Abstract
Viral hepatitis-induced oxidative stress accompanied by increased levels of transforming growth factor-β (TGF-β) and hepatic fibrosis are hallmarks of hepatitis C virus infection. The present study was designed to investigate the potential protective effect of propolis against liver injury induced by concanavalin A (Con A), a T-cell-dependent model that causes an immune-mediated hepatitis in a similar pattern to the one induced by viral infections. In the present study, rats were randomly divided into four groups. The first group (control) was administered the vehicle of Con A (i.v.) for 24 h. The second group received Con A (12 mg/kg body weight i.v.) for 24 h. The third group received propolis (300 mg/kg by oral gavage) 5 days before and concurrently with Con A for 24 h. The last group received propolis alone. Following a single injection of Con A, histopathological changes as well as significant reduction in albumin level were observed. In addition, serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin were significantly increased. These increases correlated with an increase in lipid peroxidation and downregulation of reduced glutathione (GSH) as well as superoxide dismutase (SOD) and catalase activities in liver tissue. Furthermore, these changes were associated with an increase in serum levels of the inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) as well as the profibrotic cytokine TGF-β. Moreover, TGF- β activation was accompanied with an increase in Smad phosphorylation. Interestingly, concomitant administration of propolis along with Con A significantly attenuated all these negative effects and improved liver function indicating that propolis has the ability to protect rats from Con A-induced hepatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation of the liver is a common finding during a variety of liver diseases. The inflammatory phenotype can be attributed to the innate immune response. The adaptive immune system is also influenced by the innate immune response leading to liver injury. In hepatitis, interleukins (IL-1, IL-6), interferon-γ (IFN-γ), and tumor necrosis factor α (TNF-α) have been shown to mediate liver injury in animal models (Cao et al. 1998). Viral infection has been shown to affect the cell redox equilibrium by inducing reactive oxygen species (ROS) and by interfering with the synthesis of antioxidant enzyme systems (Ruggieri et al. 2013). Moreover, ROS production in the hepatocytes may lead to Kupffer cell activation and subsequent production of proinflammatory or profibrotic cytokines such as transforming growth factor-β (TGF- β) (Poli 2000). Chronic liver injury induced by hepatitis B and C is usually the cause of liver fibrosis. It has been reported that oxidative stress induced by viral hepatitis and subsequent TGF-β release play an important role in liver fibrosis (Paik et al. 2014). Concanavalin A (Con A)-induced hepatitis is an experimental model of human autoimmune hepatitis. It causes an immune-mediated hepatitis in a similar pattern to the one induced by viral infections (Tiegs et al. 1992). It is characterized by massive hepatic injury and lymphocyte activation and accumulation in the liver leading to secretion of proinflammatory cytokines including TNF-α, IFN-γ, IL-1, IL-2, and IL-6 (Tiegs et al. 1992; Gantner et al. 1995; Mizuhara et al. 1996; Nicoletti et al. 2000). It has been reported that TNF-α and IFN-γ released from activated T cells are considered to play critical roles in the development of Con A-induced hepatic injury. The Con A model is a proper animal model to investigate most forms of acute liver injury in humans such as autoimmune hepatitis, acute liver failure, and acute viral hepatitis in which T cell infiltration is observed (Ballegeer and Libert 2016). In addition, it has been shown that ROS play an important role in immune-mediated Con A-induced hepatitis. Malondialdehyde (MDA) level in the liver increases only 12 h after Con A injection, so scavenging of reactive oxygen species by antioxidants prevents hepatitis and may be a new therapeutic target in this experimental model (Shirin et al. 2010). Recently, identification and isolation of new antioxidants from natural sources has become an active area of research. One of the most important natural antioxidants is propolis. It is a resinous natural product collected by honeybees from various plant sources. It has a wide range of biological activities including anti-inflammatory and antioxidant properties (Kolankaya et al. 2002; Tan-No et al. 2006). Propolis generally composed of 50% resins and balsams, 30% wax, 10% essential and aromatic oils, 5% pollen, and 5% other substances (Pujirahayu et al., 2014). The main chemical groups present in propolis resin comprise phenolic acids or their esters, flavonoids (flavones, flavaonones, flavonols, dihydroflavonols and chalcones), terpenes, fatty acids, stilbenes, and β-steroids (Mannaa et al., 2011). Phenolic compounds in their many forms are the main components responsible for the functional properties associated with many foods, such as antioxidant capacity (Viuda-Martos et al., 2008). Propolis has been shown to increase the antioxidant capacity in animals and humans (Jasprica et al., 2007; Zhao et al. 2009). Flavonoids and various phenolics in propolis have been reported to be free radical scavengers (Seven et al., 2009). Also, it has been reported that the antioxidant activity of propolis is due to the free radical scavenging property of its constituents, caffeic acid, galangin, chrysin (5,7-dihydroxyflavone), and quercetin (Capucho et al., 2012). In addition, it has been shown that daily intake of propolis inhibits lipid peroxidation and increases SOD activity in healthy humans (Jasprica et al., 2007). Furthermore, propolis has been shown to inhibit macrophage apoptosis via effect on glutathione (GSH) (Claus et al., 2000). Moreover, it has been reported that propolis has the ability to inhibit NADPH-oxidase and subsequent ROS production (Silva et al. 2011). Therefore, it was interesting to investigate the possible protective effect of propolis against liver injury induced by Con A, a T-cell-dependent animal model of liver injury.
