Abstract
Spontaneous microcontractions and electrical field stimulation (EFS)-evoked contractions in isolated rat bladder strips from normal and from 6 weeks partial bladder outflow obstruction (pBOO) animals were studied to identify the potential site of action for the β3-adrenoceptor (AR) agonist mirabegron in detrusor overactivity in rats. For this, effects of the β-AR agonist isoprenaline and mirabegron were tested in presence or absence of selective antagonists for β-AR subtypes, namely CGP-20712A for β1-AR, ICI-118,551 for β2-AR, and L-748,337 for β3-AR. In detrusor strips from both normal and obstructed animals, EFS-induced contractions were weakly affected by isoprenaline and even less so by mirabegron. In contrast, microcontraction activity was more potently reduced by isoprenaline (pIC50 7.3; Emax ±85 %), whereas mirabegron showed a small effect. In pBOO strips, concentration response curves for isoprenaline and mirabegron at inhibition of EFS and spontaneous microcontractions were similar to those in normal strips. Isoprenaline-induced inhibition of microcontractions and EFS was antagonized by the β1-AR antagonist, but not by the β2- and β3-AR antagonists. In the context of β3-AR-mediated bladder functions for mirabegron in other experiments, the current data question a role for effects at spontaneous microcontractions, or neurogenic detrusor stimulation in the mode of action for mirabegron in vivo, since functional bladder effects for mirabegron are reported to occur at much lower concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The subtype of β-adrenoceptor (AR) that mediates relaxation of the urinary bladder has been well studied and varies among mammalian species. In humans, β3-AR activation has been shown to produce relaxation of basal bladder tension in bladder that has been pre-contracted with carbachol or KCl (Takeda et al. 1999; Rouget et al. 2014). This bladder relaxant mechanism was proposed to be the basis to explain the activity of the recently introduced selective β3-AR agonist mirabegron (Takasu et al. 2007; Hatanaka et al. 2013) in the treatment of symptomatic bladder overactivity (OAB; Sacco and Bientinesi 2012). OAB is considered a disease of impaired bladder storage, with bladder instability as a prominent feature (Andersson 2003). Studies of filling cystometry in humans with and without OAB and in animals under different conditions, including partial bladder outlet obstruction (pBOO), have shown patterns of small non-voiding bladder contractions, increasing in intensity during bladder filling. These patterns of non-voiding activity (NVA) are associated with urinary frequency in both animals and humans and with frequency and urgency in humans. In pBOO rats, NVA was found to be modulated by anticholinergics (Lluel et al. 1998), and later Gillespie et al. (Gillespie et al. 2012) showed that mirabegron also decreased NVA in pBOO rats. Since NVA in vivo is likely to be the result of afferent bladder activity and efferent motor activity as proposed earlier (Drake et al. 2003), the possibility exists that the phenomena of NVA and bladder relaxation may be two separate components and may be differentially modulated by this new drug in conditions of OAB. An extra level of complexity may arise from findings that in isolated rat bladder tissue mounted in organ baths under non-stimulated conditions intrinsic motor activity was found on top of tonic activity. This activity is expressed as spontaneous microcontractions (MC) with amplitudes in the range of 0.1–0.6 mN, and it was found to be augmented by the cholinergic agonist carbachol and reduced by the β-adrenergic agonist isoprenaline (Gillespie et al. 2015). This points at the bladder being involved in complex motor-sensory inhibitory systems.
In this paper, we studied one part, namely, the β-AR pharmacology of inhibition of microcontractions in rat bladder as a presumably local phenomenon in rat bladder strips and compared this with electrical field stimulation (EFS)-induced contractions in the same strips in normal rat bladders as well as in bladders obtained from 6 weeks obstructed rats (pBOO).
