Abstract
Reproductive deficiency is a major outcome of pesticide exposure sequel to cellular oxidative damage to sex organs. Flavonoid possess potent antioxidant capacities to mitigate pesticide related cellular injury. The present investigation examined the mitigative effect of micronized purified fractions of diosmin and hesperidin on reproductive hormones, sperm parameters, and testicular glycogen in male Wistar rats after sub-chronic Chlorpyriphos (CPF) exposure. Twenty-five male Wistar rats (120-145 g) were randomly allocated five rats per group. Group I (DW) received distilled water (2 ml/kg), Group II (S/oil) received soya oil (2 ml/kg), Group III (DAF) received Daflon at 1000 mg/kg, Group IV (CPF) received Chlorpyriphos (7.74 mg/kg), and Group V (DAF + CPF) received Daflon (1000 mg/kg) followed by CPF (7.74 mg/kg) after 30 min of Daflon. This regimen was administered daily for 60 days. After cervical venesection under light chloroform anesthesia, blood samples were examined for levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone. Each rat's testicular tissue was quickly cut, collected, and glycogen evaluated. Sperm concentration, motility, morphology, and viability were measured in the right caudal epididymis. Results revealed that the untreated CPF group had significantly lower FSH, LH, testosterone, testicular glycogen, and sperm concentration. Additionally, CPF group sperm characteristics were abnormal compared to other groups. These reproductive hormones, testicular glycogen, and sperm parameters improved in the Daflon-treated groups. Hence, pre-treatment with flavonoid fractions of diosmin and hesperidin mitigated CPF-induced reproductive toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental chemicals have been demonstrated to impair fertility in both humans and animals (Louis et al. 2016; Pizzorno 2018). With a 50% incidence during the last few years, infertility continues to be a global health concern (Chevrier et al. 2013). There has been an emphasis on the negative reproductive consequences associated with pesticide contamination that are extensive and permanent (Umosen and Uchendu 2014). Chronic pesticide exposure may threaten the lives of non-target creatures (Zhu 2015). Previous studies have shown that pesticides contribute to reproductive impairment and consequently reduce fertility (Kenfack et al. 2015; Shittu et al. 2014). Organophosphate (OP) chemicals are well-known for their dual function as household insecticides as well as agricultural productivity boosters (Oates et al. 2014). Chlorpyriphos (CPF) is a cheap and widely available OP pesticide that has serious health risks. CPF, like other OPs, causes toxicity by decreasing the activity of acetylcholinesterase (AChE) (Ambali et al. 2010). Other cellular pathways of CPF toxicity include increased reactive oxygen species (ROS) production and oxidative stress (OS) (Ambali and Ayo 2011; Olatunji et al. 2022). CPF exposure has been associated with oxidative damage to cellular membranes of important organs in the body, including the male reproductive system, as well as disruption of hormonal balance (Shittu et al. 2014). Pesticide-induced reproductive hormone imbalance, which reduces sperm production and quality, may lead to infertility (Joshi et al. 2007). CPF also depletes glycogen levels in the testes, impairing spermatozoa's capacity to swim and migrate efficiently enough to fertilize an egg (Umosen and Uchendu 2014). ROS causes DNA damage in spermatozoa, altering concentration, motility, morphology, and potential viability (Beam et al. 2014). The development of oxidative damage from OP exposure has been associated with decreased sperm function and poor male fertility (Leong et al. 2013).
Previous animal and human research have demonstrated the relevance of plant-derived antioxidants in the treatment of acute and chronic disorders (Heikal et al. 2013; Shittu et al. 2014; Surai and Fisinin 2014). Daflon (Daflon 500 mg®) is a micronized flavonoid derived from the plant Rutaceae aurantiae that includes 90% diosmin and 10% hesperidin (Rizk and Sabri 2009). Diosmin and hesperidin are powerful flavonoid antioxidants with high ROS removal capacity that can be employed to treat oxidatively induced infertility (Olatunji et al. 2022). Therefore, the purpose of this study was to assess the protective effects of flavonoid fractions of diosmin and hesperidin on alterations in reproductive hormones, testicular glycogen, and sperm characteristics in male albino rats sub-chronically exposed to Chlorpyriphos.
Materials and methods
Laboratory animals
For this experiment, 25 male albino rats weighing 120–145 g were obtained from the National Veterinary Research Institute in Jos (Vom), Nigeria. They were confined in the animal holding facility at Ahmadu Bello University in Zaria, Nigeria. They were given commercially prepared grower pellets and had unlimited access to water. Before the experiment began, participants were allowed two weeks of acclimatization. The laboratory animal care guide was followed (CCAC 1993), and the experimental procedures were carried out in compliance with the Animal Care and Use Committee rules established at Ahmadu Bello University.
