 2014). Nodakenin is a furanocoumarin and possesses various pharmacological activities such as anti-inflammatory and antioxidant activity by downregulating multiple pro-inflammatory cytokines and various transcriptional factors (Kim et al. 2007). To the best of our understanding, this is the first research work to showcase the effectiveness of nodakenin in combating acute ulcerative colitis.
TNBS is a hapten molecule that depicts certain responses that are characterized by an increase in macrophage activation and release of a variety of pro-inflammatory mediators that cause the colon to become transmurally inflamed, body weight to drop, rectal bleeding, watery diarrhea, and shorter colon length (Oh et al. 2014; Randhawa et al. 2014; Jiminez et al. 2015). Similar characteristics were observed in the current investigation MC group; nevertheless, pretreatment with nodakenin at a higher dose significantly inhibited colon length shortening by 24% and DAI elevation by 25% as compared to the MC group.
Researchers stated that UC leads to many changes in the colonic architecture which is characterized as a damaged mucosal barrier, an increase in neutrophil infiltration in the mucosa, damaged goblet cells, accumulation of red blood cells, and severe inflammation (Yang et al. 2012; Cury et al. 2013; Lopes de Oliveira et al. 2019). Similar changes were observed in the present study, and the histopathology of the colon depicted all such changes, and the treatment with nodakenin averted the neutrophil infiltration and decreased the damage to colonic mucosa and the goblet cells demonstrating its colonoprotective effect. The study was supported by the shreds of evidence of many naturally derived compounds that mitigated colitis with all such changes (Peng et al. 2019); matrine (1, 5, 10 mg/kg) alleviated colonic injury and inflammation in TNBS-induced colitis in mice (Li et al. 2019).
TNBS leads to an increase in cell permeability causing neutrophil infiltration and macrophage activation in the mucosa of the colon when it is inflamed. Nitric oxide acts on smooth muscle and leads to increased permeability causing infiltration of pro-inflammatory cells and leading to inflammation; it is a classical marker of acute and chronic inflammation (Salas et al. 2002). TNBS leads to an increase in nitric oxide levels in the colon illustrating inflammation in the colon; the present study illustrated acute inflammation by raising NO level. Various natural compounds possessed the ability to reduce cell permeability and inflammation depicting a decrease in NO levels; quercetin and piperine reduced the NO and iNOS levels in the TNBS colitis model (Romero et al. 2017; Guo et al. 2020). Similarly, nodakenin depicted a decrease in NO levels showcasing its anti-inflammatory activity.
An increase in cell permeability marks the entry of several pro-inflammatory cells. MPO is an enzyme highly expressed in monocytes, macrophages, and neutrophils. It is regarded as a unique biomarker of acute inflammation because of reports that its elevated activity indicates neutrophil infiltration into the tissue (Kim et al. 2012). MPO levels in TNBS-induced colitis get elevated illustrating an increase in neutrophil infiltration in the colonic mucosa (Wang et al. 2016). Similarly, the present study depicted the rise in neutrophil infiltration by an increase in MPO level by 112% in the MC group. Nonetheless, nodakenin prevented the neutrophil infiltration illustrated by 25% decreased MPO levels and depicted its protective effect on the colon.