A potential limitation to our choice for the rat to study β-AR pharmacology of phenomena such as NVA and MC in bladder function is that in rats both β2- and β3-AR are shown to be involved in relaxation of bladder smooth muscle, while mRNA for all β-AR subtypes was identified in rat bladder (Fujimura et al. 1999). This is a general problem assessing β3-AR functions across species and is extensively discussed by Michel et al. (2010). Nevertheless, this limits our conclusions for the present work to the rat. Further, because of limitations in funding and time, we used single concentrations for β-AR antagonists with selectivity for the rat β1-AR: CGP-20712A, the β2-AR ICI-118,551, and the β3-AR L-748,337, based on published work by Hatanaka et al. (2013) and Van Wieringen et al. (2013).
Methods
All experiments were carried out at UROsphere, Toulouse, in accordance with the European Community Council Directive 86/609/EEC and in accordance with a French Ministry for Agriculture and Fisheries license which allows to use the type of animal studies as described below.
Animals
Female Sprague-Dawley rats (body weight 200–270 g) were obtained from Charles River Laboratories (Saint-Germain-sur-l’Arbresle, France). All animals were group-housed in cages at least 4 to 5 days before the experiments, with free access to food and water.
Surgical procedures for bladder outlet obstruction
Rats were anesthetized with isoflurane (3 %). The abdominal wall was opened through a midline incision, and the bladder and urethra were exposed. A constant degree of urethral obstruction was produced by a ligature around the urethra and a metal rod with an outer diameter of 1 mm. The abdominal wall was closed with sutures. Food and water were given ad libitum. Following partial urethral obstruction, animals were treated with one injection of long-acting tetramycine 5 % (v/v) in physiological saline to prevent infections (0.1 mL/rat/day s.c.). During the first 3 days after obstruction, the urinary bladder was squeezed once a day to avoid urinary retention and renal failure (two major causes of death for acutely obstructed rats) and each animal received an injection of 1 % ketamine (w/v) (0.1 mL/rat/day s.c.). Control animals were normal animals without surgery. In this study, data from a total number of 53 normal rats and 13 pBOO rats were used.
Preparations and experimental setup
Rats were killed by CO2-induced asphyxiation. The whole urinary bladder was isolated and freed from connective and fat tissues, the dome and base removed, and the bladder bisected into two equally sized strips without attempts to remove urothelium/mucosa, with mean size approximately 5–6 mm length and 3–4 mm width. Strips were mounted in 5-mL glass organ baths containing a Krebs-Henseleit solution of the following composition (mM): NaCl 114, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11.7, ascorbic acid 1.1 (pH 7.4), and gassed with 95 % O2 and 5 % CO2 at 37 °C. Experimental setup was performed as depicted in Fig. 1: (1) normal or pBOO strips were allowed to equilibrate with repeated washings and tension adjustment under a resting tension of 9.8 mN for approximately 90 min and were then exposed to electrical field stimulation (EFS: positive polarity, 25 V, frequency of 15 Hz, square pulses of 0.1 ms in trains of 5 s). (2). Accordingly, EFS were generated as a frequency response curve by stimulating at 1, 3, 10, 20, 30, and 40 Hz (one stimulation at each frequency) with 6-min interval between stimulations. (3). Analysis of spontaneous (basal) MC with/without antagonist or solvent was done after spontaneous activity resumed in a stable fashion following EFS. (4) At this point, the first data were collected for normalization of the MC responses, and subsequently, experiments to modulate spontaneous MC with the β-AR agonists (isoprenaline or mirabegron) were done. (5) EFS frequency response (1, 3, 10, 20, 30, and 40 Hz and finally at (6) forskolin 10 μM) was tested to obtain full relaxation. Steps 1–6 were done in each preparation with one agonist/strip, usually in n = 6–9. Isometric force measurements were done with calibrated EMKA transducers (EMKA Technologies, Paris, France) and recorded using a data acquisition system (PowerLab 16s, AD Instruments, Sydney, Australia).