Purchase and preparation of chemicals
Soya oil and Chlorpyriphos 20% EC (Termikill®, Gujurat, India) were mixed to make a 10% solution. Before administration, 500 mg/tablet of Daflon (Daflon-500®; Les Laboratoires Servier, France) was dissolved in 5 mL of distilled water to form a 100 mg/mL daily solution.
Sub-chronic reproductive toxicity
Five groups of five rats each were randomly chosen from a total of 25 rats. Distilled water (2 ml/kg), Soya oil (2 mL/kg), Daflon (1000 mg/kg [Olatunji et al. 2022]), and CPF (7.74 mg/kg [Olatunji et al. 2022]) were given to Groups I (DW), II (S/oil), III (DAF), and IV (CPF). Group V got 1000 mg/kg of Daflon before being subjected to CPF (7.74 mg/kg) 30 min later (Olatunji et al. 2022). Each regimen was given via gavage once a day for 60 days. Following a brief chloroform anesthesia, the rats were terminated via cervical venipuncture. To analyze FSH, LH, and testosterone levels, 2 mL of blood sample was collected from the medial canthus of the eyes of each rat into sterile plain sample bottles, and were centrifuged at 1000 rpm for 10 min to obtain serum. Each rat's testicular tissue was promptly cut, collected, and measured for glycogen content. Sperm concentration, motility, morphology, and viability were assessed in the right caudal epididymis.
Evaluation of follicle-stimulating, luteinizing, and testosterone hormone levels
Following the manufacturer’s instructions, serum FSH, LH, and Testosterone concentrations were measured using enzyme immunoassay (EIA) kits; K-Assay ELISA Kit, KT-15332 (Kamiya Biomedical Company, USA), E-EL-R0026 (Elabscience, USA), and Testosterone.
Evaluation of testicular glycogen levels
The gravity method of Good et al. (1993) was used to assess testicular glycogen levels using the BioVision Glycogen Colorimetric Assay Kit II (K648-100), as directed by the manufacturer. The data were given as grams of glycogen per 100 g of testicular tissue.
Evaluation of sperm characteristics
Shortly after the testes were carefully removed, the right epididymis was examined for sperm count following Yokoi et al. (2003). Sperm were extracted by slicing the right caudal epididymis into 5 ml of physiological saline, which was then let to sit at room temperature for two minutes after being rocked for ten minutes. Following the incubation period, the supernatant fluid was diluted 1:100 using a solution that contained 25 mg of eosin stain, 1 mL of formalin (35%), and 5 g of sodium bicarbonate per 100 mL of water. The total number of sperm was counted using a hemocytometer magnified × 200 under a light microscope. Sperm motility was assessed according to Sonmez et al. (2005) guidelines. Using a pipette, remove the fluid from the cauda epididymis, and dilute it to 2 mL with Tris buffer solution. An aliquot of this solution was placed on a slide under a phase contrast microscope, and the % motility was assessed at an enlargement of × 400 using a light microscope. For every sample, estimates of motility were obtained from three distinct fields. The final motility score was calculated using the mean. Sperm cell morphology was evaluated in compliance with the guidelines provided by Atessahin et al. (2006). India ink was used to produce slides that were smeared with spermatozoa. The morphological anomalies of 500 sperm cells were examined. Spermatozoa were observed under a light microscope at a × 400 magnification and classified using phase-contrast optics. Sperm viability was assessed using the Siegel (1993) technique. To distinguish between living and dead sperm, 50 µL of liquid sperm and 50 µL of Eosin-Nigrosin stain were mixed in a microtube. One drop of the mixture was distributed on a microscope slide, and after 30 s, it was allowed to air dry. A bright field microscope was used to examine each slide with an immersion oil drop at a total magnification of 1000. Vital spermatozoa were identified by white or light pink sperm heads.
Statistical analysis
Data were given as mean ± SEM and statistically evaluated using one-way ANOVA and Tukey's post-hoc multiple comparison test. All analyses were conducted using Graphpad Prism version 4.0, San Diego, California, USA (www.graphpad.com). Microsoft excel (2012) was used to plot charts of data obtained in this study. p-values < 0.05 were considered significant.
Results
Follicle stimulating concentration
The concentration of FSH was significantly (P < 0.05) lower in the CPF group (10.81 ± 0.43 ng/mL) when compared to S/oil (14.03 ± 0.63 ng/mL), DAF (15.65 ± 0.56 ng/mL) and DAF + CPF (14.29 ± 0.64 ng/mL) groups (Fig. 1).
