Abstract
Phenethyl isothiocyanate (PEITC) is a natural isothiocyanate with anticancer activity against many drug-resistant cancer cells. A body of evidence suggests that PEITC enhances oxidative stress leading to cancer cell death. Cholangiocarcinoma (CCA) is an aggressive bile duct cancer with resistance to chemotherapeutic drugs. PEITC rapidly kills KKU-100 CCA cells with concurrent induction of cellular glutathione depletion, superoxide formation, and loss of mitochondrial transmembrane potential. The loss was associated with increased Bax and decreased Bcl-xl proteins followed by the release of cytochrome c and the activation of caspase-9 and -3. Although TEMPOL could prevent superoxide formation, it did not prevent the disruption of glutathione (GSH) redox, mitochondrial dysfunction, and cell death. On the other hand, N-acetylcysteine could prevent the events and cell death. It was concluded that disruption of GSH redox but not superoxide formation may be an initial step leading to mitochondrial injury. PEITC could be a promising chemopreventive agent for CCA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isothiocyanates (ITCs) are hydrolysis products of a group of naturally occurring thioglucoside and glucosinolate compounds which are found in cruciferous vegetables and others including broccoli, cauliflower, cabbage, brassicas, watercress, and kale (Cheung and Kong 2010). The ITCs rather than their parent compounds, the glucosinolates, are highly active. The ITCs including phenethyl isothiocyanate (PEITC), sulforaphane, and allyl isothiocyanate are very effective antioxidants and potent suppressors of tumor growth (Hayes et al. 2008). The compounds possess dual effects of antioxidant activity and also generation oxidative stress. The antioxidant is demonstrated by the radical scavenging activity and indirectly by induction of the expression of antioxidant and cytoprotective enzymes, whereas the oxidative stress is generated via several cellular mechanisms (Cheung and Kong 2010). The antitumor activities in vitro and in vivo confer a collective activity so-called cancer chemoprevention (Keum et al. 2003; Khor et al. 2008; Xiao et al. 2010). Several epidemiological studies have suggested that dietary intake of cruciferous vegetables is inversely related to the development of cancer (Chan et al. 2005). PEITC, an isothiocyanate, found abundantly in cruciferous vegetables has been shown effectively inhibit the growth of cancers of prostate, colon, lung, and leukemic cells (Chiao et al. 2004; Cheung et al. 2008; Mi et al. 2011b). Clinical trials of PEITC on the treatment of lung cancer and leukemia are currently undertaken (www.clinicaltrials.gov).
The cancer chemopreventive effects of PEITC are expressed via several cell signaling pathways at multiple levels. The induction of reactive oxygen species (ROS) formation plays a pivotal role in oxidation of cellular redox-sensitive molecules and disruption of the mitochondrial function leading to the death of leukemic and prostate cancer cells (Xiao et al. 2010; Kluza et al. 2011). However, PEITC-mediated apoptosis of HepG2 or multiple myeloma cells was suggested to be independent of ROS formation (Rose et al. 2003; Mi et al. 2011a). It is interesting to note that PEITC mediates apoptotic cell death of various drug-resistant cancer cells including imatinib-, Gleevec-, and fludarabine-resistant leukemic cells and prostate cancer (Trachootham et al. 2008; Mukherjee et al. 2009; Kluza et al. 2011). Moreover, the effects of PEITC on multiple steps of cancer growth make this compound highly versatile and promising candidate for cancer chemoprevention and chemotherapy.
Bile duct cancer or cholangiocarcinoma (CCA) is a highly malignant adenocarcinoma originating from the cholangiocytes (Patel 2011). CCA is a rare type of cancer worldwide; however, CCA is the most common type of liver cancer in some regions in Asia, particularly the Mekong Basin subregion, where the incidence of CCA is highest in the world (Sripa et al. 2012). In the Western countries, the incidence and the mortality rate of intrahepatic CCA are reportedly increasing at an alarming rate (Khan et al. 2005; Patel 2011). The prognosis of CCA patients is very poor because most patients are already in the advanced stage at diagnosis. Chemotherapy and radiotherapy show modest benefit for prolonged overall survival in patients with unresected CCA (Hezel and Zhu 2008). Currently, there is no standard regimen of chemotherapy for CCA (Patel 2011). Despite of the many recent advances in cancer chemotherapy, management of CCA is still an immense challenge, and several strategies in sensitizing resistant CCA cells to respond to chemotherapeutic drugs have been attempted (Buranrat et al. 2010; Kongpetch et al. 2012).