Study protocol for in vitro experiments with normal and pBOO rat strips. Explanatory text is given in the “Methods” section
Data collection and statistics
Briefly, EFS data sets for normal and pBOO strips were generated as frequency response curve at 1, 3, 10, 20, 30, and 40 Hz (one stimulation at each frequency). Following this treatment force data (in mN) were generated for pBOO and normal strips and analyzed by two-way ANOVA in GraphPad 6.0 (GraphPad Inc., La Jolla, USA). Data on agonist effects for cumulative concentrations of isoprenaline or mirabegron (10-9 to 10−4 M) on MC and 15 Hz EFS with/without β-AR antagonists (steps 3–5) were expressed as follows: MC activity was expressed as integral of area under the MC versus time registrations for min-max during 400 ms in mN s, as motivated in a previous paper (Gillespie et al. 2015). As noticed in this study, basal MC is very variable between strips yet stable within each strip. This prompted to compare treatments within each strip, using normalization to basal MC. In the present study for normal bladders mean MC for 72 strips was 75.7, with range of 5.4–194.2, and SD of 40.2 mN s. Responses to 15 Hz EFS for isoprenaline and mirabegron were determined by measuring the amplitude of responses and expressed as a percentage of control response (before agonist addition). Individual data per strip for MC and 15 Hz EFS were fitted for log concentration-response analysis. Data were fitted in GraphPad using the “Sigmoidal 4PL” option as the most adequate in this package, since this fitting procedure allowed to statistically compare curves for top and bottom values as well as IC50 and Hill slope. Where concentration-response curves for agonist with/without antagonist had Hill slopes not different from unity, statistical significance for difference in pIC50 values was calculated with an F test for the sum of squares. Accordingly, pK B values were estimated using the Schild equation according to MacKay (1978). These procedures were used to generate the data given in Table 1. Graphical interpolations using the “Sigmoidal 4PL” method were used for all concentration-response curves for isoprenaline, while those for mirabegron were fitted with the “log inhibitor versus response” option in GraphPad. Since both methods are based on least square regression, the choice of method was based on the D’Agostino and Pearson test for Gaussian scatter. Graphs are depicted in Figs. 4, 5, and 6. Because different curve-fitting procedures were used to fit the data for isoprenaline and mirabegron and difference in maximum inhibitory effect was noted for these agonists, an unpaired two-tailed t test with Welch correction in GraphPad was used for testing differences in column statistics for the data points for both curves, with P < 0.05 as criterion for significance.
Drugs and chemicals used
Isoflurane was purchased from Baxter (Maurepas, France) and saline (NaCl 0.9 %), tetramycine, and ketamine from Centravet (Lapalisse, France). Salts for preparing the Krebs-Henseleit solution (NaCl, KCl, CaCl2, MgSO4, KH2PO4, glucose) were obtained from Prolabo-VWR international (Fontenay-sous-bois, France). NaHCO3 and isoprenaline were purchased from Sigma-Aldrich (Saint Quentin Fallavier, France). CGP-20712A ((±)-2-hydroxy-5[2-{[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl0-1H-imidazol-2-yl)phenoxy]propyl]amino]ethoy}-benzamide] methanesulfonate, ICI-118,551 ((−)-1-(2,3-[dihydro-7-methyl-1H-inden-4-yl] oxy)-3-([1-methylethyl]-amino)-2-butanol, and L-748,337 (S)-N-[4-[2-{[3-[3-(acetamidomethyl)phenoxy]-2-hydroxypropyl]amino}-ethyl]phenyl] benzenesulfonamide were purchased from Tocris (Bristol, UK). Mirabegron was provided by Astellas.
Results
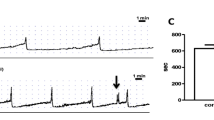
Normal and pBOO preparations responded to EFS in a frequency-dependent manner (Fig. 2); however, the amplitudes of EFS-induced contractions, normalized for bladder weight differences, were significantly reduced (P < 0.0001 for 3–40 Hz) in pBOO preparations (Fig. 3).
