Abstract
Besides their deleterious action on cardiac muscle, anthracycline-type cytostatic agents exert significant neurotoxic effects on primary sensory neurons. Since cardiac sensory nerves confer protective effects on heart muscle and share common traits with cutaneous chemosensitive nerves, this study examined the effects of cardiotoxic doses of adriamycin on the function and morphology of epidermal nerves. Sensory neurogenic vasodilatation, plasma extravasation, and the neural CGRP release evoked by TRPV1 and TRPA1 agonists in vitro were examined by using laser Doppler flowmetry, the Evans blue technique, and ELISA, respectively. Carrageenan-induced hyperalgesia was assessed with the Hargreaves method. Immunohistochemistry was utilized to study cutaneous innervation. Adriamycin treatment resulted in profound reductions in the cutaneous neurogenic sensory vasodilatation and plasma extravasation evoked by the TRPV1 and TRPA1 agonists capsaicin and mustard oil, respectively. The in vitro capsaicin-, but not high potassium-evoked neural release of the major sensory neuropeptide, CGRP, was markedly attenuated after adriamycin treatment. Carrageenan-induced inflammatory hyperalgesia was largely abolished following the administration of adriamycin. Immunohistochemistry revealed a substantial loss of epidermal TRPV1-expressing nociceptive nerves and a marked thinning of the epidermis. These findings indicate impairments in the functions of TRPV1 and TRPA1 receptors expressed on cutaneous chemosensitive nociceptive nerves and the loss of epidermal axons following the administration of cardiotoxic doses of adriamycin. Monitoring of the cutaneous nociceptor function in the course of adriamycin therapy may well be of predictive value for early detection of the deterioration of cardiac nerves which confer protection against the deleterious effects of the drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthracycline derivatives are cytotoxic agents widely used for the treatment of various malignancies, although they have significant adverse effects. Their serious side-effects include their cardiotoxicity, which may compromise the cardiac function and result in cardiomyopathy and even heart failure. Congestive dilatative cardiomyopathy develops in a dose-dependent manner both in man (Minow et al. 1975; Lefrak et al. 1975; Praga et al. 1979; Suzuki et al. 1979) and in animal models (Schwarz et al. 1998b; Katona et al. 2004). Another adverse effect of anthracyclines is the neurotoxicity. Systemic or local administration of adriamycin to peripheral nerves may result in degenerative alterations or even the death of sensory ganglion cells (Bigotte and Olsson 1982; Kondo et al. 1987). Interestingly, recent findings suggest that the neurotoxic effect of anthracyclines may be of relevance with respect to their adverse effects on the heart function. Indeed, experiments in an established rat model of anthracycline cardiomyopathy indicated that chemosensitive afferent nerves, which express the transient receptor potential vanilloid type 1 receptor (TRPV1), may confer cardioprotection against adriamycin-induced cardiomyopathy. Several lines of evidence indicate that the cardioprotection provided by sensory nerves is mediated through the release of calcitonin gene-related peptide (CGRP) from cardiac chemosensitive afferent nerves (Ferdinandy et al. 1997; Csont et al. 2003; Li et al. 2009; Liu et al. 2011). Hence, these findings suggest that, besides its direct toxic effect on cardiac muscle, adriamycin may also contribute to the development of cardiac pathologies by virtue of its neurotoxic effect in eliminating the protective role of sensory nerves.
Chemosensitive C-fiber primary afferent neurons are a unique population of sensory ganglion cells (Jancsó et al. 1977), which express the TRPV1 (Caterina et al. 1997) and/or the transient receptor potential ankyrin type 1 (TRPA1) receptors (Story et al. 2003; Jordt et al. 2004). In addition to the transmission of nociceptive information toward the central nervous system, these primary sensory neurons are involved, through the release of peptides from their peripheral nerve endings, in local regulatory functions of the innervated organs and tissues (Jancsó 1960; Jancsó et al. 1968; Jancsó 1984; Jancsó et al. 1987; Maggi and Meli 1988; Szolcsányi 1988; Holzer 1998b; Sántha et al. 1998; Sántha et al. 2000; Nagy et al. 2004; Jancsó et al. 2009). Of the many facets of these local regulatory functions, sensory neurogenic vasodilatation and plasma extravasation, collectively designated neurogenic inflammation, are particularly important in the modulation of local blood flow, vascular permeability, inflammatory processes, and tissue defense mechanisms (Jancsó 1960; Jancsó et al. 1968; Rózsa et al. 1986; Maggi et al. 1987; Brain 1997; Holzer 1998a; Holzer and Holzer-Petsche 2001; Jancsó et al. 2009; Jancsó 2009). A quantitative assessment of the vascular reactions elicited through activation of TRPV1 and/or TRPA1 receptors localized on chemosensitive afferent nerves serves as a reliable indicator of the functional condition of these afferent nerves in both man and animals (Jancsó and Janka 1981; Jancsó et al. 1985; Baron et al. 1988; Lynn et al. 1996; Dux et al. 2003; Bernardini et al. 2004; Namer et al. 2005). Further, measurements of the capsaicin- or mustard oil-induced release of the sensory neuropeptide CGRP in ex vivo preparations provide valuable information on the functional integrity of chemosensitive afferent nerves (Gamse et al. 1980; Saria et al. 1987; Lundberg et al. 1992; Dux et al. 2007; Fuchs et al. 2010).
Chemosensitive afferent nerves innervating different organs and tissues share many common traits in terms of their morphology, neurochemistry, and function. The cutaneous chemosensitive afferent nerves are the best-characterized class of this particular population of sensory nerves (Roosterman et al. 2006; Jancsó et al. 2009). Through application of a treatment paradigm previously shown to produce congestive cardiomyopathy in the rat, the primary aim of the present work was therefore an explanation of the neurotoxic effects of adriamycin on chemosensitive afferent nerves through studies of cutaneous neurogenic sensory vasodilatation, neurogenic plasma extravasation, inflammatory hyperalgesia and innervation, and measurement of CGRP release from sensory nerves.
Materials and methods
The experiments were approved by the Ethical Committee for Animal Care at the University of Szeged and were carried out in accordance with the European Communities Council Directive (86/609/EEC). All efforts were made to minimize animal suffering. The number of experimental animals was kept as low as possible.