Materials and methods
Animals
Male Wistar albino rats weighing 150–200 g were housed in a 12 h dark/light cycle animal facility with controlled humidity and constant temperature. The animals were fed a standard diet pellets. Water was supplied ad libitum. The animals were kept under observation for 1 week before the treatments for adaptation. The experimental protocol used in this study was approved by the Institutional Animal Ethics Committee.
Drugs and chemicals
Concanavalin A (Con A) were purchased from Sigma-Aldrich Chemicals Co. (St. Louis, MO, USA). Propolis was purchased from Sigma Pharma Ind., Egypt. Reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and thiobarbituric acid reactive substance (TBARS) assay kits were purchased from Bio-diagnostic, Egypt. Alanine amino transferase (ALT), aspartate amino transferase (AST), albumin, and bilirubin were purchased from Spectrum Diagnostics, Egypt. Rat interleukin-6 (IL-6) enzyme-linked immunosorbent assay (ELISA) kit, rat tumor necrosis factor-α (TNF-α) ELISA kit and rat transforming growth factor-β1 (TGF-β1) ELISA kit were purchased from Immuno-Biological Laboratories, Minnesota, USA, Ray Biotech, Inc., Norcross, Georgia, USA, and Kamiya biomedical Co, WA, USA, respectively. An antibody raised against phospho-Smad-2/3 and anti-rabbit HRPlinked IgGs were obtained from Santa Cruz Biotechnology, USA.
Experimental design
Twenty-four male Wistar albino rats were used in this study. The animals were randomly divided into four groups, 6 animals in each. The first group (control) received a single injection of phosphate-buffered saline via the tail vein. The second group received a single injection of Con A via the tail vein (12 mg/kg i.v.) (RaviKumar et al. 2011). The third group was administered propolis (300 mg/kg/day by oral gavage) (Al-Hariri et al. 2011) 5 days before and concurrently during Con A administration. The fourth group received propolis alone (as previously described in the third group). Twenty-four hours after treatment, blood samples were collected for the determination of serum levels of ALT, AST, albumin, and bilirubin as well as IL-6, TNF-α, and TGF-β. After terminal bleeding, animals were sacrificed and liver tissues were dissected immediately after death, washed with ice-cold phosphate-buffered saline (PBS), and kept at −20 °C till used. In addition, specimens from the three major lobes were fixed in 10% neutral-buffered formal saline for histopathological investigation as well as immunohistochemical analysis.
Assessment of hepatotoxicity markers:
Assessment of serum ALT and AST levels
The serum levels of ALT and AST were determined by commercial kits according to the manufacturer’s instructions (Spectrum Diagnostics, Egypt). The colorimetric determination of ALT activity depending on a chemical reaction, where the amino group is enzymatically transferred by ALT present in the sample from alanine to the carbon atom of 2-oxoglutarate yielding pyruvate and L-glutamate. ALT activity is measured by monitoring the concentration of pyruvate hydrazone formed with 2,4-dinitrophenylhydrazine. Also, the colorimetric determination of AST activity depending on a chemical reaction, where the amino group is enzymatically transferred by AST present in the sample from Laspartate to the carbon atom of 2-oxoglutarate yielding oxaloacetate and L-glutamate. AST activity is measured by monitoring the concentration of oxaloacetate hydrazone formed with 2,4-dinitrophenylhydrazine.
Assessment of albumin and total bilirubin levels
Albumin and total bilirubin levels in serum were determined by commercial kits according to the manufacturer’s instructions (Spectrum Diagnostics, Egypt). Measurement of albumin is based on its binding to the indicator dye bromocresol green (BCG) in pH 4.3 to form a blue-green colored complex. The intensity of the blue-green color is directly proportional to the concentration of albumin in the sample. The estimation of total bilirubin is based on the reaction of the pigment with diazotized sulfanilic acid to yield colored azobilirubin compounds. Azobilirubin compounds can then be measured spectrophotometrically. The intensity of the produced color is directly proportional to the bilirubin concentration in the sample.
Assessment of inflammatory cytokines:
Assessment of IL-6
The level of IL-6 in serum was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Immuno-Biological Laboratories, Minnesota, USA). The kit is based on standard sandwich enzyme-linked immune-sorbent assay technology. Rat IL-6-specific polyclonal antibodies were pre-coated onto 96-well plates. The rat-specific detection polyclonal antibodies were biotinylated. The test samples and biotinylated detection antibodies were added to the wells subsequently and then followed by washing with PBS. Next, Avidin conjugated to horseradish peroxidase (HRP) is added to each microplate well and incubated. After 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution is added, only those wells that contain IL-6, biotin-conjugated antibody, and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of acidic stop solution, and the color change is measured spectrophotometrically.