The effects of electrical field stimulation on bladder strips isolated from normal bladders and from bladders following 6 weeks of partial bladder outflow obstruction (pBOO); a and b show original records from control and pBOO bladder strips, respectively. Strips were stimulated where indicated by the arrows at frequencies shown
EFS-response histograms for normal (diagonal striped) and pBOO (checkered) bladder strips (n = 20 and 15, respectively), corrected for bladder weight. Statistical difference for P < 0.0001 is indicated with * (two-way ANOVA multiple comparisons test). At 1 Hz, no statistical significance resulted (P = 0.06)
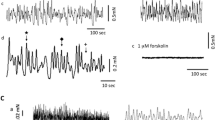
A distinct feature of all preparations under resting and non-stimulated conditions was microcontractile (MC) activity (see Fig. 2 for MC activity in between EFS-induced contractions). While the recordings from Fig. 2 may suggest differences in MC activity between pBOO and control preparations, a comparison of the integral of MC activity between control and pBOO preparations normalized for bladder weight differences in 24 strips for each category revealed no statistical differences (P > 0.05). In addition, the ratio for integral of MC activity versus EFS at 40 Hz for pBOO and control rats (n = 24) was also not statistically significant (P > 0.05).
In concentration-response curves in normal bladder strips (Figs. 4 and 5), the MC curves for isoprenaline and mirabegron (straight curves) were significantly different (P < 0.05; unpaired t test), and the different pharmacological characteristics for isoprenaline and mirabegron show clearly: isoprenaline acted as a potent agonist, with almost full decrease of MC at the highest concentrations tested, while mirabegron showed a low potency and low efficacy. Hill slopes for concentration-response for isoprenaline were not different from unity, while those for mirabegron were different from unity. On EFS contractions (dashed curves), isoprenaline only partially inhibited tonic contractile effects, while mirabegron showed similarly shallow concentration-response curves for reduction of EFS and MC. The potency of isoprenaline to reduce MC was apparently higher than for EFS contractions (see Table 1 for pIC50 values). The β3-AR blocker L-748,337 at 10−6 M did not antagonize isoprenaline or mirabegron effects at EFS and MC (see Table 1 for pIC50 values for isoprenaline and mirabegron where appropriate). A statistically significant shift to the right for the concentration-response curve for isoprenaline at MC was observed with the β1-AR blocker CGP-20712A at 10−7 M (P < 0.0001), but not for the β2-AR blocker ICI-118,551 at 10−7 M (Fig. 4). Estimation of a pK B value for CGP-20712A on isoprenaline-induced effects on MC resulted in a value of 7.58 (calculated according to MacKay 1978). Remarkably, increasing the concentration of CGP-20712A to 10−6 M did not further increase the shift of the concentration-response curve for inhibition of MC by isoprenaline (data not shown). For isoprenaline concentration-response curves at 15 Hz EFS, a significant shift of concentration-response curves after pre-incubation with CGP-20712A (P = 0.0074), with calculated pK B of 7.51. For ICI-118,551 at 10−7 M, no statistically significant shift resulted.
Normal rat strips: agonist-induced inhibition of microcontractions (straight lines) and EFS contractions at 15 Hz (dashed lines). Curves for isoprenaline control (circle) and after antagonist pre-incubation with CGP-20712A 10−7 M (triangle); ICI-118,551 10−7 M (square). Data are depicted as mean ± SD, with n = 6–9
Normal rat strips: a Agonist-induced inhibition of microcontractions (straight lines) and b EFS contractions at 15 Hz (dashed lines). Curves for isoprenaline are given in open symbols and for mirabegron in filled symbols, with isoprenaline control indicated as open circle and mirabegron control as filled circle. Isoprenaline and mirabegron after pre-incubation with L-748,337 10−6 M are indicated as (open diamond and filled diamond, respectively. Data are depicted as mean ± SD, with n = 6–10
In pBOO strips, concentration-response curves for isoprenaline as well as for mirabegron for MC and EFS shown in Fig. 6 appeared to be more shallow and SD values for mean data points were higher than in normal control rats. For isoprenaline MC and EFS curves the best fit was obtained with GraphPad’s sigmoidal 4PL curve fitting option, while for mirabegron the “log inhibitor versus response” package was used based on the D’Agostino and Pearson test results (Fig. 6b). Concentration-response curves for isoprenaline with and without CGP20712A at 10−6 M at MC had Hill slopes not different from unity, and the displacement to the right was significant (P = 0.0002). Unfortunately, data for isoprenaline with CGP20712A at 10−7 M did not survive QC check and was deleted from the data set. Isoprenaline with L748,337 at 10−6 M did not show significant displacement versus isoprenaline control curves (Table 1).