Adriamycin treatment
Adult male Wistar rats weighing 250–280 g at the beginning of the experiments were used in the study. Groups of animals received cumulative doses of 7.5 or 15 mg/kg adriamycin (doxorubicin hydrochloride, TEVA, Debrecen, Hungary) through intraperitoneal injection of 2.5 mg/kg of the drug three times a week for 1 or 2 weeks, respectively. The control rats received equivalent amounts of the vehicle (saline). All measurements were made 2–7 days after the cessation of the adriamycin treatment.
Measurement of chemically induced neurogenic cutaneous vasodilatation
Animals were anesthetized with chloral hydrate (400 mg/kg, i.p., Reanal, Budapest, Hungary). Body temperature was maintained at 37.2 ± 0.5 °C with a heating pad. A small silicone chamber (5 mm in diameter) was mounted on the middle region of the dorsal skin of the hind paw to avoid the lateral spreading of the applied solutions. Cutaneous blood flow (CuBF) was monitored with a Laser Doppler Blood Flow (LDF) Monitor (type MBF3, Moor Instruments Ltd., Axminster, Devon, UK) by positioning the probe over the surface of the skin in the center of the chamber. After a stable LDF signal was attained, 20 μl of mustard oil (0.2 %, allyl-isothiocyanate, Merck, Darmstadt, Germany) was applied onto the skin through a polyethylene cannula built into the wall of the chamber and the CuBF was recorded for 15–20 min. This procedure was repeated 45 min later with a higher concentration of mustard oil (1 %). We also studied the vasodilatatory effect of capsaicin (1 %, Fluka, Buchs, Switzerland) in a similar set of experiments. The effects of pretreatments with the specific TRPA1 and TRPV1 antagonists, HC-030031 (Tocris, Bristol, UK) and capsazepine (Sigma-Aldrich GmbH, Germany), respectively, were also tested. Capsaicin was dissolved in ethanol. Stock solutions of HC-030031 (100 mM) and capsazepine (10 mM) were prepared with dimethyl sulfoxide and ethanol, respectively. Mustard oil was diluted with ethanol. Relative increases in CuBF were calculated by comparing the 3-min pre-drug mean CuBF with the mean of consecutive 1-min LDF signal values recorded after the application of test agents.
Measurement of chemically induced cutaneous plasma protein extravasation
Neurogenic plasma extravasation was evoked and quantified as described previously. Briefly, animals were anesthetized with chloral hydrate and injected intravenously with Evans blue dye (50 mg/kg, 1 % in saline). Ten minutes later, the dorsal skin of the hind paws was painted with a solution of 5 % mustard oil, 1 % capsaicin or their solvent (liquid paraffin and ethanol, respectively). The effects of pretreatments with the specific TRPA1 and TRPV1 antagonists, HC-030031 and capsazepine, respectively, were also tested. After 20 min, the animals were perfused transcardially with saline, and samples of the dorsal paw skin were removed, weighed, and placed into formamide to extract the dye for quantitative photometric determination (Jancsó et al. 1977). Tissue Evans blue contents were expressed in μg dye/g tissue as mean ± SD.
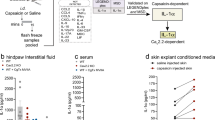
Measurement of CGRP release in vitro
The animals were anesthetised with chloral hydrate, sacrificed by decapitation, and the sciatic nerves were removed. The epineurium was carefully removed from the nerves under microscopic control, and the samples were washed with synthetic interstitial fluid (SIF, containing in mM: 107.8 NaCl, 26.2 NaHCO3, 9.64 Na-gluconate, 7.6 sucrose, 5.55 glucose, 3.48 KCl, 1.67 NaH2PO4, 1.53 CaCl2, and 0.69 MgSO4) gassed with 95 % O2 and 5 % CO2 to a pH of 7.4 at room temperature for 30 min. Thereafter, the SIF was replaced with 400 μl of the release buffer consisting of SIF supplemented with 0.1 % bovine serum albumin and 16 μM thiorphan (Price et al. 2005). After an incubation period of 10 min to measure the basal CGRP release, the buffer was replaced either with a release buffer containing 10 μM capsaicin or with a modified release buffer containing 60 mM KCl. The tissue samples were then further incubated for 10 min to determine the capsaicin- and the depolarization-induced CGRP release. The effect of capsazepine, a specific TRPV1 antagonist, on the capsaicin-induced CGRP release was also studied. Samples were collected at the end of each incubation period into silicone-coated Eppendorf cups, frozen and stored at −70 °C until the determination of their CGRP content. The wet weights of the nerves were also measured. The CGRP concentrations of the samples were determined with an enzyme-linked immunoassay kit (ELISA, Bertin Pharma, Montigny-le-Bretonneux, France) according to the protocol provided by the manufacturer. The absorbances of the samples were measured photometrically with a microplate reader (DY-NEX MRX, Dynex Technologies, Chantilly, USA), and the CGRP contents were calculated and corrected for the tissue weights. The results (mean ± S.D.) are expressed as percentages of the basal CGRP release.
Carrageenan-induced thermal and mechanical hyperalgesia
The nociceptive paw withdrawal response to radiant heat stimulation was studied by the Hargreaves method (Hargreaves et al. 1988). The animals were placed on a glass surface in a transparent plastic cage and were allowed to adapt to their environment for 15 min before testing. The heat stimulus was directed onto the plantar surface of the hind paw. The withdrawal latency was measured three times on both hind paws of each rat, the means of the measured data serving as baseline for the left and right hind paws. The apparatus was calibrated to give a withdrawal latency of about 10 s in control animals. To prevent tissue damage, a cutoff-time of 20 s was chosen. For the assessment of carrageenan-induced thermal hyperalgesia, rats received an intraplantar injection of carrageenan (3 mg in 0.1 ml saline, Sigma-Aldrich GmbH, Steinheim, Germany) into the right hind paw. The paw withdrawal latencies were measured 3 h after the carrageenan injection, and the data were expressed as percentage changes (mean ± SD) from the control values.