Luteinizing hormone concentration
LH concentration in the CPF group (11.77 ± 1.34 mIU/mL) was significantly (P < 0.05) lower than those recorded in S/oil, DAF, and DAF + CPF groups with values of 22.05 ± 1.28, 22.74 ± 0.74 and 24.94 ± 0.92 mIU/mL, respectively (Fig. 2).
Testosterone hormone concentration
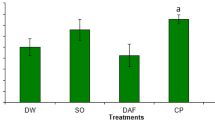
In Fig. 3, a significant (P < 0.05) decrease in testosterone concentration was recorded in the CPF group (2.03 ± 0.18 ng/mL), when compared to DAF + CPF (3.67 ± 0.26 ng/mL). Also, a significant increase in testosterone concentration in the DAF (3.35 ± 0.11 ng/mL) and DAF + CPF (3.67 ± 0.26 ng/mL) groups was recorded when compared to the DW (1.85 ± 0.12 ng/mL) group.
Testicular glycogen concentrations
Testicular glycogen concentration was significantly (P < 0.05) lower in the CPF group (1.38 ± 0.46 ng/mL) when compared to that of DAF, S/oil, and DAF + CPF groups with values of 3.18 ± 0.27, 2.52 ± 0.24 and 2.17 ± 0.04 ng/mL, respectively (Fig. 4).
Sperm concentration
A significant (P < 0.05) decrease in sperm concentration in the CPF group (40.43 ± 0.26 count × 106 cells/mL) was recorded when compared to S/oil (67.63 ± 2.27 count × 106 cells/mL) and DAF + CPF (62.43 ± 1.36 count × 106 cells/mL). Sperm concentration in the DAF + CPF was significantly higher when compared to the DW (48.20 ± 1.95 count × 106 cells/mL) group (Table 1).
Sperm motility
Progressive sperm motility
The progressive motility of spermatozoa significantly (P < 0.05) decreased in the CPF group (31.33 ± 1.76%) compared to those of DW and DAF with values of 55.67 ± 6.22% and 61.00 ± 4.04% respectively. Also, a significant (P < 0.05) increase in progressive sperm motility in the DAF group (61.00 ± 4.04%), was observed when compared to S/oil (48.67 ± 2.33) and DAF + CPF (43.00 ± 2.08%) groups (Table 1).
Non-progressive sperm motility
A significant (P < 0.05) increase in non-progressive sperm motility in the CPF group (34.33 ± 2.33%) was recorded when compared to S/oil and DAF groups, with 23.67 ± 2.33 and 15.00 ± 1.52% values respectively. The non-progressive sperm motility in the DAF group (15.00 ± 1.52%) decreased when compared to that of DAF + CPF (32.67 ± 1.76%) (Table 1).
Non-motile spermatozoa
There was a significant (P < 0.05) increase in non-motile sperms in the CPF group (34.33 ± 0.88%) was recorded, when compared to DW (20.00 ± 0.57%), DAF (28.33 ± 1.20%) and DAF + CPF (27.33 ± 1.76%) groups (Table 1).
Sperm morphology
Sperm head abnormality
Head abnormality of spermatozoa significantly (P < 0.05) increased in the CPF group (37.33 ± 5.45%) than in rats given DW, S/oil, DAF, and DAF + CPF groups with values of 18.00 ± 1.15, 21.67 ± 2.60, 18.33 ± 1.20 and 21.67 ± 0.88%, respectively (Table 1).
Sperm head and mid-piece abnormality
There was no significant (P > 0.05) difference in head and mid-piece abnormality in all the groups, but DAF (2.33 ± 0.66%) group had the lowest percentage of head and mid-piece abnormality when compared to the DW (3.66 ± 0.33%), S/oil (4.33 ± 1.20%), CPF (4.33 ± 0.88%) and DAF + CPF (3.33 ± 0.33%) groups respectively (Table 1).
Sperm tail abnormality
The number of spermatozoa with tail abnormalities was significantly (P < 0.05) higher in the CPF group (13.33 ± 1.45%), compared to DW, S/oil, DAF, and DAF + CPF groups with values of 4.00 ± 0.57, 3.66 ± 1.20, 8.33 ± 0.88 and 8.00 ± 0.57%, respectively (Table 1).
Normal spermatozoa
The number of normal sperm cells significantly (P < 0.05) decreased in the CPF group (52.67 ± 2.40%), when compared to DW, S/oil, DAF, and DAF + CPF groups, with values of 73.00 ± 1.52, 68.67 ± 1.45, 70.00 ± 0.57 and 68.00 ± 1.15%, respectively (Table 1).