pBOO rat strips: a, b Agonist-induced inhibition of microcontractions (straight lines) and EFS contractions at 15 Hz (dashed lines). As for Fig. 5, curves for isoprenaline are indicated with open symbols and for mirabegron with filled symbols. Curves for agonists after pre-incubation with the β-AR antagonist L-748,337 10−6 M are depicted as open diamond or filled diamond and for CGP-20712A 10−6 M as inverted filled triangle
Data with β1 and β3-AR antagonists at pBOO bladder strips did not reveal significant differences over those obtained in normal control rat bladder strips.
Discussion
The current data set confirms the observations on the occurrence of microcontractions in bladder strips from normal rats and the capability for inhibition of this phenomenon by isoprenaline and mirabegron as shown earlier by our group (Gillespie et al. 2015). The present study was conducted to reveal the pharmacological identity of the β-AR subtype involved in inhibition of spontaneous MC to contribute to the over-arching question in this series of manuscripts as follow-up to our earlier work (Gillespie et al. 2012) on what phenomenon would be leading in the mode of action for the β3-AR agonist mirabegron in modulating bladder hyperactivity in animals as mimic for bladder overactivity in man. The implications of local bladder processes versus afferent-efferent modulation of the bladder in establishing the mode of action for mirabegron are discussed elsewhere (Eastham et al. 2015). The design of our studies was to perform β-AR agonist/antagonist experiments on MC activity under stable isometric conditions with frequency-response of EFS to check strip homogeneity and submaximal neurogenic contractions to compare the drug effects seen on MC without otherwise changing the baseline of the preparation as with direct-acting contractile cholinergic agonists or KCl. In addition, EFS stimulates neuronal release of mediators, and for similar stimulation conditions to our protocol, detrusor strips from normal rats are reported to be non-adrenergic but mainly cholinergic, with a smaller purinergic component (Rouget et al. 2014). For pBOO rat bladder strips subjected to 16 Hz EFS contractions, the cholinergic component was found to shift a little more towards a cholinergic dominance (Pinna et al. 2006). The non-adrenergic nature of EFS responses in rat detrusor is in agreement with histochemical findings (Watanabe and Yamamoto 1979) showing little, if any, adrenergic nerve terminals. The EFS frequency-contraction response data with our study protocol were consistent with those of Rouget et al. (Rouget et al. 2014), and inhibition of EFS by isoprenaline was comparable with data in mentioned paper. Our data indicate that the isoprenaline inhibition of EFS is likely a β1-AR-mediated response, since concentration-response curves were shifted by the β1-AR antagonist CGP-20712A at 10−7 M and not by 10−6 M of the β3-AR antagonist L-748,337. We assume that the low efficacy and potency of mirabegron for inhibition of EFS contraction could also be a β1-AR-mediated effect, since mirabegron is a selective β3-AR agonist with high potency and efficacy in CHO cells with rat transfected β3-ARs, but with a measurable but low potency and efficacy at transfected rat β1-ARs (Hatanaka et al. 2013). Data for another β-AR agonist, CL-316,243, a relatively selective and potent agonist at rat β3-AR (Woods et al. 2001), with a similar EFS protocol as we have used showed low-potency inhibitory responses at EFS in Rouget’s study. In addition, their pA2 for the β3-AR antagonist L-748,337 with CL-316,243 on EFS-stimulated rat detrusor was lower than that obtained with mirabegron and L-748,337 in rat bladder tissue (Van Wieringen et al. 2013). In our understanding, these observations can be interpreted as effects via the β1- rather than the β3-AR component of this drug.