Paw withdrawal thresholds for mechanical stimulation were measured in both hind paws with an automated apparatus suitable for the application of reproducible mechanical stimuli (Dynamic plantar aesthesiometer, Ugo Basile, Gemonio, Varese, Italy). Rats were placed on a metal mesh table and allowed to adapt to their new compartments. With the help of an adjustable angled mirror, the mechanical stimulus was directed to the plantar surface of the hind paw from below the floor. When the unit was started, a steel rod was pushed against the plantar surface of the hind paw with increasing force of 0 to 50 g over a 20-s period. As the animal withdrew its hind paw or an applied force of 50 g was reached, the stimulation was stopped and the force at which the response occurred was recorded. This procedure was repeated three times, and the withdrawal responses were averaged. Mechanical hyperalgesia was assessed after an intraplantar injection of carrageenan as described above. The latency of withdrawal responses were measured 3 h after carrageenan. The data were expressed as percentage changes (mean ± SD) from the control.
Kinematic analysis of hindlimb locomotor function
Experimental setup
For the kinematic analysis of the locomotion of intact and treated animals a transparent plexiglass runway (100 cm [L] × 15 cm [W]) with a tilted mirror fixed under the floor plate was used. Another mirror system was fixed behind the runway in order to observe the movements of the contralateral limb, too. A square grid pattern with 1 cm intervals was scratched into the front panel for calibration purposes. The rats were trained prior to the measurements to walk from one end of the runway to the other reaching a shelter. The hair of the animals was shaved off from the hindlimbs and the skin was marked with permanent black ink above the major joints. The locomotor pattern of the rats was recorded with high resolution and high speed digital cameras. The analysis of locomotor function was performed on basis of the observations published earlier by other laboratories (Fey et al. 2010; Ribotta et al. 2000) and of our own observations on animals that had suffered from an ongoing motoneuron degeneration and/or loss (Nógrádi and Vrabová 2001; Nógrádi et al. 2007; Pájer et al. 2014). A methodological manuscript detailing the description of the above set up and analysis is currently being prepared for publication.
Locomotor data analysis
We recorded three runs of each animal in every session and examined five steps complying two criteria: one similar step was required before and after the measured step and the head of the animal had to point to the direction of walk. The appropriate video sequences were divided into single frames using the VirtualDub software and the selected frames where the animals that were in a defined phase of movement were analyzed by using the ImageJ software (NIH, USA). The following parameters were determined and used in the analysis of locomotion in this study. Lateral view parameters were defined as follows. Step length: the length of the step cycle measured between the first moments of the consequent stance phases expressed in cm; tarsus off angle: the angle enclosed by the tarsus and the floor plate at the last moment of the stance phase expressed in degrees; ankleflexion: the angle enclosed by the tarsus and the tibia at the first moment of the stance phase expressed in degrees; knee flexion: the angle enclosed by the tibia and the femur at the first moment of the stance phase expressed in degrees; lateral placing: the angle enclosed by the tarsus and the longitudinal axis of the animal. We measured this parameter at the first moment of the stance phase expressed in degrees. Ankle lifting: the highest point reached by the ankle joint during the swing phase compared to its lowest position on the ground (in mm); knee lifting: the highest point reached by the knee joint during the swing phase compared to its lowest position on the ground (in mm). Rear-view parameters were defined as follows. Metatarsus-surface angle: the angle enclosed by the metatarsus and the surface (expressed in degrees) at the first moment of the swing phase when the foot has just left the ground; spreading of toes: the angle opening between the 2nd and the 4th toes is determined in degrees at the first moment of the swing phase when the foot has just left the ground.
Demonstration of cutaneous innervation by immunohistochemistry and quantitative morphometry
For immunohistochemical studies, adriamycin- or vehicle-treated rats were perfused via the left heart ventricle with 4 % buffered formaldehyde solution 2–7 days after the completion of treatment. The plantar skin of the hind paws was removed and, after a post-fixation period of 3 h, was placed into a buffer solution and stored at 4 °C until sectioning. Skin samples were taken from the mid-plantar region of the paw and transverse sections were cut at a thickness of 25 μm and processed for immunohistochemical staining with the indirect immunofluorescence technique by using antisera raised against the TRPV1 receptor (rabbit polyclonal, 1:1000–1:1500, Alomone Laboratories, Jerusalem, Israel) and β-tubulin (mouse monoclonal, 1:1000, Sigma-Aldrich GmbH, Steinheim, Germany). Donkey anti-rabbit and anti-mouse IgGs labeled with Cy3, DL-488 (1:500, Jackson Immunoresearch Laboratories, West Grove, PA, USA) were used as secondary antibodies. Control procedures for immunolabeling were performed by replacing the primary antisera by normal donkey serum. To test the specificity of the TRPV1 antibody we have also performed a preadsorption test by applying a blocking peptide (supplied by the manufacturer of the TRPV1 antibody) representing the immunogenic fragment of TRPV1 against which the antibody was generated. No staining was observed in either case. Slides were covered with ProLong Gold Antifade Mountant with or without DAPI (Invitrogen, ThermoFisher Scientific, Budapest, Hungary).
The specimens were viewed under a Zeiss confocal fluorescence microscope. Z-stack image series were collected from sections of plantar skin samples obtained from control and adriamycin-treated rats. The density of epidermal axons was estimated with the vertical projection method. This design-based stereological approach allows an unbiased estimation of the length density of linear structures in a given volume (Gokhale 1990). Systemic random projection images of the epidermis were obtained from 25-μm-thick cryostat sections of the plantar skin of the hind paw. By using the filter grid cycloid arc plug-in of the Image-J image analysis software (Image Processing and Analysis in Java, National Institutes of Health, USA), a counting template containing cycloid curves was defined and superimposed onto the images. The major axis of the cycloid curves was aligned perpendicular to the skin surface. The total length of the cycloid curves and the number of counting grid points were optimized by performing pilot experiments on control skin sections. The factor l/p (length of test line per grid point) was set at 12.046 μm. Intersections of the epidermal nerve fibers with the cycloid arcs, and the number of grid points coinciding with the area representing the cross-section of the epidermal layer were determined on each image. The length density (Lv) of the intraepidermal nerve fibers was determined via the equation Lv = 2 x li/d x Pi x (l/p), where li is the number of cycloid intersections, d is the thickness of the section and Pi is the number of grid points hitting the cross section area of the epidermis.
The epidermal thickness was also measured along a line perpendicular to the skin surface with the aid of Image-J software in sections prepared for immunohistochemistry and covered with a mounting medium containing DAPI to visualize cell nuclei. Epidermal thickness was calculated as the average of six measurements on each sample from control and adriamycin-treated rats.