The cancer chemopreventive effect of PEITC in CCA is still unknown. In this study, we examined the inhibitory effect of PEITC on KKU-100, a CCA cell line derived from human tumor tissue with poorly differentiated adenocarcinoma. To elucidate the possible mechanisms of the effects of PEITC on CCA cells, ROS formation, glutathione (GSH) redox stress, and mitochondrial function were evaluated for PEITC-treated CCA cells. The results show that the PEITC-mediated killing of CCA cells was not associated with the generation of superoxide anion. The disruption of GSH redox may be an important step eliciting mitochondrial dysfunction, a common pathway leading to cell death.
Materials and methods
Chemicals and reagents
PEITC, 4-hydroxy-TEMPO (TEMPOL), N-acetyl-l-cysteine (NAC), dihydroethidium (DHE), sulforhodamine B (SRB), and buthionine sulfoximine (BSO) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). 1-Methyl-2-vinylpyridinium triflate (M2VP) was obtained from Fluka Chemical (Buch, Switzerland). 5,5′,6,′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1) was obtained from Clayman Chemical (Ann Arbor, MI, USA). Caspase-9 substrate I (Ac-LEHD-AFC), granzyme B substrate II caspase 8 (Z-IETD-AFC), and glutathione monoethyl ester (GSH-MEE) were purchased from Calbiochem (EMD Millipore, Billerica, MA, USA). EnzCheck® Caspase-3 Assay kit#1 was purchased from Molecular Probes (Eugene, OR, USA). Antibodies used for immunoblotting were from Santa Cruz Biotechnology (Sandiego, CA, USA).
Cell cultures
A human CCA cell line, KKU-100 established in our institute (Sripa et al. 2005), were grown in Ham’s F12 medium supplemented with 4 mM L glutamine, 1 mM sodium pyruvate, 12.5 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES; pH 7.3), 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 10 % fetal calf serum and maintained under 5 % CO2 in air at 37 °C. The cells were subcultured every 2–3 days before cultured cell confluence using 0.25 % trypsin–EDTA, and the medium was changed after overnight incubation.
Cytotoxicity assay
CCA cells were seeded onto 96-well culture plates at a density of 7,500 cells/well. After an overnight culture, the serum-free medium was replaced with an addition of PEITC, and the cells were cultured for various time intervals. The cytotoxicity was examined by the SRB assay. In brief, cultured cells were fixed with ice-cold trichloroacetic acid and stained with 0.4 % SRB in 1 % acetic acid for 30 min. Excess dye was removed by rinsing several times with 1 % acetic acid, and protein-bound dye was dissolved with 10 mM Tris base solution for determination of absorbance with a microplate reader with filter wavelength of 570 nm.
To determine if cells have undergone apoptosis, cultured cells were stained with fluorescent dyes as previously described (Buranrat et al. 2010; Suphim et al. 2010). In brief, the cells were washed once with phosphate-buffered saline (PBS) and stained with acridine orange and ethidium bromide (AO/EB) in PBS with the trace amount of hemoglobin. The cells were examined using a Nikon Eclipse TS100 inverted microscope with the excitation and emission filters of 480 and 535 nm, respectively. The number of viable, apoptotic, and necrotic cells which were stained with green fluorescence with intact nuclei, green fluorescence with the appearance of cell shrinkage, nuclear condensation and fragmentation, and bright orange fluorescence, respectively, were enumerated. The apoptotic cells were calculated as the percent apoptotic cells over a total number of cells in the same area.