Sperm viability
Live spermatozoa
A significant (P < 0.05) decrease in live sperm cells in the CPF group (53.33 ± 0.88%) was recorded, when compared to DW, DAF, and DAF + CPF groups, with values of 70.67 ± 0.88, and 63.67 ± 2.33 and 61.00 ± 3.05%, respectively (Table 1).
Dead spermatozoa
Table 1 depicts a significant increase in dead sperm cells in the CPF group (51.33 ± 3.84%) when compared to DW, DAF and DAF + CPF groups, with values of 26.33 ± 2.18, 36.33 ± 2.33 and 39.00 ± 3.05%, respectively.
Discussion
The lower level of reproductive hormones found in this study is in line with research by Shittu et al. (2013) and Umosen and Uchendu (2014), who discovered that rats exposed to chlorpyriphos had decreased serum concentrations of testosterone, luteinizing hormone, and follicle-stimulating hormone. Research has demonstrated that pesticides impact endocrine functions across the entire spectrum of hormonal regulation, encompassing hormone production, release, storage, transit, clearance, receptor identification, and binding (Mathur et al. 2009). Other pathways of CPF toxicity include anti-gonadal activities in the pituitary gland, which may impair gonadotropin levels and function in human and experimental animals (Faraga et al. 2010; Heikal et al. 2014). FSH, LH, and testosterone are the primary regulators of germ cell growth. FSH enhances spermatogenesis by binding to Sertoli cell receptors, whereas LH stimulates testosterone production in Leydig cells, which then stimulates spermatogenesis in the Sertoli and peritubular cells of the seminiferous tubules (O’Donnel et al. 1994). Thus, a decrease in LH, as seen in this study, leads to a decrease in testosterone levels. The failure of the pituitary gland to release FSH and LH clearly reveals CPF's inhibitory action on the pituitary gland, hence reducing gonadotrophin production and influencing steroidogenesis (Heikal et al. 2013; Shittu et al. 2014). Pre-treatment with Daflon reversed the hormonal imbalance observed in the CPF group, indicating that the flavonoids diosmin and hesperidin can increase gonadotropin output from the pituitary gland. This conclusion is consistent with previous findings where the efficiency of antioxidant-containing flavonoids is documented in restoring hypothalamic-pituitary function by reducing oxidative damage within its axis (Shittu et al. 2013). Olatunji et al. (2022) reported a CPF-induced increase in pituitary and testicular MDA concentrations, as well as decreased antioxidant enzyme activity (superoxide dismutase and catalase), indicating that ROS-induced damage to the pituitary and testes is mediated by oxidative stress induction. As a result, DAF's antioxidant ability to counteract ROS created by CPF's harmful effects may have contributed to the increased levels of FSH, LH, and testosterone.
The CPF group exhibited a notable reduction in testicular glycogen concentration, while the DAF group showed an increase, in comparison to the control group (DW). These results align with previous studies conducted by Shittu et al. (2013) and Al-Amoudi (2015), which also reported a decrease in testicular glycogen levels due to exposure to CPF. Glycogen is an important source of energy for overall body metabolism (Rajawat et al. 2014). Protein synthesis in spermatogenic cells is glucose-dependent, therefore a decrease in glycogen concentration impacts protein synthesis and, as a result, inhibits spermatogenesis (Bhushan et al. 2013; Gawish 2014). In this study, exposure to CPF resulted in substantial glycogen depletion in the testes, indicating decreased glucose storage. The reduction in total glycogen concentration could be attributed to its use to detoxify the pesticide or its metabolites via the process of glucuronidation, which involves harmful metabolites combined with glucose phosphate and being expelled from the liver via bile. Furthermore, the decrease in glycogen content could be related to an increase in enzyme activity (phosphorylase-A and phosphorylase-B), which are involved in glycogenolysis for the glucuronidation process. The increased amounts of testicular glycogen in the groups treated with DAF alone and with DAF + CPF demonstrate that this antioxidant can protect the liver and, as a result, the testes from CPF-induced damage.