Assessment of TNF-α
The serum level of TNF-α was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Ray Biotech, Inc., Norcross, Georgia, USA). This assay employs an antibody specific for rat TNF-α coated on a 96-well plate. Standards and samples are pipetted into the wells, and TNF-α present in a sample is bound to the wells by the immobilized antibody. The wells are washed and biotinylated anti-rat TNF-α antibody is added. After washing away unbound biotinylated antibody, HRP-conjugated streptavidin is pipetted to the wells. The wells are again washed, a TMB substrate solution is added to the wells, and color develops in proportion to the amount of TNF-α bound. The stop solution changes the color from blue to yellow, and the intensity of the color is measured at 450 nm.
Assessment of profibrotic markers:
Assessment of TGF-β
The level of TGF-β in serum was determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (Kamiya biomedical Co, WA, USA). The microtiter plate provided in this kit has been pre-coated with an antibody specific to TGF-β1. Calibrators and samples are then added to the appropriate microtiter plate wells with a biotin-conjugated polyclonal antibody preparation specific for TGF-β1. Next, Avidin conjugated to HRP was added to each microplate well and incubated. Then, the TMB substrate solution was added to each well. Only those wells that contain TGF-β1, biotin-conjugated antibody, and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of a sulfuric acid solution, and the color change was measured spectrophotometrically at a wavelength of 450 nm.
Immunohistochemical detection of p-Smad2/3
Immunohistochemical detection of p-Smad2/3 was performed as described previously (Afify et al. 2016). Paraffin-embedded tissue sections of 4 μm thickness were deparaffinized in xylene and rehydrated in graded ethanol solutions. The slides were then incubated with 5% bovine serum albumin in Tris-buffered saline (TBS) for 2 h for blocking of nonspecific immunoreactions. The sections were then incubated with the primary antibody p-Smad-2/3 (Santa Cruz Biotechnology, USA, Cat No. sc-11769-R) in a dilution of 1:125 at 4 °C overnight for immunostaining. After washing the slides with TBS, they were incubated with the secondary antibody (goat anti-rabbit) for 1 h at room temperature. The slides were then washed and incubated with diaminobenzidine (DAB) for 5 min at room temperature. The counterstaining was performed using hematoxylin. The slides were visualized under a light microscope for positive immunoreactions. Negative control slides were included.
Determination of oxidative stress markers
Determination of lipid peroxides
Malondialdehyde (MDA) level in liver tissues was measured using a TBARS assay kit according to the manufacturer’s instructions (Bio-diagnostic, Egypt). Briefly, the colorimetric determination of TBARS calculated as MDA is based on the reaction of one molecule of MDA with two molecules of TBARS at low pH and boiling water temperature for 30 min. The resultant pink color was extracted with n-butanol, and the absorbance was determined at 534 nm spectrophotometrically. The MDA content in unknown samples was determined by comparison with the predetermined MDA standard curve.
Determination of endogenous antioxidants
Determination of GSH content
Hepatic content of GSH was determined using Ellman’s reagent (Ellman 1959). The method depends on the reduction of Ellman’s reagent by the SH group in GSH to form an intense yellow product which can be measured spectrophotometrically at 405 nm.
Determination of SOD activity
Superoxide dismutase activity in liver tissues was determined by assay kit according to the manufacturer’s instructions (Bio-diagnostic, Egypt). The method depends on the ability of SOD enzyme to inhibit the phenazine methosulfate-mediated reduction of nitroblue tetrazolium dye.
Determination of CAT activity
Catalase activity in liver tissues was determined by assay kit according to the manufacturer’s instructions (Bio-diagnostic, Egypt). Briefly, catalase reacts with a known quantity of hydrogen peroxide. After 1 min, the reaction was stopped using CAT inhibitor. The remaining hydrogen peroxide reacts with 3,5-dichloro-2-hydroxybenzene sulfonic acid and 4-aminoantipyrine in the presence of peroxidases to form a chromophore with a color intensity inversely proportional to the amount of CAT in the sample.
Histopathological examination
Histopathological examination was performed as described previously (Akool 2015).
Statistical analysis
Data are presented as means ± standard error (SEM). One-way ANOVA followed by Tukey-Kramer as a post-hoc test was performed to analyze statistical significance among groups. P values below 0.05 were considered as indication for statistically significant differences between conditions compared.
Results
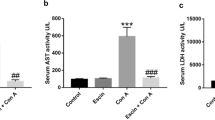
Propolis reduced ALT and AST levels induced by Con A
As shown in Fig. 1a, b, treatment of rats with Con A resulted in hepatic damage as indicated by significant increase in ALT and AST levels by 860 and 850%, respectively, as compared to control. However, concomitant administration of propolis along with Con A improved the liver damage as indicated by significant reduction in ALT and AST levels by 53 and 50%, respectively, compared with Con A alone treated animals. No significant changes were observed in animals treated with propolis alone.
Propolis enhances albumin production in Con A-treated animals
Albumin production was highly reduced in animals treated with Con A alone by 45% compared with control indicating hepatic function deterioration. However, concomitant administration of propolis along with Con A significantly improved liver function as indicated by an increase in albumin level by 26% compared with animals treated with Con A alone (Fig. 1c). The albumin level in animals treated with propolis alone was not changed.