Assays for ROS and glutathione
Intracellular ROS generation was measured by cell-permeable fluorescent probe, DHE, staining, which is specific for superoxide anion. Briefly, 5 × 104 cells were seeded in 96 black well plates and cultured overnight. Then, the medium was removed, and the cells were washed with PBS and were treated with PEITC, 25 μM DHE with or without 2 mM NAC or 0.5 mM TEMPOL in serum-free medium and kept under an atmosphere of 5 % CO2 at 37 °C for 90 min. The fluorescence intensity was read and quantified in a Gemini XPS fluorescent plate reader with excitation and emission wavelength of 518 and 605 nm, respectively.
Glutathione was assayed by the method previously described (Suphim et al. 2010) using M2VP as a glutathione scavenger. In brief, cell lines were trypsinized and washed three times with cold PBS, and cell suspensions were reacted with M2VP (1 mM) for determination of GSSG. Another aliquot of cell suspension was used for the assay of total GSH. The cultured media were collected, centrifuged, and supernatants were saved for total GSH assay.
Measurement of mitochondrial transmembrane potential
The dissipation of the mitochondrial electrochemical potential gradient is known as an early event in apoptosis. The assay of mitochondrial transmembrane potential (∆Ψ m) was performed according to the previously described method (Kongpetch et al. 2012) using the cationic, lipophilic dye, JC-1 staining. The cells were seeded in 96 black well plates at the density of 1 × 104 cells/well and cultured overnight before treatment with PEITC at varied concentrations for 3 and 24 h. Images were captured by a fluorescence microscope with excitation wavelength of 485 nm and long-pass emission wavelength of 535 nm. JC-1 forms J-aggregates in healthy mitochondrial matrix, which can be visualized as red fluorescence. In depolarized mitochondria, JC-1 effluxes to the cytoplasm and exists as monomers with green fluorescence. The shift of red to green fluorescence is an indicative of depolarization of ∆Ψ m.
Western blot analysis
Whole cell lysates were prepared as described previously (Buranrat et al. 2010). The protein was electrophoretically separated on 10 % SDS–polyacrylamide gel and was transferred to polyvinylidene difluoride (PVDF) membranes by semidry blotting. The PVDF membranes were blocked with 5 % (w/v) skimmed milk powder in PBS and incubated overnight at 4 °C with primary antibodies including rabbit polyclonal IgG against cytochrome c (sc-13560), Bax (sc-493) and AIF (sc-5586), and mouse monoclonal IgG against Bcl-xl (sc-8392) and β-actin (sc-1616). The primary antibody was removed, and the blots were incubated with the respective horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse or anti-rabbit IgG). The blots were incubated with ECL substrate solution. The densities of bands of specific cytochrome c, Bcl-xl, Bax, AIF, and β-actin were visualized and captured by ImageQuant™ 400.
Measurements of caspase activities
After treatment with PEITC for 3 and 6 h, the cultured cells were trypsinized, washed in PBS, and adjusted to 106 cells for each reaction. Cell pellets were stored frozen at −80 °C for analysis at a later time. Cell pellet was lysed with the cell lysis buffer and incubated on ice for 10 min. After centrifugation to pellet the cellular debris, the supernatant was transferred to individual microplate wells and processed according to the manufacturers’ instructions using Ac-LEHD-AFC as caspase-9 substrate, Z-IETD-AFC as caspase-8 substrate, and Z-DEVD-AMC as caspase-3 substrate. The fluorescent signals were read using Gemini XPS fluorescent plate reader with the excitation and emission wavelengths of 400 and 505 nm, respectively, to assess caspase-9 and -8 activities, and 340 and 440 nm, respectively to caspase-3 activity.
Statistical analysis
All results were presented as the mean ± SEM. Comparison between control and treated group performed with student’s t test or ANOVA with student–Newman–Keuls test where appropriate. The level of significance was set at p < 0.05.