Low sperm count, serum gonadotropins, and testosterone commonly accompany abnormal spermatogenesis (Babu et al. 2004). Sperm count is one of the most sensitive assays for spermatogenesis, which is linked to fertility (El-bendary et al. 2014). This study found that the CPF group's caudal epididymal sperm concentration was significantly lower than other groups. Other researchers found that herbicides like CPF reduced sperm count in animals (Shittu et al. 2013; Umosen and Uchendu 2014). Narayana et al. (2006) found that methyl parathion, an organophosphate, decreased adult male rat sperm concentration. In this study, gonadotrophin suppression may have decreased testicular sperm density, altering the caudal epididymis biochemical environment. Sertoli cell activity is directly affected by toxicants, which can cause epithelial disorganization, decreased spermatogenesis, and tubular atrophy (Bedwal et al. 1994; Olorunshola et al. 2011), lowering sperm count.
In their study, Pallav and Rajdeb (2014) discovered that pesticide exposure has a detrimental impact on spermatozoa, disrupts the functioning of Sertoli or Leydig cells, and reduces the quality of semen. DAF + CPF treatment may have increased FSH, LH, and testosterone, which may explain the considerable increase in sperm count. This shows the new spermatogenetic property of this antioxidant agent in protecting and restoring the testes from CPF damage. This work shows for the first time that DAF can increase sperm concentration after sub-chronic CPF exposure. Other sperm properties including motility, morphology, and viability govern fertilizing capacity, hence unfavorable changes affect conception (W.H.O. 2010).
Based on movement patterns, WHO guidelines classify sperm motility as sperm progressive motility (SPM), sperm non-progressive motility (SNPM), or non-motile spermatozoa (NMS). In this study, the CPF group had significantly lower SPM and higher SNPM and NMS than the treated groups, thus agreeing with previous work (El-Kashoury and Tag El-Din 2010; Kenfack et al. 2007). Negative sperm motility impairs fertilization. An optimum ATP pool is needed for spermatozoa movement, therefore even a little shortage lowers motility and may cause infertility. The decreased glycogen concentration in the CPF group may explain the considerable inhibition of sperm motility in this study. Because glycogen stores glucose, it produces ATP, which is needed for sperm motility (Gawish 2014). Thus, greater glycogen levels in DAF and DAF + CPF groups may explain their increased sperm motility.
Pesticide-induced testicular toxicity causes teratospermia (Baykalir et al. 2016; Sai et al. 2014). Other researchers (El-bendary et al. 2014; Kenfack et al. 2015) found similar abnormalities in the CPF group, including a large increase in head and tail sperm and less normal spermatozoa. Along with the parent substance, OP oxon metabolites like CPF were discovered to cause organophosphorus sperm genotoxicity (Salazar-Arrendo et al. 2008). In groups exposed to CPF, Faraga et al. (2010) found a decrease in morphologically normal spermatozoa and sperm count. This study's CPF group showed pesticide-induced free radical formation in spermatozoa, which may cause teratospermia (Baykalir et al. 2016). Due to their high membrane polyunsaturated fatty acid content, spermatozoa may be predisposed to problems due to increased testicular oxidant indicators (Olatunji et al. 2022). Sperm lipid matrix peroxidation causes rapid ATP loss, axonemal damage, reduced sperm viability, and increased morphological abnormalities, and in extreme cases, spermatogenesis is completely prevented (Sanocka and Kurpisz 2004). These morphological abnormalities may explain the CPF group's lower live spermatozoa rate.
Golec et al. (2003) found that agricultural workers exposed to OPs have a higher incidence of sperm morphological abnormalities. Observations in post-CPF exposure showed spermatozoa morphological abnormalities. These aberrations distinguish fertile and infertile males (Abdelaziz et al. 2010; Golec et al. 2003). According to Dutta and Sahu (2013) and El-bendary et al. (2014), CPF can modify testicular DNA and sperm chromatin structure, creating morphological defects in mitotic and meiotic chromosomes. Pesticide-induced sperm toxicity may cause OP mutagenicity and spermatotoxicity (Topham 1980). Oxidative stress and a decrease in local antioxidant defense might damage sperm DNA, notably during epididymal development, causing morphological defects (Dutta and Sahu 2013). Dysmature cells, defined by excess residual cytoplasm, persistent nuclear histones, inadequate zona binding, and altered chaperone content, were found in CPF-exposed sperm cells by Abdelaziz et al. (2010). The vaso-protective components (hesperidin and diosmin) of Daflon reduce cell membrane hyperpermeability, protecting blood vessels from supplying the testes and reducing toxicant passage through the blood-testes barrier, minimizing sperm abnormalities. As an antioxidant, Daflon may have protected the shield surrounding the sperm cell plasma membrane and lipid layer structure, enhancing the sperm's ability to tolerate ROS attack. This may explain the CPF + DAF group's significant increase in normal spermatozoa.