Statistics
For the statistical comparisons of the mustard oil-induced vasodilatatory responses two-way ANOVA was performed with pairwise comparisons based on Estimated Marginal Means using the Bonferroni post-hoc analysis, whereas one-way ANOVA was used for comparisons of the capsaicin-induced blood flow changes. One-way ANOVA was used to compare Evans blue contents of skin samples. The Student t test (unpaired) was applied for the statistical analyses of the data concerning the paw withdrawal responses, the in vitro CGRP release and the immunohistochemical experiments. In all groups, normality was proved by the Shapiro-Wilk test and homogeneity of variances was confirmed by Levene’s test in advance of performing two-way ANOVA. One-way ANOVA was applied to analyze the results of the hindlimb locomotor parameters. In all comparisons, a p value of <0.05 was accepted as a significant difference.
Results
Effects of adriamycin on neurogenic sensory vasodilatation
In control rats, applications of mustard oil at concentrations of 0.2 % and 1 %, and 1 % capsaicin produced significant increases in the CuBF (Fig. 1a–d). Subcutaneous (s.c.) injection of HC-030031 (100 μM, 50 μl) 20 min before the epicutaneous application of mustard oil significantly inhibited the vasodilatatory response. The increase in CuBF evoked by mustard oil (1 %) amounted to 121.80 ± 24.19 %, whereas the increase in CuBF after the prior administration of the TRPA1 antagonist, HC030031 amounted to 14.47 ± 10.44 % (p < 0.01, n = 6/group). The epicutaneous application of capsaicin (1 %) resulted in a marked elevation in CuBF, which amounted to 167.23 ± 53.36 %. Administration of the specific TRPV1 antagonist, capsazepine (10 μM, 50 μl, s.c.) 20 min before the epicutaneous application of capsaicin resulted in a significant inhibition of the vasodilatatory effect which amounted to 27.94 ± 8.95 % (p < 0.01, n = 6/group). In the adriamycin-treated animals, vasodilatatory responses were measured 2 days after cessation of the treatment. After the administration of a cumulative dose of 7.5 mg/kg adriamycin, the CuBF increases elicited with 1 % mustard oil and 1 % capsaicin were reduced by 51 % (p < 0.01, n = 10) and 64 % (p < 0.01, n = 6), respectively, whereas the vasodilatatory effect of 0.2 % mustard oil was not affected significantly (n.s., n = 10). However, in animals treated with a cumulative dose of 15 mg/kg adriamycin, the increase in CuBF was markedly and significantly reduced in response to both 0.2 and 1 % mustard oil (Fig. 1c), and to 1 % capsaicin (Fig. 1d), amounting to 43, 29, and 29 %, respectively, of the control values (p < 0.01, n = 6–10/group, Table 1).
Effects of adriamycin treatment on chemically-evoked cutaneous sensory neurogenic vasodilatation (a, c, d), plasma extravasation (e, f), and axonal CGRP release (b). a The graph demonstrates percentage changes of blood flow of the dorsal paw skin after the application of mustard oil (0.2 %, arrow) in control and adriamycin-treated animals as assessed by laser Doppler flowmetry. Each marker indicates changes of blood flow measured in consecutive 1-min periods expressed as mean + SD of six animals. b The graph shows the effect of adriamycin treatment (cumulative dose: 15 mg/kg) on capsaicin- and high KCl-induced release of CGRP from rat sciatic nerves in vitro. (n = 6–8/group). c, d The graphs show the effect of adriamycin treatments on mustard oil- and capsaicin-induced cutaneous vasodilatation. (n = 6–10/group). e, f The graphs show mustard oil- and capsaicin-evoked plasma extravasation in control and in adriamycin-pretreated rats (n = 8 for each group). The effects of capsaicin and mustard oil were inhibited significantly by the specific TRPV1 and TRPA1 antagonists capsazepine and HC-030031, respectively. In graphs b–f, each marker indicates an individual value and the horizontal lines show the mean of the group. * Significantly different from the control, p < 0.05
Effects of adriamycin on neurogenic plasma protein extravasation
Neurogenic plasma protein extravasation was studied with the quantitative Evans blue technique following the epicutaneous application of mustard oil or capsaicin. In control rats, following the epicutaneous application of mustard oil at a concentration of 5 %, skin Evans blue dye content amounted to 180 ± 68 μg/g, which was markedly and significantly reduced to 32.67 ± 17.98 μg/g by the prior s.c. administration of the specific TRPA1 antagonist HC-030031 (p < 0.01, n = 6/group). Similarly, the specific TRPV1 antagonist capsazepine almost completely abolished the vascular permeability increasing effect of 1 % capsaicin; the Evans blue dye content of the skin amounted to 205.50 ± 60.90 μg/g after the application of 1 % capsaicin, which was reduced to 32.73 ± 19.75 μg/g by the prior administration of capsazepine (p < 0.01, n = 6/group). Following the administration of a cumulative dose of 7.5 mg/kg adriamycin, the mustard oil-induced increase in tissue Evans blue content was markedly reduced (72.85 ± 20.84 μg/g, p < 0.01, n = 8), and after a cumulative dose of 15 mg/kg, the low Evans blue dye content of the skin (24.95 ± 10.84 μg/g) indicated an almost complete abolition of the neurogenic inflammatory response (p < 0.01, n = 8; Fig. 1e). Similarly, after the administration of adriamycin at cumulative doses of 7.5 and 15 mg/kg, tissue Evans blue contents were markedly and sifgnificantly reduced to 109.93 ± 38.16, and 51.08 ± 17.43 μg/g after the application of capsaicin (for both comparisons p < 0.01, n = 8; Fig. 1f).