Propolis decreased bilirubin level induced by Con A
As demonstrated in Fig. 1d, administration of Con A significantly increased bilirubin level by 381% as compared to control indicating liver function deterioration. On the other hand, concomitant administration of propolis along with Con A significantly reduced bilirubin level by 39% as compared to Con A alone treated animals indicating hepatic function improvement. No significant changes were observed in animals treated with propolis alone.
Propolis inhibits lipid peroxidation induced by Con A
Administration of Con A significantly induced lipid peroxidation as indicated by an increase in MDA content (by-product of lipid peroxidation) by 558% (Fig. 2a), indicating that Con A induced oxidative damage in the liver. However, lipid peroxidation is significantly attenuated in animals treated with Con A in combination with propolis by 40% (Fig. 2a). The basic level of MDA in animals treated with propolis alone was not changed.
Propolis increases GSH content in animals treated with Con A
One of the most important cellular antioxidant defense systems against free radical overproduction is GSH. The decrease in cellular concentration of GSH impairs cellular defense against oxidative stress. As shown in Fig. 2b, administration of Con A significantly reduced GSH content in liver tissues by 91%. However, GSH level in rats treated with Con A in combination with propolis was significantly increased compared with Con A alone treated animals by 208%. No significant changes were found in animals treated with propolis alone.
Propolis enhances SOD and CAT activities in Con A-treated rats
Also, SOD and CAT play an important role in cell protection by scavenging ROS from the cell. As shown in Fig. 2, treatment of animals with Con A significantly reduced SOD and CAT activities by 91 and 86%, respectively. On the other hand, SOD and CAT activities were highly increased in animals treated with Con A in combination with propolis by 242 and 336%, respectively, compared with Con A alone treated animals (Fig. 2c, d). SOD and CAT activities were not changed in propolis alone treated rats.
Propolis reduced the levels of the inflammatory cytokines IL-6 and TNF-α
Administration of Con A significantly increased serum levels of the inflammatory cytokines IL-6 and TNF-α by 829 and 1050%, respectively (Fig. 3a, b). However, concomitant administration of propolis along with Con A significantly reduced IL-6 and TNF-α levels by 36 and 34%, respectively, compared with animals treated with Con A alone (Fig. 3a, b). The levels of IL-6 and TNF-α were not changed in rats treated with propolis alone.
Propolis inhibits TGF-β activation and subsequent Smad phosphorylation induced by Con A
Activation of TGF-β and subsequent Smad phosphorylation play an important role in tissue fibrosis. As shown in Fig. 4, Con A administration significantly induced TGF-β activation as indicated by an increase in serum level of active TGF-β by 733%. On the other hand, concomitant administration of propolis along with Con-A significantly reduced TGF-β activation by 33% as compared to Con A alone treated rats (Fig. 4a). Furthermore, Smad phosphorylation was observed in liver sections obtained from rats treated with Con A as indicted by a marked immunostaining of phosph-Smad 2/3. However, Smad-2/3 phosphorylation induced by Con-A was highly reduced in rats pretreated with propolis (Fig. 4b).
Con A-triggered TGF-β activation and Smad-2/3 phosphorylation is abrogated in the presence of propolis. a Plasma levels of activated TGF-β in rats treated with either vehicle or propolis or Con A alone or in combination with propolis. Data represent means ± S.D. (n = 6), ***p < 0.001 versus control, ###p < 0.001 versus Con A alone-treated animals. b Immunohistochemical staining of p-Smad-2/3 in liver tissues from rats treated with either vehicle or propolis or Con A alone or in combination with propolis. a Control group shows negative immunostaining (×600). b Con A group shows phosphorylated Smad2/3 (p-Smad2/3) as indicated by extensive immunostaining in the smooth muscle fiber and stellate cells (×600). c Con A + propolis group: p-Smad2/3 immunostaining induced by Con A was highly attenuated in the presence of propolis (×600). d Propolis group shows negative immunostaining (×600)
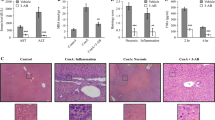
Propolis attenuates histopathological changes induced by Con A
Hepatic damage was further assessed by histological analysis of liver sections. As demonstrated in Fig. 5, histopathological investigation showed severe dilatation and congestion in the central veins associated with inflammatory cell infiltration in the adjacent hepatic parenchyma as well as degeneration in the hepatocytes surrounding the dilated and congested central vein in animals treated with Con A alone (Fig. 5b). On the other hand, liver specimens from rats pretreated with propolis showed only dilatation in central vein with mild degeneration in hepatocytes (Fig. 5c). Liver sections of either normal rats or rats treated with propolis alone revealed normal histological structure of hepatic lobules (Fig. 5a, d).