This study similarly found a substantial drop in sperm live ratio and an increase in sperm dead ratio in the CPF group, supporting Golec et al. (2003) and Dutta and Sahu (2013). Sperm viability is a key indicator of fertilizing potential (Joshi et al. 2007; Dutta and Sahu 2013). Therefore, any detrimental effect on motility and morphology will severely reduce sperm viability (Ben et al. 2012; El-bendary et al. 2014). Pesticide-induced lipid peroxidation destroys the sperm membrane's lipid matrix, causing fast ATP loss, axonemal damage, and reduced sperm viability (Baykalir et al. 2016). Direct spermicidal effects of CPF may reduce sperm viability (Dutta and Sahu 2013; El-bendary et al. 2014). Micronization of daflon (Simsek et al. 2007; Osman et al. 2013) made it more likely to act intracellularly and at the membrane level, protecting the cell from ROS and toxic metabolites, and this may have allowed the antioxidant to cross the blood-testes barrier, hence protecting the testes from CPF-induced damage as reported in the histology of our earlier published work (Olatunji et al. 2022).
Conclusion
Inference from this research demonstrated that Daflon treatment improved the level of reproductive hormones, glycogen content, sperm count, motility, morphology, and viability in CPF-exposed rats. Therefore, pretreatment with Daflon or ingestion of food rich in diosmin and hesperidin may be beneficial to mitigate CPF-induced reproductive deficiency in farmers and household users.
Limitations
Our investigation was limited by the lack of more sophisticated technology to measure sperm parameters coupled with the high cost of hormonal assay kits which limited the number of hormones that could have been assayed. Further studies should be performed on the molecular interplay involved in the combination therapy between chlorpyriphos and the flavonoid compounds, diosmin and hesperidin.
Data availability
No datasets were generated or analysed during the current study
References
Abdelaziz KB, El Makawy AI, Abd Elsalam AE, Darwish AM (2010) Genotoxicity of chlorpyrifos and the antimutagenic role of lettuce leaves in male mice. Comun Sci 1(2):137–145
Al-Amoudi WM (2015) Effect of propolis on the reproductive toxicity of deltamethrin in male albino rats. J Anat Physiol 5(5):1–7
Ambali SF, Ayo JO (2011) Sensorimotor performance deficits induced by chronic chlorpyrifos exposure in Wistar rats: mitigative effect of vitamin C. Toxicol Environ Chem 93(6):1212–1226
Ambali SF, Abubakar AT, Shittu M, Yaquub LS, Kobo PI, Giwa A (2010) Ameliorative effect of zinc on chlorpyrifos-induced erythrocyte fragility in Wistar rats. N Y Sci J 3:117–122
Atessahin A, Karahan I, Turk G, Gur S, Yilmaz S, Ceribasi AO (2006) Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod Toxicol 21(1):42–47
Babu SR, Sadhnani MD, Swarna M, Padmavathi P, Reddy PP (2004) Evaluation of FSH, LH and testosterone levels in different subgroups of infertile males. Indian J Clin Biochem 19(1):45–49
Baykalir BG, Seven PT, Gur S, Seven I (2016) The effects of propolis on sperm quality, reproductive organs and testicular antioxidant status of male rats treated with cyclosporine-A. Anim Reprod 13(2):105–111
Beam J, Botta A, Barendregt R, Ghosh S (2014) Dietary fatty acids, redox signaling, and the heart. Syst Biol Free Radic Antioxidants 111:1497–1522
Bedwal RS, Edwards MS, Katoch M, Bahuguna A, Dewan R (1994) Histological and biochemical changes in testis of zinc deficient BALB/c strain of mice. Indian J Exp Biol 32:243–247
Ben SA, Ben AF, Keskes-Ammar L, Mallek Z, El Feki A (2012) Embryonic exposure to dimethoate and/or deltamethrin impairs sexual development and programs reproductive success in adult male offspring mice. Andrologia 44(1):661–666
Bhushan B, Saxena PN, Saxena N (2013) Biochemical and histological changes in rat liver caused by cypermethrin and beta-cyfluthrin. Arch Indust Hygiene Toxicol 64(1):57–67
Canadian Council on Animal Care (CCAC) (1993) Guide to the Care and Use of Experimental Animals, vol 1: 2nd edn®