The activated neutrophils and macrophages lead to the production of reactive oxygen species (ROS) within the intestinal mucosa, causing oxidative stress and contributing to the pathophysiology of colitis (Elmaksoud et al. 2021). Lipid peroxidation is caused by free radicals removing a hydrogen atom from polyunsaturated fatty acids in cell membranes. One crucial stage in the process of colonic mucosal damage is thought to be lipid peroxidation. MDA is the byproduct of lipid peroxidation and is frequently employed as a biomarker of the lipid peroxidation process (Ohkawa et al. 1979). An antioxidant called SOD is necessary for cells to defend themselves against ROS and the byproducts of free radical chain reactions. In the TNBS-induced colitis model, MDA increases and SOD level is decreased depicting an increase in ROS generation and oxidative damage (Zhou et al. 2006). Similarly, the study demonstrated 208% elevated MDA levels and 50% depleted SOD levels in TNBS-induced colitis demonstrating oxidative damage. Manganese superoxide dismutase (10, 20, and 40 mg/kg) prevented the increase in MDA level and decrease in SOD level in TNBS-induced colitis (Wang et al. 2016). Similarly, nodakenin pretreatment prevented the 32% rise in MDA level and 12% decline in SOD levels preventing ROS generation and protecting from oxidative damage. Lim et al. have demonstrated the anti-oxidant effect of nodakenin at 30 mg/kg dose in LPS-induced liver injury in mice by protecting the decrease in the SOD and GSH levels (Lim et al. 2021). Rim et al. stated the antioxidant effect of nodakenin on LPS-induced inflammation in RAW 264.7 cells by decreasing the NO levels depicting the fall in ROS generation and having an antioxidant effect at the dose of 100 µM (Rim et al. 2012). Another study by Liao et al. stated that nodakenin at the dose of 20 mg/kg dramatically suppressed the production of reactive oxygen species and subsequent NLPR3 inflammasome activation in ischemia reperfusion-induced renal injury in mice (Liao et al. 2021b). Similarly, the present study supported the previous studies and depicted the effect of nodakenin on various oxidative stress parameters showcasing its anti-oxidant activity.
The precise mechanism of nodakenin responsible for the anti-inflammatory activity and colonoprotective activity in mitigating IBD is unclear. Recent studies have concentrated on identifying the transcription factors and signaling mechanisms that bind to gene promoter regions, control the production of pro-inflammatory cytokines, and mediate gene transcription in ulcerative colitis. The immune system and inflammatory processes are regulated by NFƙB, a crucial transcription factor of lymphocytes and macrophages, as evidenced by recent studies (Li et al. 2005; Tsang et al. 2015). Various pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, iNOS, COX-2, and IL-18, are expressed in greater quantities once NFƙB is activated. TNF-α is one of these pro-inflammatory cytokines that is crucial to TNBS-induced colitis and is probably the primary regulator of the inflammatory cascade in ulcerative colitis (Zhou et al. 2006; Natarajan et al. 2018). The current investigation verifies a noteworthy rise in colon tissue TNF-α, IL-6, and NFƙB levels TNBS model group. Lim et al. reported the protective effect of nodakenin (10 and 30 mg/kg) against LPS-induced liver damage by downregulating the expression of NFƙB and pro-inflammatory mediators such as TNF-α, IL-6, IL-1β, iNOS, and COX-2 (Lim et al. 2021). Another study by Rim et al. reported that nodakenin (10 and 20 mg/kg) suppressed the LPS-induced inflammatory response in RAW 264.7 murine macrophage cells and in mice and also protected mice from endotoxin shock by targeting NFƙB pathway and downregulating various pro-inflammatory mediators such as TNF-α, IL-6, IL-1β, iNOS, and COX-2 (Rim et al. 2012). Similarly, in the present investigation, following nodakenin (10, 20, and 40 mg/kg) treatment, there was a noticeable decrease in the production of these pro-inflammatory cytokines in colon tissues, which could be linked with the anti-inflammatory effect of nodakenin.
Inducing the transcriptional expression of NLRP3, NFƙB is a crucial mediator of the priming signal of NLRP3 inflammasome activation in UC. Activated NFƙB transcriptionally induces the expression of the pro‑IL‑1β and pro-IL-18, but its maturation to IL‑1β and IL-18, respectively, and further its secretion would require activation of NLRP3 inflammasome (Zhen and Zhang 2019; Mustafa 2022; Kim et al. 2022). The present investigation demonstrated the upregulation of mRNA expression of NLRP3, IL-1β, and IL-18. Nevertheless, nodakenin (20 and 40 mg/kg) downregulated the mRNA expression of all the transcriptional factors and pro-inflammatory mediators. A study by Liao et al. demonstrated the effect of nodakenin (20 mg/kg) alleviated renal ischemia-reperfusion injury by suppressing reactive oxygen species–induced NLRP3 inflammasome activation (Liao et al. 2021a).