Although the magnitude of spontaneous MC and EFS presents differently in the tracings of normal and pBOO bladders (Fig. 2a, b), expressing the data on a mg/tissue basis (pBOO rat bladders are usually three- or more-fold heavier than those for control) shows that there is a pronounced loss of response for EFS with pBOO conditions, but MC activity is similar for both conditions. While we did not test tetrodotoxin sensitivity of the EFS conditions we employed, to check the neurogenic nature of the contractile responses, our data for EFS in this respect confirm published data: A reduced neurogenic response following EFS in pBOO animal detrusors and in detrusor muscle strips from obstructed patients has been published widely, and this phenomenon is supposed to be due to a reduced cholinergic nerve density in obstructed urinary bladders, decreased mitochondrial function, and decreased ability of the sarcoplasmic reticulum to store and release Ca2+, which apparently are mechanisms extending over species (Sibley 1997; Levin et al. 1999). In spite of this, in our data set the efficacy of the β-adrenergic mechanism for inhibition of responses remained stable as seen by a similar pIC50 for isoprenaline at EFS in pBOO and control rat bladder strips. To our understanding, this indicates that the gross product of β-AR numbers activated and the sensitivity of the cellular transduction machinery that determines the efficacy of the inhibitory mechanism are unaltered for EFS in pBOO conditions. For MC apparently similar reasons apply, since pIC50 values for IPE were similar for normal and pBOO strips as well.
Isoprenaline was found to have >0.5 log unit higher potency and a higher efficacy for the inhibition of spontaneous MC than for neurogenic contractions induced by EFS at rat detrusor in our study (Table 1; Figs. 4, 5, and 6). Findings of higher potency for β-AR agonists to induce relaxation under “resting” tension compared to relaxation of agonist- or depolarisation-induced contractions is a well documented phenomenon in various smooth muscle tissues, including bladder (Michel and Sand 2009) and airways (Naline et al. 2007). Usually, reasons for such phenomena are related to difference in receptor reserve as discussed by Ruffolo (1982) and Gunst et al. (1989). Unfortunately, our data set does not allow in-depth discussion of this observation but suggests that since our observation of differences in potency for inhibition of β-AR are in the same tissue and with the same full agonist (isoprenaline) MC and EFS are two different systems with different sensitivity for β-AR modulation of rat bladder functions.
Like for EFS, inhibition of spontaneous MC at rat detrusor was not sensitive to blockade by the β2-AR antagonist ICI-118,551 and the β3-AR antagonist L-748,337. Based on the observations of a rightward shift of the concentration-response curve to isoprenaline by the β1-AR blocker CGP-20712A in both normal and pBOO strips, it seems plausible to assume that the inhibition of isoprenaline in both spontaneous MC and EFS contraction is mediated by the same β-AR, which is neither a β2 nor a β3-AR. In this study, the shift of the inhibition curve to isoprenaline in the presence of CGP-20712A was parallel, but the pK B value was low compared to the pK B value of 8.81 reported for CGP-20712A at human recombinant β1-AR (Baker 2005). Based on similar observations, Mallem et al. (2004) proposed a low affinity state of the β1-AR for studies in endothelium-denuded rat aortic rings and other rat tissues where CGP12177 was used as β-AR agonist and 10 μM of CGP20712A as the antagonist. A paper by the same group suggested that CGP20712A can reveal different affinity states of the β1-AR (Baker et al. 2003). In data by Granato et al. (2015), PGE2-augmented MC at rat bladder strips were found to be inhibited by isoprenaline via a β1-AR as concluded by a pK B value for the antagonism found for the selective β1-AR blocker metoprolol of 7.25, in line with literature data for the affinity of metoprolol for the β1-AR (Baker 2005). In addition, in the latter experiments also no shift for a β2- or a β3-AR antagonist on isoprenaline was found. Therefore, we assume that the molecular identity of the β-AR modulating MC in rat bladder is a β1-AR.