Results
PEITC induced cytotoxicity and depletion of GSH
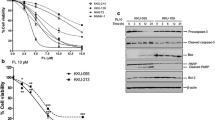
To examine the cytotoxic effect of PEITC, KKU-100 cells were exposed to various concentrations (0.3, 1, 3, 10, and 30 μM) of PEITC for 24 and 48 h (Fig. 1a). The viability of CCA cells reduced rapidly after exposure to PEITC in a dose–response manner and the percentage of cytotoxicity at 48 h was not significantly different from that at 24 h. The IC50 values were not different between 24 and 48 h of incubation (inset, Fig. 1a). Moreover, PEITC induced apoptosis very rapidly within the first 3 h and in a dose-dependent manner (Fig.1b). In contrast, PEITC induced only few number of necrotic cell death at every time points (data not shown).
PEITC induced cytotoxicity and GSH depletion. KKU-100 cells were incubated with various concentrations (0.3, 1, 3, 10, 30 μM) of PEITC for 24 and 48 h before the cytotoxicity of the cells was determined by SRB assay (a) and the number of apoptotic cells was assessed by AO/EB method at 24 h of incubation (b). GSH levels were measured in cell pellet as intracellular GSH (c) and in the medium as efflux from the cells. Each value represents the mean ± SEM of three experiments. Significantly different from controls, *p < 0.05
As cellular GSH is the major cellular redox buffer, the mechanism of PEITC-induced cytotoxicity was suggested to be associated with disturbance of GSH (Trachootham et al. 2008). GSH in KKU-100 cells was decreased rapidly within the first 3 h and continuously suppressed through 24 h of incubation (Fig. 1c). The efflux of total GSH from the cells into the medium was also determined to observe how oxidative stress was handled by CCA cells. Total GSH levels in the medium were increased with time of incubation and concordant with the decrease of cellular GSH levels (Fig. 1c).
Effects of antioxidants on PEITC-induced ROS formation and GSH depletion
As PEITC suppressed cell growth and induced cell death in association with oxidative stress manifested as GSH depletion, we evaluated whether the GSH redox stress was causally related with the formation of superoxide anion by using TEMPOL, a superoxide scavenging agent and NAC, a thiol antioxidant and redox reagent. Treatment of 3 or 10 μM of PEITC rapidly induced an increase of fluorescent signals of superoxide formation within 90 min (Fig. 2a). Co-treatment of the cells with PEITC and 0.5 mM TEMPOL or 2 mM NAC almost completely suppressed PEITC-induced ROS formation in the cells. Treatment with NAC or TEMPOL alone did not change the basal ROS levels when compared with controls. GSH in the cells was determined after concurrent treatment of PEITC with TEMPOL or NAC. The PEITC (10 μM) suppressed the cellular GSH after incubation for 3 h. It was only NAC that could completely stabilize GSH levels when cells were treated with PEITC (Fig. 2b). The treatment of TEMPOL could not protect losses of cellular GSH and even induced loss at low concentration of PEITC.
Effect of antioxidants on superoxide formation and GSH levels KKU-100 cells were incubated with PEITC at indicated concentrations [3 and 10 μM, shown as P3 and P10] with or without TEMPOL (TEM; 0.5 mM) or N-acetyl-L-cysteine (NAC; 2 mM) for 90 min. Superoxide formation was quantified by dihydroethidium fluorescent method (a). The effects of TEMPOL and NAC on PEITC-induced reduction of cellular GSH were determined after incubation for 3 h (b). Each bar represents the mean ± SEM of three experiments. Significantly different from controls, *p < 0.05 and significantly different from PEITC treatment, †p < 0.05
Protective effect of TEMPOL and NAC on PEITC-induced cytotoxicity
To evaluate whether the cytotoxicity of PEITC was associated with the superoxide and GSH redox stress, KKU-100 cells were concurrently treated with PEITC and TEMPOL or NAC. The presence of TEMPOL could not suppress the cytotoxic effects of PEITC at any incubation periods, i.e., 3, 24, and 48 h. Rather, the concurrent treatment with TEMPOL tended to exacerbate the cytotoxic effect of PEITC at 24–48 h of incubation (Fig. 3, upper panel). On the other hand, NAC significantly prevented the cytotoxicity of PEITC (Fig. 3, upper panel) in association with the protection of cellular GSH (Fig. 2b).