Chevrier C, Warembourg C, Gaudreau E, Monfort C, Le Blanc A, Guldner L, Cordier S (2013) Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology 24(2):251–260
Dutta AL, Sahu CR (2013) Emblica officinalis Garten fruits extract ameliorates reproductive injury and oxidative testicular toxicity induced by chlorpyrifos in male rats. J Pharm Biol Sci 2(1):541–550
El-bendary HM, Saleh AA, Negm SA, Khadey ME, Eldeen FA (2014) Spermatogenic alterations induced by organophosporus compounds profenofos, chlorpyrifos and synthetic pyrethroid lambada-cyhalothrin in mice. Annu Res Rev Biol 4(6):856–873
El-Kashoury AA, Tag El-Din HA (2010) Chlorpyrifos (from different sources): Effects on testicular biochemistry of male Albino rats. J Am Sci 6(7):252–261
Faraga AT, Radwana AH, Sorourb F, El-Okazyc A, El-Agamyd E, El-Sebaea A (2010) Chlorpyrifos induced reproductive toxicity in male mice. Reprod Toxicol 29(1):80–85
Gawish AM (2014) The protective role of alpha lipoic acid against pesticide-induced testicular toxicity. Histopathol Histochem Stud Rep Opin 6(9):84–94
Golec J, Hanke W, Dabrowski S (2003) Fertility and occupational exposure to pesticides. Med Pr 54(5):465–472
Good CA, Krames H, Somogyi M (1993) Chemical procedures for analysis of polysaccharides. Methods Enzymol 7:34–40
Heikal TM, Mossa ATH, Abdel Rasoul MA, Marei GIK (2013) The ameliorating effects of green tea extract against cyromazine and chlorpyrifos induced liver toxicity in male rats. Asian J Pharm Clin Res 6:48–55
Heikal TM, Abdel-Tawab H, Mossa AW, Abdel-Hamid HF (2014) Oxidative damage and reproductive toxicity associated with cyromazine and chlorpyrifos in male rats: the protective effects of green tea extract. Res J Environ Toxicol 8:53–67
Joshi CS, Mathur R, Gulati N (2007) Testicular toxicity of chlorpyrifos (an organophosphate pesticide) in albino rats. Toxicol Ind Health 23(7):439–444
Kenfack A, Ngoula N, Tchoumboué J, Kamtchouing P (2007) Influence of chlorpiryphos-ethyl in some male reproductive parameters in albinos rats exposed post-natally. Int J Biol Chem 1:237–243
Kenfack A, Ferdinand N, Paul DW, Omer BN, Tsambou MA, Judith KC, Guylene MZ, Zeukeng IL, Arthenice JN, Tah PN (2015) Persistence of the reproductive toxicity of chlorpiryphos-ethyl in male wistar rat. Asian Pac J Reprod 4(1):37–40
Leong CT, D’Souza UJ, Iqbal M, Mustapha ZA (2013) Lipid peroxidation and decline in antioxidant status as one of the toxicity measures of diazinon in the testis. Redox Rep 18:155–164
Louis GMB, Barr DB, Kannan K, Chen Z, Kim S, Sundaram R (2016) Paternal exposures to environmental chemicals and time-to-pregnancy: overview of results from the LIFE study. Andrology 4(4):639–647
Mathur N, Pandey G, Jain GC (2009) Pesticides: A review of the male reproductive toxicity. J Herb Med Toxicol 4(1):1–8
Narayana K, Prashanthi N, Nayanatara A, Kumar SG, Kumar HHC, Bairy KL, D’Souza UJA (2006) A broad-spectrum organophosphate pesticide O’O-dimethyl O-4-nitrophenyl phosphorothioate (methyl parayhion) adversely affects the structure and function of male accessory reproductive organs in the rat. Environ Toxicol Pharmacol 22:315–324
O’Donnel L, Mc Lachlan RI, Wreford NG, Robertson DM (1994) Testosterone promotes the conversion of round spermatids between stages vii and viii of the rat spermatogenic cycle. Endocrinology 135(4):2608–2614
Oates L, Cohen M, Braun L, Schembri A, Taskova R (2014) Reduction in urinary organophosphate pesticides metabolites in adults after a week-long organic diet. Environ Res 132:105–111
Olatunji AO, Ayo JO, Suleiman MM, Ambali SF, Shittu M, Akorede GJ, Raji LO, Atata JA, Biobaku KT, Azeez MO (2022) Effect of daflon-500®, a flavonoid compound on chlorpyriphos-induced oxidative changes in the hypophysis and testes in adult male rats. Toxicol Res 38(3):345–353. https://doi.org/10.1007/s43188-021-00120-2
Olorunshola KV, Achie LN, Akpomiemie ML (2011) Ascorbic acid ameliorates toxic effects of chlopyrifos on testicular functions of albino rats. Br J Pharmacol Toxicol 2(5):262–269
Osman MW, Nikolopoulos I, Jayaprakasan K, Raine-Fenning N (2013) Pelvic congestion syndrome. Obstet Gynaecol 15:151–157