Effects of adriamycin treatment on the in vitro neural release of CGRP
Neurogenic sensory vasodilatation is mediated by CGRP released from chemosensitive afferent nerves in response to stimulation of the TRPV1 and TRPA1 receptors. Study of the capsaicin-induced release of CGRP is an established experimental approach through which to characterize the sensory efferent function of peptidergic nociceptors expressing the TRPV1 ion channel. Capsaicin and high potassium-induced release of CGRP was therefore measured with ELISA in ex vivo preparations of sciatic nerves from control and adriamycin-treated rats. In the control sciatic nerve preparations, both capsaicin (10 μM) and high potassium (60 mM) elicited a marked release of CGRP (325 ± 110 %, and 327 ± 77 % of the basal release, respectively, n = 6–8/group). The release of CGRP, elicited by capsaicin at concentrations of 0.1, 1 and 10 μM, was significantly inhibited by 69.54, 54.02, and 23.26 %, respectively, in the presence of the specific TRPV1 antagonist, capsazepine (10 μM; p < 0.01, n = 6/group). In preparations obtained from adriamycin-treated rats, the high potassium-induced peptide release was similar to that in the controls (303 ± 55 % of the basal release, n = 8, n.s.). In contrast, the capsaicin-induced release of CGRP was significantly reduced in the adriamycin-treated animals (164 ± 71 % of the basal release, p < 0.01, n = 6; Fig. 1b).
Effects of adriamycin on carrageenan-induced thermal and mechanical hyperalgesia
The findings on the effects of adriamycin treatment on the neurogenic sensory vascular responses were indicative of a marked functional impairment of the chemosensitive afferent nerves which express the TRPV1 and TRPA1 receptors. Since these nociceptive ion channels play a crucial role in the mechanism of inflammatory hyperalgesia (Caterina et al. 2000), the effects of adriamycin treatment were studied in the carrageenan model of paw inflammation. In the control rats that received carrageenan, the paw withdrawal latencies to radiant heat stimulation decreased by 63.2 ± 2.0 % (p < 0.05, n = 5). In contrast, in the adriamycin-treated rats that received carrageenan, the heat withdrawal latencies were barely different from the control values (16.5 ± 11.6 %, n = 5, n.s.). Similarly, the mechanical withdrawal thresholds were significantly reduced in the controls (by 61.6 ± 10.5 %, p < 0.05, n = 5), but not in the adriamycin-treated animals (by 19.4 ± 7.0 %, n.s., n = 5; Fig. 2).
Effect of adriamycin treatment (cumulative dose: 15 mg/kg) on carrageenan-induced thermal and mechanical hyperalgesia. Percentage decreases in withdrawal responses to heat and mechanical stimuli are shown. Each symbol indicates the data of an individual animal; horizontal lines show the mean values of the groups (n = 5 for each group). * Significantly different from the control, p < 0.05
Effects of adriamycin on hindlimb locomotor parameters
The treated animals walked in a similar manner as their intact controls. No marked difference in the locomotor pattern of intact and treated animals was observed before the detailed analysis. In adriamycin treated rats, thorough examination of the high resolution images failed to reveal any significant impairment in step length, ankle and knee lifting, lateral placing (Fig. 3), ankle flexion, and spreading of toes (n.s., n = 5 ). The knee flexion, tarsus off angle, and metatarsus-surface angle parameters showed moderate but significant differences for both treatment groups as compared with the controls (p < 0.05, n = 5).
Hindlimb locomotor parameters directly dependent on the integrity of motoneuron function. Box whisker plots showing the knee lifting, ankle lifting, step length and lateral placing parameters measured in control and adriamycin-treated rats. There is no indication of motor impairment after adriamycin treatment. (n = 5 for each group)
Effects of adriamycin treatment on cutaneous sensory nerves
The effects of adriamycin treatment on the cutaneous innervation were studied with immunohistochemistry, quantitative stereological methods being used to determine the epidermal nerve fiber density of the rat plantar paw skin. In control skin samples, tubulin immunostaining revealed subepithelial bundles of nerve fibers giving rise to individual epidermal axons approaching the surface. In accord with our previous findings, two morphological types of epidermal axons were seen: axons with simple morphology, exhibiting little branching and a straight course, and axons which take a tortous course and develop elaborate arborization. It was earlier established that the axons with simple morphology are peptidergic, whereas those with complex morphology are non-peptidergic (Dux et al. 1999; Zylka et al. 2005). Double immunofluorescence staining revealed that almost all the tubulin-immunoreactive epidermal axons are TRPV1-positive. The densities of tubulin- and TRPV1-immunoreactive epidermal axons were 1075 ± 120 mm/mm3 and 1065 ± 116 mm/mm3, respectively. The density of epidermal axons decreased by roughly half following adriamycin treatment; the densities of tubulin- and TRPV1-positive axons were 503 ± 76 (p < 0.01, n = 5) and 417 ± 80 mm/mm3 (p < 0.01, n = 5), respectively. Interestingly, although the number of epidermal axons was greatly reduced, bundles of nerve fibers were seen subepidermally. It appeared that the epidermal axons were truncated at the epidermal-subepidermal border (Fig. 4). Adriamycin treatment also resulted in a significant reduction in epidermal thickness (control: 70.41 ± 7.52 μm; adriamycin-treated: 47.47 ± 6.75 μm; p < 0.01, n = 5; Fig. 5).
Effects of adriamycin treatment (cumulative dose: 15 mg/kg) on the distribution of intraepidermal axons of the rat plantar skin. Z-stack confocal microscopic images were taken from specimens double immunostained for tubulin (b, e) and TRPV1 (a, d). Note the colocalization of tubulin and TRPV1 (c, f) and the massive loss of intraepidermal axons after adriamycin treatment (d, e, f). Subepidermal nerves (arrowheads) can still be seen after adriamycin treatment (d, e, f). The scale bar in f holds for all microphotographs
Z-stack confocal microscopic images of the plantar skin of control (a) and adriamycin-treated (b, cumulative dose: 15 mg/kg) rats. Epidermal nuclei are visualized with DAPI and intraepidermal axons with TRPV1 immunohistochemistry. Note the marked thinning of the epidermis after adriamycin treatment. The scale bar in b holds for both microphotographs
Discussion
Pathologies affecting peripheral sensory nerves are commonly observed both in patients and in animals treated with antineoplastic compounds, manifested as neuropathic changes of variable severity, ranging from mild paresthesias to profound cutaneous and visceral sensory deficits (Rosenthal and Kaufman 1974; Scripture et al. 2006; Kanbayashi et al. 2010). Although the effects of antitumor agents on sensory functions have been the subject of extensive investigations (Grisold et al. 2012), studies on the effects of these compounds on nociceptor functions are scarce. The present study revealed profound changes in the afferent, nociceptive and sensory-efferent, local vascular functions of cutaneous nociceptive primary sensory neurons, which may be explained, at least in part, by structural changes associated with adriamycin treatment.