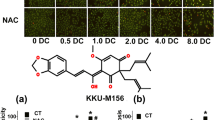
Effects of antioxidants on cytotoxicity and changes in mitochondrial transmembrane potential KKU-100 cells were incubated with PEITC at indicated concentrations [P3 and P10 (μM)] with or without TEMPOL (TEM) (0.5 mM) or NAC (2 mM) for 3, 24, and 48 h. The cytotoxicity was assayed by SRB method (bar graph in upper panel). Each bar represents the mean ± SEM of three experiments. Significantly different from control, *p < 0.05 and significantly different from PEITC-treated group, †p < 0.05. The ∆Ψ m was assessed using JC-1 staining method after the cells were treated with PEITC for 24 h. Fluorescent images were captured by a fluorescence microscope. The experiment was done twice with similar results. The representative images from one experiment are shown (images in lower panel)
PEITC-induced depolarization of mitochondrial transmembrane potential
To elucidate the underlying mechanisms of PEITC-induced cytotoxicity in relation to oxidative stress, the effect of PEITC treatment on mitochondrial function was investigated. The mitochondrial transmembrane potential (∆Ψ m) was determined by JC-1 assay in cells treated with PEITC for 3 and 24 h. In control cells, mitochondria predominantly exhibited red fluorescence indicating the intact ∆Ψ m (Fig. 3a, lower panel). PEITC rapidly depolarized ∆Ψ m as revealed by green fluorescence staining. The depolarization of ∆Ψ m was apparent within the first 3 h of PEITC treatment and persisted up to 24 h. When cells were concurrently treated with PEITC and antioxidants, NAC but not TEMPOL showed protective effect on the ∆Ψ m (Fig. 3c, d). Treatment with TEMPOL or NAC alone did not affect ∆Ψ m (data not shown). The changes in ∆Ψ m were correlated well with the cytotoxicity of PEITC.
Effects of GSH replenishment and GSH depletion
Since there was a relationship between GSH depletion and cell death, we further evaluated whether depletion of GSH by PEITC was an important cause of cell death. For this purpose, the cultured cells were treated with BSO (0.5 mM), an inhibitor of GSH synthesis. Treatment with BSO caused a rapid depletion of cellular GSH (data not shown) but did not induce any cytotoxic effect after incubation for 24 h (Fig. 4a). However, BSO strongly potentiated PEITC-induced cytotoxicity. The result indicates that GSH depletion alone is insufficient to cause cell death. To demonstrate if the protective effect of NAC is related to GSH redox regulation, the cells were treated with GSH-MEE, a cell-permeable GSH concurrent with high concentration of PEITC to cause massive cell death. Treatment with GSH-MEE could almost completely prevent cell death (Fig. 4b).
Effects of GSH replenishment and GSH depletion on PEITC-induced cytotoxicity. Cultured cells of KKU-100 cells were treated with combination of PEITC and buthionine sulfoximine (BSO) for 24 h (a) or PEITC with glutathione monoethyl ester (GSH-MEE) (b). Analysis of cell death was performed by fluorescent staining method. Each bar represents the mean ± SEM of three experiments. Significantly different from controls, *p < 0.05
Effects of PEITC on cytochrome c, Bcl-2 proteins, and caspase activity
The involvement of mitochondria in PEITC-induced cytotoxicity was examined further. After PEITC treatment for 3 and 6 h, the remarkable release of cytochrome c was observed (Fig. 5, left panel) along with the increases in AIF and Bax levels, while Bcl-xl expression was suppressed.
PEITC induced the release of cytochrome c and Bcl2 proteins, and activation of caspases KKU-100 cells were treated with PEITC (10 μM) for 3 and 6 h. The cytosol protein was analyzed for cytochrome c, Bax, Bcl-xl, and AIF by Western immunoblotting using β-actin as loading control. Values beneath images indicated the relative protein intensity as a ratio of the treatment to the corresponding vehicle-treated control. Each experiment was done at least twice with similar results, and the representative bands from one experiment are shown (images at left panel). The total cell lysates were assayed for caspase activities using specific fluorogenic substrates (bar graph at right panel), caspase-9 (a), caspase-8 (b), and caspase-3 (c). Each bar represents the mean ± SEM of three experiments. Significantly different from corresponding controls, *p < 0.05
Along with the cytochrome c release, the activation of caspases was observed. PEITC treatment activated caspase-9 and -3, but not caspase 8, in KKU-100 cells (Fig. 5, right panel).