In the present study, we illustrated the colonoprotective effect of nodakenin in combating TNBS-induced colitis in mice by suppressing various pro-inflammatory mediators such as TNF-α, IL-6, IL-1β, IL-18, iNOS, COX-2, and various transcriptional factors such as NFƙB and NLRP3. Nodakenin also demonstrated its anti-inflammatory activity and anti-oxidant activity by protecting the colonic damage caused by to generation of ROS by preventing the rise in MPO, MDA, and NO levels while increasing SOD levels. It is expected that the development of lead compounds targeting the NLRP3 inflammasome can be developed for the treatment of IBD (Xue et al. 2023). To sum up, the study demonstrated strong evidence of nodakenin alleviating TNBS-induced colitis through NFƙB mediated NLRP3 inflammasome pathway.
Conclusion
Taking together, the study has provided evidence that nodakenin has the potential effect against TNBS-induced colitis in mice probably by modulating NFƙB-associated NLRP3 pathway as it halted the overexpression of MPO, MDA, NO, iNOS, COX-2, NFƙB, and NLRP3 and downregulated the expression of various pro-inflammatory mediators such as TNF-α, IL-6, IL-1β, and IL-18 (Fig. 6). Our present research work provides the basis for the development of a novel therapeutic agent in combating a complex inflammatory disease such as ulcerative colitis.
Proposed mechanism of nodakenin in mitigating TNBS-induced colitis in mice through downregulating NFƙB-mediated NLRP3 inflammasome pathway. TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α; TRADD, TNFR1-associated death domain protein; RIP, receptor-interacting protein; TRAF2, the tumor necrosis factor (TNF) receptor-associated factor; TNBS, 2,4,6-trinitrobenzene sulfonic acid; NFƙB, nuclear factor kappa B; ROS, reactive oxygen species; NLRP3, NOD-like receptor protein-3; IL-6, interleukin-6; IL-1β, interleukin 1β; IL-18, interleukin IL-18; iNOS, inducible nitric oxide; COX-2, cyclooxygenase-2; MPO, myeloperoxidase; MDA, malondialdehyde; NO, nitric oxide; SOD, superoxide dismutase. The image was created using BioRender.com
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CD:
-
Crohn’s disease
- COX-2:
-
Cyclooxygenase-2
- DAI:
-
Disease activity index
- ELISA:
-
Enzyme-linked immunosorbent assay
- IBD:
-
Inflammatory bowel disease
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- LPS:
-
Lipopolysaccharides
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase
- NFKB:
-
Nuclear factor kappa B
- NLRP3:
-
Nucleotide-binding domain receptor protein-3
- NO:
-
Nitric oxide
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNBS:
-
2,4,6-Trinitrobenzene sulfonic acid
- TNF-α:
-
Tumor necrosis factor-alpha
- UC:
-
Ulcerative colitis
References
Abhirami NR, Laksmi VV, Deepitha AM (2022) A review on prevalence of inflammatory bowel disease in India. J Drug Deliv Ther 12:219–223. https://doi.org/10.22270/JDDT.V12I6.5403
Alatab S, Sepanlou SG, Ikuta K et al (2020) The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 5:17–30. https://doi.org/10.1016/S2468-1253(19)30333-4
Cai Z, Wang S, Li J (2021) Treatment of inflammatory bowel disease: a comprehensive review. Front Med (Lausanne) 8: 765474
Carneiro A, Matos MJ, Uriarte E, Santana L (2021) Trending topics on coumarin and its derivatives in 2020. Molecules 26:501
Cury DB, Mizsputen SJ, Versolato C et al (2013) Serum calprotectin levels correlate with biochemical and histological markers of disease activity in TNBS colitis. Cell Immunol 282:66–70. https://doi.org/10.1016/j.cellimm.2013.04.004