The cutaneous neurogenic sensory vasodilatation elicited by capsaicin, the archetypal activator of the TRPV1 receptor, or by mustard oil, a TRPA1 receptor agonist, was significantly inhibited by the systemic administration of adriamycin. Importantly, the adriamycin treatment schedule was earlier applied to produce congestive cardiomyopathy in the rat (Schwarz et al. 1998a; Katona et al. 2004). In this experimental model and other models of heart failure, the protective role of chemosensitive afferent nerves which express the TRPV1 receptor has been demonstrated and suggested to be mediated through the release of CGRP from cardiac sensory nerves (Ferdinandy et al. 1997; Katona et al. 2004; Wang and Wang 2005; Ferdinandy and Jancsó 2009; Wang et al. 2012). Although the mechanisms of the cardioprotective effect of CGRP-containing sensory nerves are not fully understood, the experimental evidence suggests that impairment of these particular peptidergic sensory nerves aggravates the development and course of cardiac pathologies, such as drug-induced congestive cardiomyopathy and heart failure; conversely, the TRPV1 receptor-activated increased release of CGRP (Zhong and Wang 2008) from cardiac sensory nerves confers protection against heart failure.
By demonstrating markedly reduced vasodilatatory responses to TRPV1 and TRPA1 agonists, the present findings reveal a similar impairment of the cutaneous chemosensitive sensory nerves after adriamycin treatment, since cutaneous sensory neurogenic vasodilatation is mediated by CGRP released from afferent nerves (Gamse et al. 1987; Brain et al. 1986; Jancsó et al. 1987; Holzer 1998b). The results of CGRP measurements in in vitro sciatic nerve preparations furnished further support for this assumption. The data disclosed a significant reduction of capsaicin-, but not high potassium-induced release of CGRP in preparations from adriamycin-treated rats. Since the high potassium-induced release was not affected by adriamycin, it appears reasonable to assume that the decreased release may be accounted for, at least in part, by an impairment of the activation of the TRPV1 receptor, as observed in diabetic neuropathy (Dux et al. 2007).
Chemosensitive primary sensory neurons play an essential role in the mechanisms of inflammatory hyperalgesia. The present findings furnished evidence of a profound inhibition of the development of carrageenan-induced inflammatory hyperalgesia by chronic adriamycin treatment. This suggests an impairment of the afferent, nociceptive function of chemosensitive sensory nerves expressing the TRPV1/TRPA1 receptors, which have been shown to be critically involved in the mechanism of inflammatory hyperalgesia (Davis et al. 2000; Pogatzki-Zahn et al. 2005). The results of the quantitative locomotor analysis failed to reveal major motor impairments in adriamycin-treated rats. Except three parameters all measurements yielded results similar to the controls. Locomotor parameters directly dependent on the integrity of spinal motoneuron function, such as step length, ankle and knee lifting, and lateral placing remained unaffected by the treatment (Nógrádi et al. 2007; Pájer et al. 2014). The significant differences observed in parameters tarsus off angle, metatarsus-surface angle and knee flexion, suggest disturbances in proprioception rather than a true impairment of motor function. Hence, the increased latency of the nociceptive withdrawal reflex following the administration of carrageenan in adriamycin treated animals may be attributed to a toxic damage of the antitumor agent on nociceptive afferent nerves, rather than an impairment of motor function/performance.
The mechanisms of the impairments in both the classical afferent, nociceptive and sensory-efferent, local vascular functions of chemosensitive afferent nerves expressing the TRPV1 and TRPA1 receptors cannot be fully explained on the basis of the present findings. However, the results do indicate that degenerative changes of cutaneous, and in particular epidermal sensory axons may be responsible, at least partially, for these functional disturbances. Examination of cutaneous innervation by means of immunohistochemistry and a quantitative stereological approach revealed a marked loss of epidermal axons in adriamycin-treated animals. In contrast, the density and distribution of subepidermal nerve fibers appeared normal, suggesting a selective loss of epidermal nerve endings. This might explain both the marked reduction of the sensory neurogenic vascular reactions and the inflammatory hyperalgesia in adriamycin-treated rats, since activation of the epidermal chemosensitive nerves is essential in the initiation of these responses (Maggi and Meli 1988; Szolcsányi 1988; Holzer 1998b; Davis et al. 2000; Caterina et al. 2000; Jancsó 2009). It is noteworthy that similar structural changes, i.e., a selective loss of terminal, epidermal, but not preterminal nerve fibers was also observed in the toxic neuropathies elicited by paclitexel (Boyette-Davis et al. 2011) and vincristine (Siau et al. 2006) and, interestingly, a human bacterial disease, leprosy (Miko et al. 1993).
Epidermal thinning was pronounced after the administration of adriamycin. This may well be explained by a direct antiproliferative action of the drug on the germinative cells of the epidermis, but the contribution of neuronal mechanisms cannot be excluded. Indeed, sensory denervation-induced thinning of the plantar skin has been demonstrated in the rat (Chiang et al. 1998). Hence, the marked loss of epidermal axons observed in the present study may also play a role in this phenomenon.
In conclusion, the present study has revealed significant functional impairments of cutaneous afferent nerves which express the TRPV1 and TRPA1 nociceptive ion channels following the administration of cardiotoxic doses of adriamycin. Cardiomyopathy is a severe adverse effect of adriamycin and related anticancer agents, and the primary management strategy of anthracycline cardiac toxicity is prevention and early detection (Remesh 2012). The functional impairments of the nociceptive afferent neurons observed in the present study precede the commencement of cardiomyopathic changes (Katona et al. 2004). Hence, the findings raise the possibility of using specific sensory testing, such as the detection and quantification of the axon reflex flare response, the human equivalent of neurogenic sensory vasodilatation, to predict the risk of adriamycin-induced cardiac injury in clinical practice.