Discussion
PEITC is a natural compound of isothiocyanate group. It has been advocated as a chemopreventive agent for leukemia, pancreatic, prostate, and lung cancers (Mi et al. 2007; Hayes et al. 2008; Trachootham et al. 2008; Xiao et al. 2010). The mechanism of actions of PEITC is multifaceted, including ROS formation, interacting with redox signaling and mitochondrial proteins, leading to oxidative stress and mitochondrial damage (Cheung et al. 2008; Kluza et al. 2011; Mi et al. 2011b). Although CCA is a malignant tumor highly resistant to chemotherapy (Eckel and Schmid 2007; Hezel and Zhu 2008), PEITC potently inhibited CCA cell growth and induced apoptosis. The ROS was generated by PEITC, but it does not seem to play a critical role in the induction of cell death. On the other hand, GSH depletion and oxidative stress seem to increase cellular redox stress and alter expression of Bcl-xl and Bax protein expressions with subsequently initiating an intrinsic mitochondrial cell death pathway leading to the release of cytochorome c, activation of caspase enzymes, and ensuing cell death.
PEITC-induced GSH depletion in association with cell death is consistently observed in various tumor cells (Lee et al. 2002; Hayes et al. 2008; Trachootham et al. 2008). GSH depletion might be caused by the increased ROS formation. Alternatively, GSH depletion by PEITC may be caused by rapid adduction of GSH. The PEITC adducts efflux from the cells via multidrug resistance-associated protein 2 (MRP2), whereas PEITC could reversibly be hydrolyzed and retaken up into the cells to generate a wasting cycle of GSH (Hayes et al. 2008). This was consistent with the observed rapid increase of GSH efflux into the medium. However, GSH depletion itself could not induce cell death, as BSO, which could deplete GSH by large, did not induce cell death. However, depletion of GSH may sensitize the cells to the other concurrent effect of PEITC leading to cell death. This is consistent with our results that NAC and GSH-MEE can almost completely abolish cytotoxicity of PEITC. Because NAC is not an efficient radical scavenging agent (Parasassi et al. 2010), it serves as a precursor for GSH synthesis. More importantly, GSH and NAC have been shown to play critical roles in modulating a number of redox-sensitive enzymes, where these proteins involve in cellular signaling for survival and cell death (Lee et al. 2012). It is probable that PEITC may exert multiple effects probably via redox stress in induction of cell death.
In these studies, TEMPOL, a superoxide scavenger (Krishna et al. 1996), abolished superoxide anion without cytoprotective effect. The role of ROS in PEITC-induced cell death may be cell type-specific because PEITC caused cell death of chronic lymphocytic and chronic myelogenous leukemic cells in a ROS-dependent manner (Trachootham et al. 2008; Xiao et al. 2010). On the other hand, PEITC and its electrophilic adducts may cause oxidative stress by interacting with an active cysteine thiol moiety of vital cellular molecules, particularly redox regulating and signaling regulating proteins such as thioredoxin, 14-3-3 protein, and HSP where those proteins are ultimately involved in the regulation of cell death (Mi et al. 2011b). Our results suggested a mechanistic dissociation between superoxide production and cellular redox disturbance, in the regulation of cell death.
Cellular GSH redox plays important roles in the complex process of apoptosis involving with Bcl2 family proteins where Bcl2 proteins modulate the mitochondrial permeability transition (MPT) and the activity of mitochondrial complexes (Xiao et al. 2010). The mode of action of PEITC to CCA cells, which is common to some tumor cells, may be an induction of mitochondrial damage (Xiao et al. 2010; Kluza et al. 2011). The mitochondrial dysfunction is evidenced by the loss of ∆Ψ m which is closely related to the cytotoxic effect of PEITC. The MPT pore is a protein pore that is formed in the inner membrane of mitochondria under certain stimuli. The opening of the MPT pores leads to the loss of proton gradient across the inner membrane rendering mitochondria into a depolarized state while increasing the number of MPT pores leads to apoptosis and necrosis (Kim et al. 2003). As NAC prevented the loss of ∆Ψ m and protected cell death, this suggests that redox stress may be an early event leading to mitochondrial damage, whereas NAC may alleviate the redox stress.