Photomicrographs of liver sections stained by hematoxylin and eosin (×200). a Section taken from control group showing normal histological structure of the central vein (CV) and surrounding hepatocytes (h). b Section taken from Con A-intoxicated rat showing severe dilatation of CV with inflammatory cells (m) in the surrounding adjacent hepatic parenchyma and degeneration in the hepatocytes surrounding the congested CV. c Section taken from a rat treated with propolis + Con A showing only dilatation in CV with mild degeneration in hepatocytes. d Sections taken from propolis group showing normal histological structure of the CV and surrounding hepatocytes (h)
Discussion
Acute liver injury is a significant cause of patient morbidity and mortality. The most frequent causes include viral hepatitis, alcohol metabolites, drugs, and toxins. Hepatitis C virus (HCV) infection is one of the main causes of chronic liver disease. Despite the full understanding of the mechanisms by which HCV causes cellular damage, several reports confirm that ROS production increases while antioxidant capacity decreases in all types of liver damage. Furthermore, it has been reported that the high amount of HCV-structural or nonstructural proteins induce oxidative stress and disrupt the antioxidant equilibrium in hepatocytes. The constant production of ROS is a challenge that the cell has to overcome to survive (Lozano-Sepulveda et al. 2015). The Con A model is a proper animal model to investigate most forms of acute liver injury in humans. It has been reported that ROS play an important role in Con A-induced acute liver injury (Shirin et al. 2010). In the present study, we demonstrated that propolis has the ability to protect rats from Con A-induced acute liver injury. In the present work, Con A treatment was found to be associated with structural damage and hepatic dysfunction as indicated by significant increase in serum levels of ALT, AST, and bilirubin as well as significant reduction in albumin level and clear histopathological alterations. These data are in agreement with previous study (RaviKumar et al. 2011). Interestingly, the present study demonstrates that propolis has the ability to antagonize the hepatotoxic effect of Con A as indicated by significant reduction in the serum levels of ALT and AST as well as total bilirubin level. In addition, albumin level was significantly increased. These results are in accordance with that of Su et al. (2014). In addition, this is in harmony with the findings of Lin et al. (1997) who recorded the hepatoprotective effect of propolis on chronic alcohol-induced liver injuries. Furthermore, histopathological examination of liver sections revealed that propolis has the ability to improve histopathological changes induced by Con A. More evidence links ROS as an important factor in Con A-induced acute liver injury (Shirin et al. 2010), where SOD, GSH, and CAT protect against the deleterious effect of ROS (De Andrade et al. 2015; Genestra 2007). The present work shows that Con A significantly reduced the liver content of GSH as well as SOD and CAT activities. Moreover, MDA content was significantly increased. These results agree with a previous study performed in mice (Liu et al. 2014). Interestingly, it was found that propolis has the ability to antagonize oxidative stress induced by Con A as indicated by an increase in GSH content as well as SOD and CAT activities. In addition, MDA content was highly reduced in presence of propolis. These results are in line with El-Khatib et al. (2002) and Al-Amoudi. (2015) who stated that propolis has the ability to reduce the level of MDA and increase the activity level of antioxidant enzymes. This reduction in the by-product of lipid peroxidation (MDA) indicates that propolis attenuated liver damage that may be attributed to its main components, such as flavonoids and caffeic acid phenethyl ester (CAPE). Flavonoids in the cell membrane protect the unsaturated fatty acids against oxidants (Havsteen, 2002). Also, it was found that CAPE decreased MDA levels by blocking ROS production (Hoşnuter et al., 2004). Previously, it has been reported that the imbalance between ROS production and elimination plays an important role in cell damage (Li et al. 2015). In addition, it has been shown that pretreatment of mice with free-radical scavengers prevented Con A-induced hepatitis, indicating a crucial role for ROS in the development of immune-mediated experimental liver damage (Shirin et al. 2010). Concanavalin A has the ability to activate T-cells and secrete a variety of hepatotoxic cytokines (TNF-α and IL-6) (Genestra 2007). These cytokines stimulate and activate liver cells which contribute to the development of hepatocyte damage (Liu et al. 2012; Bozza et al. 1999). TNF-α is a central mediator in inflammation-induced hepatocytes apoptosis (Gantner et al. 1995). Also, it has been shown that the continued production of IL-6 results in more extensive damage to hepatocytes (Tagawa et al. 2000). Previous studies revealed that TNF-α and IL-6 contributed to the development of hepatocyte damage through Toll-like receptors (TLRs) signal pathway which are involved in innate immunity and inflammation (Zhou et al. 2013; Xu et al. 2013). The present study demonstrates that Con A administration significantly increases the pro-inflammatory cytokines TNF-α and IL-6. These results are in accordance with those of other investigators (Gantner et al. 1995; Bruck et al. 1997; Klein et al. 2003; Liang et al. 2013; Xia et al. 2014). Interestingly, pretreatment with propolis significantly attenuated the serum level of TNF-α and IL-6 induced by Con A. The anti-inflammatory activity of propolis was supported by a previous study on arthritis in rats (DU Toit et al. 2009). Furthermore, propolis has been shown to have hepatoprotective effects against TNF-α-induced cell death (Alencar et al. 2007). Previously, it has been demonstrated that the ongoing inflammation in the liver is associated with formation of TGF-β (Schierwagen et al. 2013), which is a profibrogenic cytokine that has been shown to regulate multiple fundamental cellular processes, including cell growth, differentiation, migration, adhesion, and ECM deposition. It is secreted by all immune cell lineages, including B, T, and dendritic cells as well as macrophages (Moustakas et al. 2002). Oxidative stress has been shown to be one of the major factors involved in TGF-β activation via oxidation of the latency-associated peptide, resulting in conformational change that releases TGF-β (Barcellos-Hoff and Dix 1996). In the present work, administration of Con A significantly increased TGF-β activation. These results resemble those obtained from previous studies on mice (Kimura et al. 1999) and rats (Liang et al. 2013). Furthermore, it was found that the increase in TGF-β activation by Con A is accompanied by a marked increase in Smad2/3 phosphorylation. These results are in agreement with the previous study of Qin et al. (2014). Most interestingly, propolis was found to interfere with TGF-β activation induced by Con A. Furthermore, this reduction in TGF-β activation is accompanied by a strong reduction of Con A-induced Smad2/3 phosphorylation in the presence of propolis. Propolis was previously shown to reduce TGF-β level (Kao et al., 2013 and Lima et al., 2014). Also, it has been reported that propolis has the ability to inhibit TGF-β-induced epithelial-mesenchymal transition (EMT) and lung fibrosis (Kao et al., 2013). The inhibitory effect of propolis on TGF-β-induced EMT may be due to its suppressive effect on TGF-β/Smad signaling pathway. Moreover, propolis prevents the TGF-β-induced downregulation of peroxisome proliferator-activated receptor gamma (PPAR-γ) that inhibits hepatic fibrosis, suggesting that the anti-fibrotic effect of propolis may partly function through PPAR-γ activation (Wang et al., 2011; Kao et al., 2013).