References
Baron JF, Payen D, Coriat P, Edouard A, Viars P (1988) Forearm vascular tone and reactivity during lumbar epidural anesthesia. Anesth Analg 67:1065–1070
Bernardini N, Neuhuber W, Reeh PW, Sauer SK (2004) Morphological evidence for functional capsaicin receptor expression and calcitonin gene-related peptide exocytosis in isolated peripheral nerve axons of the mouse. Neuroscience 126:585–590
Bigotte L, Olsson Y (1982) Retrograde transport of doxorubicin (adriamycin) in peripheral nerves of mice. Neurosci Lett 32:217–221
Boyette-Davis J, Xin W, Zhang H, Dougherty PM (2011) Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain 152:308–313
Brain SD (1997) Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology 37:133–152
Brain SD, Tippins JR, Morris HR, MacIntyre I, Williams TJ (1986) Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Investig Dermatol 87:533–536
Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288:306–313
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Chiang HY, Huang IT, Chen WP, Chien HF, Shun CT, Chang YC, Hsieh ST (1998) Regional difference in epidermal thinning after skin denervation. Exp Neurol 154:137–145
Csont T, Csonka C, Kovács P, Jancsó G, Ferdinandy P (2003) Capsaicin-sensitive sensory neurons regulate myocardial nitric oxide and cyclic GMP signaling. Eur J Pharmacol 476:107–113
Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA (2000) Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405:183–187
Dux M, Rosta J, Pinter S, Sántha P, Jancsó G (2007) Loss of capsaicin-induced meningeal neurogenic sensory vasodilatation in diabetic rats. Neuroscience 150:194–201
Dux M, Sann H, Jancsó G (1999) Changes in fibre populations of the rat hairy skin after selective chemodenervation by capsaicin. Eur J Neurosci 10:299
Dux M, Sántha P, Jancsó G (2003) Capsaicin-sensitive neurogenic sensory vasodilatation in the dura mater of the rat. J Physiol Lond 552:859–867
Ferdinandy P, Csont T, Csonka C, Török M, Dux M, Németh J, Horváth LI, Dux L, Szilvássy Z, Jancsó G (1997) Capsaicin-sensitive local sensory innervation is involved in pacing induced preconditioning in rat hearts: role of nitric oxide and CGRP? Naunyn Schmiedeberg’s Arch Pharmacol 356:356–363
Ferdinandy P, Jancsó G (2009) Capsaicin-sensitive sensory nerves in myocardial ischemia-reperfusion injury and ischemic stress adaptation: role of nitric oxide and calcitonin-gene related peptide. In: Jancsó G (ed) Neurogenic inflammation. Elsevier Science, Amsterdam, pp. 267–288
Fey A, Schachner M, Irintchev A (2010) A novel motion analysis approach reveals late recovery in C57BL/6 mice and deficits in ncam-deficient mice after sciatic nerve crush. J Neurotrauma 27:815–828
Fuchs D, Birklein F, Reeh PW, Sauer SK (2010) Sensitized peripheral nociception in experimental diabetes of the rat. Pain 151:496–505
Gamse R, Holzer P, Lembeck F (1980) Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol 68:207–213
Gamse R, Posch M, Saria A, Jancsó G (1987) Several mediators appear to interact in neurogenic inflammation. Acta Physiol Hung 69:343–354
Gokhale AM (1990) Unbiased estimation of curve length in 3-D using vertical slices. J Microsc 159:133–141
Grisold W, Cavaletti G, Windebank A (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-Oncology 14:45–54
Hargreaves KM, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Holzer P (1998a) Implications of tachykinins and calcitonin gene-related peptide in inflammatory bowel disease. Digestion 59:269–283
Holzer P (1998b) Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol 30:5–11
Holzer P, Holzer-Petsche U (2001) Tachykinin receptors in the gut: physiological and pathological implications. Curr Opin Pharmacol 1:583–590
Jancsó G, Janka Z (1981) A simple test for topographical diagnosis of sensory nervous system lesions. Eur Neurol 20:84–87
Jancsó G (1984) Sensory nerves as modulators of inflammatory reactions. In: Chahl J, Szolcsányi J, Lembeck F (eds) Antidromic vasodilatation and neurogenic inflammation. Akadémiai Kiadó, Budapest, pp. 207–222
Jancsó G (2009) Neurogenic inflammation in health and disease. Elsevier, Amsterdam
Jancsó G, Katona M, Horváth V, Sántha P, Nagy J (2009) Sensory nerves as modulators of cutaneous inflammatory reactions in health and disease. In: Jancsó G (ed) Neurogenic inflammation in health and disease. Elsevier, Amsterdam, pp. 3–36
Jancsó G, Király E, Jancsó-Gábor A (1977) Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 270:741–743
Jancsó G, Király E, Such G, Joó F, Nagy A (1987) Neurotoxic effect of capsaicin in mammals. Acta Physiol Hung 69:295–313
Jancsó G, Obál F Jr, Tóth-Kása I, Katona M, Husz S (1985) The modulation of cutaneous inflammatory reactions by peptide-containing sensory nerves. Int J Tissue React 7:449–457
Jancsó N (1960) Role of the nerve terminals in the mechanism of inflammatory reactions. Bull Millard Fillmore Hosp 7:53–77
Jancsó N, Jancsó-Gabor A, Szolcsányi J (1968) The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemother 33:32–41
Jordt SE, Bautista DM, Chuang HH, Mckemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427:260–265
Kanbayashi Y, Hosokawa T, Okamoto K, Konishi H, Otsuji E, Yoshikawa T, Takagi T, Taniwaki M (2010) Statistical identification of predictors for peripheral neuropathy associated with administration of bortezomib, taxanes, oxaliplatin or vincristine using ordered logistic regression analysis. Anti-Cancer Drugs 21:877–881
Katona M, Boros K, Sántha P, Ferdinandy P, Dux M, Jancsó G (2004) Selective sensory denervation by capsaicin aggravates adriamycin-induced cardiomyopathy in rats. Naunyn Schmiedeberg’s Arch Pharmacol 370:436–443
Kondo A, Ohnishi A, Nagara H, Tateishi J (1987) Neurotoxicity in primary sensory neurons of adriamycin administered through retrograde axoplasmic transport in rats. Neuropathol Appl Neurobiol 13:177–192
Lefrak EA, Pitha J, Rosenheim S, O’Bryan RM, Burgess MA, Gottlieb JA (1975) Adriamycin (NSC 123127) cardiomyopathy. Cancer Chemother Rep 6:203–208
Li D, Zhang XJ, Chen L, Yang Z, Deng HW, Peng J, Li YJ (2009) Calcitonin gene-related peptide mediates the cardioprotective effects of rutaecarpine against ischaemia-reperfusion injury in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 36:662–667