Danese S, Solitano V, Jairath V, Peyrin-Biroulet L (2022) The future of drug development for inflammatory bowel disease: the need to ACT (advanced combination treatment). Gut 71:2380-2387
Di Stasi LC (2021) Coumarin derivatives in inflammatory bowel disease. Molecules 26:422
Dieleman LA, Palmen MJHJ, Akol H et al (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385-91
Elmaksoud HAA, Motawea MH, Desoky AA et al (2021) Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed Pharmacother 142. https://doi.org/10.1016/j.biopha.2021.112073
Gandhi T, Patel B, Patel D et al (2021) Optimization and validation of polyherbal formulation by applying boxbehnken design for the treatment of inflammatory bowel disease in experimental animals. Curr Drug Ther 17:17–29. https://doi.org/10.2174/1574885517666211220130024
Green LC, Wagner DA, Glogowski J et al (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131-138
Guo G, Shi F, Zhu J et al (2020) Piperine, a functional food alkaloid, exhibits inhibitory potential against TNBS-induced colitis via the inhibition of IκB-α/NF-κB and induces tight junction protein (claudin-1, occludin, and ZO-1) signaling pathway in experimental mice. Hum Exp Toxicol 39:477–491. https://doi.org/10.1177/0960327119892042
Haftcheshmeh SM, Abedi M, Mashayekhi K et al (2022) Berberine as a natural modulator of inflammatory signaling pathways in the immune system: focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother Res 36:1216–1230. https://doi.org/10.1002/PTR.7407
Jain M, Venkataraman J (2021) Inflammatory bowel disease: an Indian perspective. Indian J Med Res 153:421–430
Jiminez JA, Uwiera TC, Inglis GD, Uwiera RRE (2015) Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog 7:29
Kang SY, Kim YC (2007) Neuroprotective coumarins from the root of Angelica gigas: structure-activity relationships. Arch Pharm Res 30:1368-73
Kim DH, Kim DY, Kim YC et al (2007) Nodakenin, a coumarin compound, ameliorates scopolamine-induced memory disruption in mice. Life Sci 80:1944–1950. https://doi.org/10.1016/j.lfs.2007.02.023
Kim JJ, Shajib MS, Manocha MM, Khan WI (2012) Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 3678. https://doi.org/10.3791/3678
Kim SL, Shin MW, Kim SW (2022) Lipocalin 2 activates the NLRP3 inflammasome via LPS-induced NF-κB signaling and plays a role as a pro-inflammatory regulator in murine macrophages. Mol Med Rep 26. https://doi.org/10.3892/mmr.2022.12875
Kim Y-J, Park S-J, Kim T-J (2011) Anti-allergic effects of nodakenin in IgE/Ag-induced type I hypersensitivity. J Life Sci 21:1721–1725. https://doi.org/10.5352/JLS.2011.21.12.1721
Kirsch G, Abdelwahab AB, Chaimbault P (2016) Natural and synthetic coumarins with effects on inflammation. Molecules 21:1322
Kobayashi T, Siegmund B, Le Berre C et al (2020) Ulcerative colitis. Nat Rev Dis Primers 6. https://doi.org/10.1038/s41572-020-0205-x
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87:1344–1350. https://doi.org/10.1016/0016-5085(84)90202-6
Li JH, Yu JP, Yu HG et al (2005) Expression and significance of nuclear factor κB p65 in colon tissues of rats with TNBS-induced colitis. World J Gastroenterol WJG 11:1759. https://doi.org/10.3748/WJG.V11.I12.1759
Li P, Lei J, Hu G et al (2019) Matrine mediates inflammatory response via gut microbiota in TNBS-induced murine colitis. Front Physiol 10:444299. https://doi.org/10.3389/FPHYS.2019.00028/BIBTEX
Liao Y, Lin X, Li J et al (2021a) Nodakenin alleviates renal ischaemia-reperfusion injury via inhibiting reactive oxygen species-induced NLRP3 inflammasome activation. Nephrology 26:78–87. https://doi.org/10.1111/nep.13781