In conclusion, our findings demonstrate that propolis has the ability to protect rats from Con A-induced liver injury. This significant protection against Con A-induced hepatitis by propolis can be attributed to its ability to restore the balance between oxygen radical production and the endogenous antioxidant defense system which was disturbed by Con A in the liver tissue. It may also be attributed to its ability to inhibit the inflammatory cytokines TNF-α and IL-6 as well as the profibrotic cytokine TGF-β and subsequent smad phosphorylation. Finally, since Con A-induced hepatitis is an experimental model that causes an immune-mediated hepatitis in a similar pattern to the one induced by viral infections, the use of propolis might be useful in reducing liver injury induced by viral infections. Additional clinical studies are warranted to investigate such an effect in human subjects.
References
Afify H, Abdel-Aziz HA, Balah A, Akool E-S (2016) Wheat germ oil and vitamin E inhibit TGF-β/SMAD signaling pathway induced by tacrolimus (FK506) in rat kidney. Int J Pharm Bio Sci 7:129–138
Akool E-S (2015) Molecular mechanisms of the protective role of wheat germ oil against cyclosporin A-induced hepatotoxicity in rats. Pharm Biol 53:1311–1317
Al-Amoudi W (2015) Ameliorative role and antioxidant effect of propolis against hepatotoxicity of fenvalerate in albino rats. J Cytol Histol 6:303
Alencar SM, Oldoni TL, Castro ML, Cabral ISR, Costa-Neto CM, Cury JA, Rosalen PL, Ikegaki M (2007) Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. J Ethnopharmacol 113:278–283
Al-Hariri M, Gamal Eldin T, Abu-Hozaifa B, Elnour A (2011) Glycemic control and anti-osteopathic effect of propolis in diabetic rats. Diabetes Metab Syndr Obes 4:377–384
Ballegeer M, Libert C (2016) Different cell types involved in mediating concanavalin A induced liver injury: a comprehensive overview. J Gastroenterol Hepatology Res 1:1
Barcellos-Hoff MH, Dix TA (1996) Redox-mediated activation of latent transforming growth factor-β1. Mol Endocrinol 10:1077–1083
Bozza M, Bliss JL, Maylor R, Erickson J, Donnelly L, Bouchard P, Dorner AJ, Trepicchio WL (1999) Interleukin-11 reduces t-cell-dependent experimental liver injury in mice. Hepatology 30:1441–1447
Bruck R, Shirin H, Hershkoviz R, Lider O, Kenet G, Aeed H, Matas Z, Zaidel L, Halpem Z (1997) Analysis of Arg-Gly-Asp mimetics and soluble receptor of tumour necrosis factor as therapeutic modalities for concanavalin A induced hepatitis in mice. Gut 40:133–138
Cao Q, Batey R, Pang G, Russell A, Clancy R (1998) IL-6, IF-γ and TNF-α production by liver-associated T cells and acute liver injury in rats administered concanavalin A. Immunol Cell Biol 76:542–549
Capucho C, Sette R, de Souza PF, de Castro MJ, Pigoso AA, Barbieri R, Dolder MA, Severi-Aguiar GD (2012) Green Brazilian propolis effects on sperm count and epididymis morphology and oxidative stress. Food Chem Toxicol 50:3956–3962
Claus R, Kinscherf R, Gehrke C, Bonaterra G, Basnet P, Metz J, Deigner HP (2000) Antiapoptotic effects of propolis extract and propol on human macrophages exposed to minimally modified low density lipoprotein. Arzneimittelforschung 50:373–379
De Andrade KQ, Moura FA, Dos Santos JM, De Araújo ORP, Santos JCDF, Goulart MOF, De Farias Santos JC (2015) Oxidative stress and inflammation in hepatic diseases: therapeutic possibilities of N-acetylcysteine. Int J Mol Sci 16:30269–30308
DU Toit K, Buthelezi S, Bodenstein J (2009) Anti-inflammatory and antibacterial profiles of selected compounds found in south African propolis. S Afr J Sci 105:470–472
El-Khatib AS, Agha AM, Mahran LG, Khayyal MT (2002) Prophylactic effect of aqueous propolis extract against acute experimental hepatotoxicity in vivo. Z Naturforsch C 57:379–385
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem and Biophys 82:70–77
Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G (1995) Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 21:190–198
Genestra M (2007) Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal 19:1807–1819