Liu N, Zhang LZ, Han Y, Guo Z (2011) Differential effects of the calcitonin gene-related peptide on cardiac performance in acute myocardial ischemia and reperfusion in isolated rat hearts. Minerva Anestesiol 77:789–796
Lundberg JM, Franco-Cereceda A, Alving K, Delay-Goyet P, Lou YP (1992) Release of calcitonin gene-related peptide from sensory neurons. Annu Rev Neurosci 657:187–193
Lynn B, Schütterle S, Pierau FK (1996) The vasodilator component of neurogenic inflammation is caused by a special subclass of heat-sensitive nociceptors in the skin of the pig. J Physiol 494:587–593
Maggi CA, Borsini F, Santicioli P, Geppetti P, Abelli L, Evangelista S, Manzini S, Theodorsson-Norheim E, Somma V, Amenta F, et al. (1987) Cutaneous lesions in capsaicin-pretreated rats. A trophic role of capsaicin-sensitive afferents? Naunyn Schmiedeberg’s Arch Pharmacol 336:538–545
Maggi CA, Meli A (1988) The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol 19:1–43
Miko TL, Le Maitre C, Kinfu Y (1993) Damage and regeneration of peripheral nerves in advanced treated leprosy. Lancet 342:521–525
Minow RA, Benjamin RS, Gottlieb JA (1975) Adriamycin (NSC −123127) cardiomyopathy – an overview with determination of risk factors. Cancer Chemother Rep 6:195–201
Nagy I, Sántha P, Jancsó G, Urban L (2004) The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol 500:351–369
Namer B, Seifert F, Handwerker HO, Maihöfner C (2005) TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport 16:955–959
Nógrádi A, Szabó A, Pintér S, Vrabová G (2007) Delayed riluzole treatment is able to rescue injured rat spinal motoneurons. Neuroscience 144:431–438
Nógrádi A, Vrabová G (2001) The effect of riluzole treatment in rats on the survival of injured adult and grafted embryonic motoneurons. Eur J Neurosci 13:113–118
Pájer K, Feichtinger G, Márton G, Sabitzer S, Klein D, Redl H, Nógrádi A (2014) Cytokine signaling by grafted neuroectodermal stem cells rescues motoneurons destined to die. Exp Neurol 261:180–189
Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN (2005) Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain 115:296–307
Praga C, Beretta G, Vigo PL (1979) Adriamycin cardiotoxicity: a survey of 1,273 patients. Cancer Treat Rep 63:827–834
Price TJ, Louria MD, Candelario-Soto D, Dussor GO, Jeske NA, Patwardhan AM, Diogenes A, Trott AA, Hargreaves KM, Flores CM (2005) Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: effects on neuronal survival, neurochemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci 6:4
Remesh A (2012) Toxicities of anticancer drugs and its management. Int J Basic Clin Pharmacol 1:2–12
Ribotta M, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D (2000) Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci 20:5144–5152
Roosterman D, George T, Schneider SW, Bunnett N, Steinhoff M (2006) Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86:1309–1379
Rosenthal S, Kaufman S (1974) Vincristine neurotoxicity. Ann Intern Med 80:733–737
Rózsa Z, Sharkey KA, Jancsó G, Varró V (1986) Evidence for a role of capsaicin-sensitive mucosal afferent nerves in the regulation of mesenteric blood-flow in the dog. Gastroenterology 90:906–910
Sántha P, Horváth V, Pierau FK, Jancsó G (2000) Neurogenic inhibitory modulation of histamine-induced cutaneous plasma extravasation by endogenous galanin in the pigeon. J Physiol Lond 526:159P–160P
Sántha P, Pierau FK, Jancsó G (1998) Endogenous galanin inhibits neurogenic cutaneous vasodilatation in the pigeon. Eur J Neurosci 10:299
Saria A, Martling CR, Theodorsson-Norheim E, Gamse R, Hua XY, Lundberg JM (1987) Coexisting peptides in capsaicin-sensitive sensory neurons: release and actions in the respiratory tract of the Guinea-pig. Acta Physiol Hung 69:421–424
Schwarz ER, Pollick C, Dow J, Patterson M, Birnbaum Y, Kloner RA (1998a) A small animal model of non-ischemic cardiomyopathy and its evaluation by transthoracic echocardiography. Cardiovasc Res 39:216–223
Schwarz ER, Pollick C, Meehan WP, Kloner RA (1998b) Evaluation of cardiac structures and function in small experimental animals: transthoracic, transesophageal, and intraventricular echocardiography to assess contractile function in rat heart. Basic Res Cardiol 93:477–486
Scripture C, Figg W, Sparreboom A (2006) Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives. Curr Neuropharmacol 4:165–172
Siau C, Xiao W, Benett GJ (2006) Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol 201:507–514
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112:819–829
Suzuki T, Kanda H, Kaway J (1979) Cardiotoxicity of anthracycline antineoplasitc drugs – Clinicopathological and experimental studies. Jpn Circ J 43:1000–1008
Szolcsányi J (1988) Antidromic vasodilatation and neurogenic inflammation. Agents Actions 23:4–11
Wang L, Wang DH (2005) TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 112:3617–3623
Wang LH, Zhou SX, Li RC, Zheng LR, Zhu JH, Hu SJ, Sun Y (2012) Serum levels of calcitonin gene-related peptide and substance P are decreased in patients with diabetes mellitus and coronary artery disease. J Int Med Res 40:134–140
Zhong B, Wang DH (2008) N-oleoyldopamine, a novel endogenous capsaicin-like lipid, protects the heart against ischemia-reperfusion injury via activation of TRPV1. Am J Physiol Heart Circ Physiol 295:728–735
Zylka MJ, Rice FL, Anderson DJ (2005) Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45:17–25
Acknowledgments
This work was supported in part by a research grant from the Hungarian Scientific Research Fund (OTKA K-101873). P. Sántha was supported in part by the János Bolyai research fellowship of the Hungarian Academy of Sciences. The authors are grateful to Éva Hegyeshalmi for her excellent technical assistance and Tibor Nyári for his advice on statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Boros, K., Jancsó, G., Dux, M. et al. Multiple impairments of cutaneous nociceptor function induced by cardiotoxic doses of Adriamycin in the rat. Naunyn-Schmiedeberg's Arch Pharmacol 389, 1009–1020 (2016). https://doi.org/10.1007/s00210-016-1267-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1267-x