Our results indicate that spontaneous MC and neurogenic responses in rat bladder strips behave as different systems with a different sensitivity of β1-AR-mediated inhibition, which fundamentally remain similar under conditions of pBOO, a pathophysiological mimic of detrusor instability.
Further, it should be noted that in the context of the in vivo situation it is not likely that effects at spontaneous MC or at neurogenic detrusor stimulation play a role in the mode of action for mirabegron in vivo, since all effects for mirabegron in the present paper are low-potency β1-AR-mediated effects at high concentrations. Such concentrations are well over those affecting β3-AR-mediated bladder functions in a decerebrated perfused rat model (Sadananda et al. 2013).
Therefore, the site of action of mirabegron for its inhibition of non-voiding activity described in cystometric studies in conscious pBOO rats (Gillespie et al. 2012) is probably not by direct activation of β-adrenoceptors in rat detrusor, as previously hypothesized. Further studies on location of β3-AR and on functional β3-AR-mediated effects in the rat would be necessary to understand mirabegron’s mechanism of action on the urinary bladder function in vivo.
References
Andersson KE (2003) Storage and voiding symptoms: pathophysiologic aspects. Urology 62(Suppl 2):3–10
Baker JG (2005) The selectivity of β-adrenoceptor antagonists at the human β1, β2, and β3 adrenoceptors. Br J Pharmacol 144:317–322
Baker JG, Hall IP, Hill SJ (2003) Agonist actions of “β-blockers” provide evidence for two agonist activation sites or conformations of the human β1 adrenoceptor. Mol Pharmacol 63:1312–1321
Drake MJ, Harvey IJ, Gillespie JI (2003) Autonomous activity in the isolated Guinea pig bladder. Exp Physiol 88:19–30
Eastham J, Stephenson C, Gillespie JI (2015). The expression of β3 adrenoceptor and muscarinic type 3 receptor immuno-reactivity in the major pelvic ganglion of the rat and consequences for the mode of action of mirabegron.
Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y, Kobayashi M, Yamaguchi O (1999) Expression and possible functional role of the β3 adrenoceptor in human and rat detrusor muscle. J Urol 161:680–685
Gillespie JI, Palea S, Guilloteau V, Guerard M, Lluel P, Korstanje C (2012) Modulation of non-voiding activity by the muscarinergic antagonist tolterodine and the β(3)-adrenoceptor agonist mirabegron in conscious rats with partial outflow obstruction. BJU Int 110:E132–E142
Gillespie JI, Rouget C, Palea S, Granato C, Birder L, Korstanje C (2015). The characteristics of intrinsic complex microcontractile activity in isolated strips of the rat bladder. Naunyn-Schmiedeberg’s Arch Pharmacol. doi:10.1007/s00210-015-1131-4
Granato C, Korstanje C, Guilloteau V, Rouget C, Palea S, Gillespie JI (2015). Prostaglandin E2 excitatory effects on rat urinary bladder: a comparison between the β-adrenoceptors modulation of non-voiding activity in vivo and micro-contractile activity in vitro. Naunyn-Schmiedeberg’s Arch Pharmacol. doi:10.1007/s00210-015-1139-9
Gunst SJ, Stropp JQ, Flavahan NA (1989) Muscarinic receptor reserve and β-adrenergic sensitivity in tracheal smooth muscle. J Appl Physiol 67:1294–1298
Hatanaka T, Ukai M, Watanabe M, Someya A, Ohtake A, Suzuki M, Ueshima K, Sato S, Sasamata M (2013) In vitro and in vivo pharmacological profile of the selective β3 adrenoceptor agonist mirabegron in rats. Naunyn Schmiedeberg's Arch Pharmacol 386:247–253