Havsteen BH (2002) The biochemistry and medical significance of the flavonoids. Pharmacol Ther 96:67–202
Hoşnuter M, Gürel A, Babucçu O, Armutcu F, Kargi E, Işikdemir A (2004) The effect of CAPE on lipid peroxidation and nitric oxide levels in the plasma of rats following thermal injury. Burns 30:121–125
Jasprica I, Mornar A, Debeljak Z, Somolcici-bubalo A, Medic-Saric M, Mayer L, Romic Z, Bucan K, Balog T, Sobocanec S, Sverko V (2007) In vivo study of propolis supplementation effects on antioxidative status and red blood cells. J Ethnopharmacol 110:548–554
Kao HF, Chang-Chien PW, Chang WT, Yeh TM, Wang JY (2013) Propolis inhibits TGF-β1-induced epithelial–mesenchymal transition in human alveolar epithelial cells via PPARγ activation. Int Immunopharmacol 15:565–574
Kimura K, Ando K, Ohnishi H, Ishikawa T, Kakumu S, Takemura M, Muto Y, Moriwaki H (1999) Immunopathogenesis of hepatic fibrosis in chronic liver injury induced by repeatedly administered concanavalin A. Int Immunol 11:1491–1500
Klein C, Wüstefeld T, Heinrich PC, Streetz KL, Manns MP, Trautwein C (2003) ME3738 protects from concanavalin A-induced liver failure via an IL-6-dependent mechanism. Eur J Immunol 33:2251–2261
Kolankaya D, Selmanoǧlu G, Sorkun K, Salih B (2002) Protective effects of Turkish propolis on alcohol-induced serum lipid changes and liver injury in male rats. Food Chem 78:213–217
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y (2015) The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 16:26087–26124
Liang J, Zhang B, Shen RW, Liu JB, Gao MH, Li Y, Li YY, Zhang W (2013) Preventive effect of halofuginone on concanavalin A-induced liver fibrosis. PLoS One 8:82232
Lima LD, Andrade SP, Campos PP, Barcelos LS, Soriani FM, Moura SA, Ferreira MA (2014) Brazilian green propolis modulates inflammation, angiogenesis and fibrogenesis in intraperitoneal implant in mice. BMC Complement Altern Med 14:177
Lin SC, Lin YH, Chan CF, Chung CY, Hsu SH (1997) The hepatoprotective and therapeutic effects of propolis ethanol extract on chronic alcohol-induced liver injuries. Am J Chin Med 25:325–332
Liu C, Gao S, Qu Z, Wang Q, Zhu F, Guo C, Hou L, Wu P, Shi Y, Zhang L (2012) Ncpp treatment alleviates cona-induced hepatitis via reducing CD4+ T activation and no production. Immunopharmacol Immunotoxicol 34:962–967
Liu D, Zhang X, Jiang L, Guo Y, Zheng C (2014) Epigallocatechin-3-gallate (EGCG) attenuates concanavalin A induced hepatic injury in mice. Acta Histochem 116:654–662
Lozano-Sepulveda SA, Bryan-Marrugo OL, Cordova-Fletes C, Gutierrez-Ruiz MC, Rivas-Estilla AM (2015) Oxidative stress modulation in hepatitis C virus infected cells. World J Hepatol 7:2880–2889
Mannaa F, El-Shamy KA, El-Sheikh KA, El-kassaby M (2011) Efficacy of fish liver oil and propolis as neuroprotective agents in pilocarpine epileptic rats treated with valproate. Pathophysiology 18:287–294
Mizuhara H, Uno M, Seki N, Yamashita M, Yamaoka M, Ogawa T, Kaneda K, Fujii T, Senoh H, Fujiwara H (1996) Critical involvement of interferon gamma in the pathogenesis of T-cell activation-associated hepatitis and regulatory mechanisms of interleukin-6 for the manifestations of hepatitis. Hepatology 23:1608–1615
Moustakas A, Pardali K, Gaal A, Heldin CH (2002) Mechanisms of TGF- β signaling in regulation of cell growth and differentiation. Immunol Lett 82:85–91
Nicoletti F, Di Marco R, Zaccone P, Salvaggio A, Magro G, Bendtzen K, Meroni P (2000) Murine concanavalin A-induced hepatitis is prevented by interleukin 12 (IL-12) antibody and exacerbated by exogenous IL-12 through an interferon-gamma-dependent mechanism. Hepatology 32:728–733
Paik Y-H, Kim J, Aoyama T, De Minicis S, Bataller R, Brenner DA (2014) Role of NADPH oxidases in liver fibrosis. Antioxid Redox Signal 20:2854–2872
Poli G (2000) Pathogenesis of liver fibrosis: role of oxidative stress. Mol Asp Med 21:49–98
Pujirahayu N, Ritonga H, Uslinawaty Z (2014) Properties and flavonoids content in propolis of some extraction methods of raw propolis. Int J Pharm Pharm Sci 6:338–340