Liao Y, Lin X, Li J et al (2021b) Nodakenin alleviates renal ischaemia-reperfusion injury via inhibiting reactive oxygen species-induced NLRP3 inflammasome activation. Nephrology (Carlton) 26:78–87. https://doi.org/10.1111/NEP.13781
Lim JY, Lee JH, Yun DH et al (2021) Inhibitory effects of nodakenin on inflammation and cell death in lipopolysaccharide-induced liver injury mice. Phytomedicine 81. https://doi.org/10.1016/j.phymed.2020.153411
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lopes de Oliveira GA, Alarcón de la Lastra C, Rosillo MÁ et al (2019) Preventive effect of bergenin against the development of TNBS-induced acute colitis in rats is associated with inflammatory mediators inhibition and NLRP3/ASC inflammasome signaling pathways. Chem Biol Interact 297:25–33. https://doi.org/10.1016/j.cbi.2018.10.020
Luo X, Yu Z, Deng C et al (2017) Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci Rep 7. https://doi.org/10.1038/s41598-017-12562-6
Mao L, Kitani A, Hiejima E et al (2020) Bruton tyrosine kinase deficiency augments NLRP3 inflammasome activation and causes IL-1β-mediated colitis. J Clin Investig 130:1793–1807. https://doi.org/10.1172/JCI128322
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175. https://doi.org/10.1016/s0021-9258(19)45228-9
Morris GP, Beck PL, Herridge MS et al (1989) Hapten-induced model of chronic inflammation and ulceration in the colon rat. Gastroenterology 96:795-803
Mustafa M (2022) Evaluation of NLRP3 inflammasome protein expression in ulcerative colitis. Wiad Lek 75:641–644. https://doi.org/10.36740/wlek202203113
Natarajan K, Abraham P, Kota R, Isaac B (2018) NF-κB-iNOS-COX2-TNF α inflammatory signaling pathway plays an important role in methotrexate induced small intestinal injury in rats. Food Chem Toxicol 118:766–783. https://doi.org/10.1016/j.fct.2018.06.040
Oh SY, Cho KA, Kang JL et al (2014) Comparison of experimental mouse models of inflammatory bowel disease. Int J Mol Med 33:333–340. https://doi.org/10.3892/ijmm.2013.1569
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Park SJ, Cha HS, Lee YH et al (2014) Effect of nodakenin on atopic dermatitis-like skin lesions. Biosci Biotechnol Biochem 78:1568–1571. https://doi.org/10.1080/09168451.2014.923296
Peng J, Zheng TT, Li X et al (2019) Plant-derived alkaloids: the promising disease-modifying agents for inflammatory bowel disease. Front Pharmacol 10. https://doi.org/10.3389/fphar.2019.00351
Randhawa PK, Singh K, Singh N, Jaggi AS (2014) A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol 18:279–288
Rim HK, Cho W, Sung SH, Lee KT (2012) Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-κB pathways and protects mice from lethal endotoxin shock. J Pharmacol Exp Ther 342:654–664. https://doi.org/10.1124/JPET.112.194613
Romero M, Vera B, Galisteo M et al (2017) Protective vascular effects of quercitrin in acute TNBS-colitis in rats: the role of nitric oxide. Food Funct 8:2702–2711. https://doi.org/10.1039/C7FO00755H
Salas A, Gironella M, Salas A et al (2002) Nitric oxide supplementation ameliorates dextran sulfate sodium-induced colitis in mice. Lab Investig 82:597–608
Saxena A, Kaur K, Hegde S et al (2014) Dietary agents and phytochemicals in the prevention and treatment of experimental ulcerative colitis. J Tradit Complement Med 4:203–217. https://doi.org/10.4103/2225-4110.139111
Segal JP, Jean-Frédéric LeBlanc A, Hart AL (2021) Ulcerative colitis: an update. Clin Med (Lond) 21:135–139. https://doi.org/10.7861/CLINMED.2021-0080