Levin RM, Haugaard N, Mogavero L, Leggett RE, Das A (1999) Biochemical evaluation of obstructive bladder dysfunction in men secondary to BPH: a preliminary report. Urology 53:446–450
Lluel P, Duquenne C, Martin D (1998) Experimental bladder instability following bladder outlet obstruction in the female rat. J Urol 160:2253–2257
MacKay D (1978) How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated? J Pharm Pharmacol 30:312–313
Mallem MY, Toumaniantz G, Serpillon S, Gautier F, Gogny M, Desfontis JC, Gauthier C (2004) Impairment of the low-affinity state beta1-adrenoceptor-induced relaxation in spontaneously hypertensive rats. Br J Pharmacol 143:599–605
Michel MC, Sand C (2009) Effect of pre-contraction on β-adrenoceptor-mediated relaxation of rat urinary bladder. World J Urol 27:711–715
Michel MC, Ochodnicky P, Summers RJ (2010) Tissue functions mediated by beta3-adrenoceptors -findings and challenges. Naunyn-Schmiedeberg's Arch Pharmacol 382:103--108
Naline E, Trifilieff A, Fairhurst RA, Advenier C, Molimard M (2007) Effect of indacaterol, a novel long-acting β2-agonist, on isolated human bronchi. Eur Respir J 29:575–581
Pinna C, Sanvito P, Puglisi L (2006) Altered neurogenic and mechanical responses to acetylcholine, ATP and substance P in detrusor from rats with outlet obstruction. Life Sci 79:1301–1306
Rouget C, Rekik M, Camparo P, Botto H, Rischmann P, Lluel P, Palea S, Westfall TD (2014) Modulation of nerve-evoked contractions by β3-adrenoceptor agonism in human and rat isolated urinary bladder. Pharmacol Res 80:14–20
Ruffolo Jr RR (1982) Review: important concepts of receptor theory. J Auton Pharmacol 2:277–295
Sacco E, Bientinesi R (2012) Mirabegron: a review of recent data and its prospects in the management of overactive bladder. Ther Adv Urol 6:315–324
Sadananda P, Drake MJ, Paton JFR, Pickering AE (2013) A functional analysis of the influence of β3-adrenoceptors on the rat micturition cycle. J Pharmacol Exp Ther 347:506–515
Sibley GNA (1997) Developments in our understanding of detrusor instability. Br J Urol 80(Suppl 1):54–61
Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T, Sasamata M, Miyata K, Uchida H, Yamaguchi O (2007) Effect of (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther 321:642–647
Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T, Hatano A, Takahashi K, Nomura S (1999) Evidenxe for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther 288:1367--1373
Van Wieringen JP, Michel-Reher MB, Hatanaka T, Ueshima K, Michel MC (2013) The new radioligand [3H]-L 748,337 differentially labels human and rat β3-adrenoceptors. Eur J Pharmacol 720:124–130
Watanabe H, Yamamoto TY (1979) Autonomic innervations of the muscles in the wall of the bladder and proximal urethra of male rats. J Anat 128:873–886
Woods M, Carson N, Norton NW, Sheldon JH, Argentieri TM (2001). Efficacy of the β3-adrenoceptor agonist CL-316243 at experimental bladder hyperreflexia and detrusor instability in the rat. J Urol 166: 1142-1147.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gillespie, J..., Rouget, C., Palea, S. et al. Beta adrenergic modulation of spontaneous microcontractions and electrical field-stimulated contractions in isolated strips of rat urinary bladder from normal animals and animals with partial bladder outflow obstruction. Naunyn-Schmiedeberg's Arch Pharmacol 388, 719–726 (2015). https://doi.org/10.1007/s00210-015-1136-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1136-z