Qin Y, Zhong Y, Yang G, Ma T, Jia L, Huang C, Li Z (2014) Profiling of concanavalin A-binding glycoproteins in human hepatic stellate cells activated with transforming growth factor-β1. Molecules 19:19845–19867
RaviKumar S, Gnanadesigan N, Inbaneson SJ, Kalaiarasi A (2011) Hepatoprotective and antioxidant properties of Suaeda maritima (L.) Dumort ethanolic extract on concanavaline a induced hepatotoxicity in rats. Indian J Exp Biol 49:455–460
Ruggieri A, Anticoli S, Nencioni L, Sgarbanti R, Garaci E, Palamara AT (2013) Interplay between hepatitis C virus and redox cell signaling. Int J Mol Sci 14:4705–4721
Schierwagen R, Leeming DJ, Klein S, Granzow M, Nielsen MJ, Sauerbruch T, Krag A, Karsdal MA, Trebicka J (2013) Serum markers of the extracellular matrix remodeling reflect antifibrotic therapy in bile-duct ligated rats. Front Physiol 4:195
Seven PT, Yılmaz S, Seven I, Cercı IH, Azman MA, Yılmaz M (2009) Effects of propolis on selected blood indicators and antioxidant enzyme activities in broilers under heat stress. Acta Vet Brno 78:75–83
Shirin H, Aeed H, Alin A, Matas Z, Kirchner M, Brazowski E, Goldiner I, Bruck R (2010) Inhibition of immune-mediated concanavalin A-induced liver damage by free-radical scavengers. Dig Dis Sci 55:268–275
Silva V, Genta G, Moller MN, Masner M, Thomson L, Romero N, Radi R, Fernandes DC, Laurindo FR, Heinzen H, Fierro W, Denicola A (2011) Antioxidant activity of Uruguayan propolis. In vitro and cellular assays J Agric Food Chem 59:6430–6437
Su KY, Hsieh CY, Chen YW, Chuang CT, Chen CT, Chen YL (2014) Taiwanese green propolis and propolin G protect the liver from the pathogenesis of fibrosis via eliminating TGF-β-induced Smad2/3 phosphorylation. J Agric Food Chem 62:3192–3201
Tagawa Y, Matths P, Heremans H, Dillen C, Zaman Z, Iwakura Y, Billiau A (2000) Bimodal role of endogenous interleukin-6 in concanavalin A induced hepatitis in mice. J Leukoc Biol 67:90–96
Tan-No K, Nakajima T, Shoji T, Nakagawasai O, Nijima F, Ishikawa M, Endo Y, Sato T, Satoh S, Tadano T (2006) Anti-inflammatory effect of própolis throug inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol Pharm Bull 29:96–99
Tiegs G, Hentschel J, Wendel A (1992) A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 90:196–203
Viuda-Martos M, Ruiz-Navajas Y, Fernández-López Jand Pérez-Alvarez JA (2008) Functional properties of honey, propolis, and royal jelly. J Food Sci 73:117–124
Wang Z, Xu JP, Zheng YC, Chen W, Sun YW, Wu ZY, Luo M (2011) Peroxisome proliferator-activated receptor gamma inhibits hepatic fibrosis in rats. Hepatobiliary Pancreat Dis Int 10:64–71
Xia S, Han M, Li X, Cheng L, Qiang Y, Wu S, Zhang M, Xu H, Liu X, Shao Q (2014) Dietary fish oil exacerbates concanavalin A induced hepatitis through promoting hepatocyte apoptosis and altering immune cell populations. J Toxicol Sci 39:179–190
Xu X, Hu Y, Zhai X, Lin M, Chen Z, Tian X, Zhang F, Gao D, Ma X, Lv L, Yao J (2013) Salvianolic acid A preconditioning confers protection against concanavalin Ainduced liver injury through SIRT1-mediated repression of p66shc in mice. Toxicol Appl Pharmacol 273:68–76
Zhao JQ, Wen YF, Bhadauria M, Nirala SK, Sharma A, Shrivastava S, Shukla S, Agrawal OP, Mathur R (2009) Protective effects of propolis on inorganic mercury induced oxidative stress in mice. Indian J Exp Biol 47:264–269
Zhou Y, Dai W, Lin C, Wang F, He L, Shen M, Chen P, Wang C, Lu J, Xu L, Xu X, Guo C (2013) Protective effects of necrostatin-1 against concanavalin A-induced acute hepatic injury in mice. Mediat Inflamm 2013:706156
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental protocol used in this study was approved by the Institutional Animal Ethics Committee.
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Mounieb, F., Ramadan, L., Akool, ES. et al. Propolis alleviates concanavalin A-induced hepatitis by modulating cytokine secretion and inhibition of reactive oxygen species. Naunyn-Schmiedeberg's Arch Pharmacol 390, 1105–1115 (2017). https://doi.org/10.1007/s00210-017-1410-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1410-3