Sharifi-Rad J, Cruz-Martins N, López-Jornet P et al (2021) Natural coumarins: exploring the pharmacological complexity and underlying molecular mechanisms. Oxid Med Cell Longev 2021. https://doi.org/10.1155/2021/6492346
Singh G, Kaur J, Kaur M et al (2020) Anti-nociceptive and anti-inflammatory effect of imperatorin: evidences for involvement of COX-2, iNOS, NFκB and inflammatory cytokines. Int J Neurosci 130:176–185. https://doi.org/10.1080/00207454.2019.1667789
Snell A, Segal J, Limdi J, Banerjee R (2021) Inflammatory bowel disease in India: challenges and opportunities. Frontline Gastroenterol 12:390–396
Song Y, Zhao Y, Ma Y et al (2021) Biological functions of NLRP3 inflammasome: a therapeutic target in inflammatory bowel disease. Cytokine Growth Factor Rev 60:61–75
Tong X, Chen L, He S et al (2023) Forsythia suspensa (Thunb.) Vahl extract ameliorates ulcerative colitis via inhibiting NLRP3 inflammasome activation through the TLR4/MyD88/NF-κB pathway. Immun Inflamm Dis 11. https://doi.org/10.1002/iid3.1069
Tsang SW, Ip SP, Wu JCY et al (2015) A Chinese medicinal formulation ameliorates dextran sulfate sodium-induced experimental colitis by suppressing the activity of nuclear factor-kappaB signaling. J Ethnopharmacol 162:20–30. https://doi.org/10.1016/j.jep.2014.12.035
Wang K, Lv Q, Miao Y-M et al (2018) Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol 155:494–509. https://doi.org/10.1016/j.bcp.2018.07.039
Wang YH, Dong J, Zhang JX et al (2016) Effects of mimic of manganese superoxide dismutase on 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Arch Pharm Res 39:1296–1306. https://doi.org/10.1007/s12272-016-0811-z
Witaicenis A, Seito LN, Da Silveira CA et al (2014) Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 21:240–246. https://doi.org/10.1016/j.phymed.2013.09.001
Xue JC, Yuan S, Hou XT et al (2023) Natural products modulate NLRP3 in ulcerative colitis. Front Pharmacol 14:1265825
Yang XL, Guo TK, Wang YH et al (2012) Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch Pharm Res 35:1231–1239. https://doi.org/10.1007/s12272-012-0714-6
Zhang T, Mei Y, Dong W et al (2020) Evaluation of protein arginine deiminase-4 inhibitor in TNBS- induced colitis in mice. Int Immunopharmacol 84. https://doi.org/10.1016/j.intimp.2020.106583
Zhen Y, Zhang H (2019) NLRP3 inflammasome and inflammatory bowel disease. Front Immunol 10:276
Zhou YH, Yu JP, Liu YF et al (2006) Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediators Inflamm. https://doi.org/10.1155/MI/2006/92642
Acknowledgements
The authors would like to acknowledge Charotar University of Science and Technology (CHARUSAT) for funding the research work (RP3/22) and Ramanbhai Patel College of Pharmacy for facilitating the research activities.
Funding
The research work was supported by the Charotar University of Science and Technology (CHARUSAT), (Grant no. RP3/22).
Author information
Authors and Affiliations
Contributions
B.S.: Conceptualization, design of the study, acquisition of data, data analysis, drafting the manuscript. N.S.: supervision, revising the manuscript, and proofreading of the manuscript. All authors have read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Animal Ethics Committee of Ramanbhai Patel College of Pharmacy, CHARUSAT, with the protocol number: RPCP/IAEC/2022–2023/R6.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, B., Solanki, N. Ameliorative effect of nodakenin in combating TNBS-induced ulcerative colitis by suppressing NFƙB-mediated NLRP3 inflammasome pathway. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03304-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03304-3