Pallav S, Rajdeb B (2014) Environmental toxins; Alarming impacts of pesticides on male fertility. Hum Exp Toxicol 33(10):1017–1039
Pizzorno J (2018) Environmental toxins and infertility. Integr Med (Encinitas) 17(2):8–11
Rajawat NK, Soni I, Mathur P, Gupta D (2014) Cyfluthrin-induced toxicity on testes of Swiss albino mice. Int J Curr Microbiol Appl Sci 3(3):334–343
Rizk SM, Sabri NA (2009) Evaluation of clinical activity and safety of Daflon 500mg in type 2 diabetic female patients. Saudi Pharm J 17:199–207
Sai L, Li X, Liu Y, Guo Q, Xie L, Yu G, Bo C, Zhang Z, Li L (2014) Effects of chlorpyrifos on reproductive toxicology of male rats. Environ Toxicol 29(9):1083–1088
Salazar-Arrendo E, Solis-Heredia M, Rojas-Garcia E, Hernandez-Ochoa I, Quintanilla-Vega B (2008) Sperm chromatin alteration and DNA damage by methyl-parathion, chlorpyrifos and diazinon and their own oxon metabolites in human spermatozoa. Reprod Toxicol 25(4):455–460
Sanocka D, Kurpisz M (2004) Reactive oxygen species and sperm cells. Reprod Biol Endocrinol 2:1–7
Shittu M, Ambali SF, Ayo JO, Fatihu MY, Sulaiman MM, Surakat LS (2013) Evaluation of chronic chlorpyrifos-induced reproductive toxicity in male Wistar rat: protective effects of vitamin C. J Exp Integr Med 3(1):23–30
Shittu M, Olatunji OA, Ambali SF, Oyedepo IT, Kawu MU, Peter OY, Kobo PI, Hamza II (2014) Ameliorative effect of Hibiscus sabdariffa Linn on subchronic chlorpyrifos-induced alteration in sex and thyroid hormones in male Wistar rats. Am J Pharmacol Toxicol 9(1):96–106
Siegel MS (1993) The male infertility investigation and the role of the andrology laboratory. J Reprod Med 38(5):317–334
Simsek M, Burak F, Taskin O (2007) Effects of micronized purified flavonoid fraction (Daflon) on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trial. Clin Exp Obstet Gynecol 34:96–98
Sonmez M, Turk G, Yuce A (2005) The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 63:2063–2072
Surai PF, Fisinin VI (2014) Antioxidant systems of the body: From vitamin E to polyphenols and beyond. In Proceedings of the 35th Western Nutrition Conference, Edmonton, Canada 24th–25th September 2014 Pp 265–277
Topham JC (1980) Do induced sperm head abnormalities in mice specifically identify mammalian mutagens rather than carcinogens? Mutat Res 74:379–387
Umosen AJ, Uchendu C (2014) Effect of melatonin on chlorpyrifos-induced alterations in reproductive hormones and semen characteristics in Wistar rats. Am J Phtyomedicine Clin Ther 2(6):742–753
World Health Organization (WHO) (2010) WHO laboratory manual for the examination and processing of human semen. 5th edn®. https://iris.who.int/handle/10665/44261
Yokoi K, Uthus EO, Nielsen FH (2003) Nickel deficiency diminishes sperm quantity and movement in rats. Biol Trace Elem Res 93:141–153
Zhu J (2015) Advances in pesticide use in the cocoa belts and perceptions of vegetable farmers. J Hortic 2:149–152
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Aishat Omobolanle Olatunji carried out the research work under the supervision of Joseph Olusegun Ayo, Mohammed Musa Suleiman, and Suleiman Folorunsho Ambali. Muftau Shittu and Ganiu Jimoh Akorede assisted in carrying out the lab work and sacrificing the animals. Abdulfatai Aremu and Ibrahim Yusuf Lamidi participated in the analysis of data collected. Basiru Afisu, and Olubukola Tolulope Adenubi assisted in plotting of graphs, tables and fixing of legends in the manuscript. All co-authors participated in reviewing the manuscript before submission. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC/2023/144) which complies with the Canadian Council on Animal Care Guide (CCAC 1993).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olatunji, A.O., Ayo, J.O., Suleiman, M.M. et al. Ameliorative potentials of diosmin and hesperidin fractions on chlorpyriphos-induced changes in reproductive hormones, sperm characteristics, and testicular glycogen in male Wistar rats. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03241-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03241-1