In the present study, the release of cytochrome c and AIF via mitochondrial outer membrane paralleled with the altered levels of Bax and Bcl-xl. Bax is a multidomain proapoptotic Bcl2 family protein which forms the mitochondrial outer membrane permeabilization (MOMP) upon activation (Chipuk and Green 2008). The release of cytochrome c resulted in the activation of caspase enzymes in KKU-100 cells. AIF, a caspase-independent effector of apoptosis, is located in the inner membrane of mitochondria and released upon induction of apoptosis (Norberg et al. 2010). AIF is activated by oxidative modifications and proteolysis. The release of AIF from mitochondria is regulated by Bcl2 proteins including Bcl-xl and Bax to form MOMP (Norberg et al. 2010). It is noted that, caspase-8 activity remained unchanged, suggesting that the induction of apoptosis of KKU-100 cells by PEITC may be primarily mediated through the intrinsic mitochondrial pathway.
In summary, PEITC induced ROS-independent oxidative stress, GSH depletion, and alteration of Bcl2 proteins leading to mitochondrial dysfunction and cell death. The exact mechanism of alteration of GSH affecting the changes in Bcl2 proteins remains to be elucidated. PEITC is a useful chemopreventive agent where it provides multiple modes of actions to inhibit CCA cells.
Abbreviations
- BSO:
-
Buthionine sulfoximine
- TEMPOL:
-
4-hydroxy-TEMPO
- CCA:
-
Cholangiocarcinoma
- PEITC:
-
Phenethyl isothiocyanate
- GSH:
-
Glutathione
- GSSG:
-
Glutathione disulfide
- ∆Ψ m :
-
Mitochondrial transmembrane potential
- NAC:
-
N-acetyl-L-cysteine
- ROS:
-
Reactive oxygen species
- SRB:
-
Sulforhodamine B
- JC-1:
-
5,5′,6,′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide
- MOMP:
-
Mitochondrial outer membrane permeabilization
References
Buranrat B, Prawan A, Kukongviriyapan U, Kongpetch S, Kukongviriyapan V (2010) Dicoumarol enhances gemcitabine-induced cytotoxicity in high NQO1-expressing cholangiocarcinoma cells. World J Gastroenterol 16:2362–2370
Chan JM, Wang F, Holly EA (2005) Vegetable and fruit intake and pancreatic cancer in a population-based case–control study in the San Francisco bay area. Cancer Epidemiol Biomarkers Prev 14:2093–2097
Cheung KL, Khor TO, Yu S, Kong AN (2008) PEITC induces G1 cell cycle arrest on HT-29 cells through the activation of p38 MAPK signaling pathway. AAPS J 10:277–281
Cheung KL, Kong AN (2010) Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J 12:87–97
Chiao JW, Wu H, Ramaswamy G, Conaway CC, Chung FL, Wang L, Liu D (2004) Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis 25:1403–1408
Chipuk JE, Green DR (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18:157–164
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902
Hayes JD, Kelleher MO, Eggleston IM (2008) The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr 47(Suppl 2):73–88
Hezel AF, Zhu AX (2008) Systemic therapy for biliary tract cancers. Oncologist 13:415–423
Keum YS, Owuor ED, Kim BR, Hu R, Kong AN (2003) Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC). Pharm Res 20:1351–1356
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD (2005) Cholangiocarcinoma. Lancet 366:1303–1314
Khor TO, Cheung WK, Prawan A, Reddy BS, Kong AN (2008) Chemoprevention of familial adenomatous polyposis in Apc(Min/+) mice by phenethyl isothiocyanate (PEITC). Mol Carcinog 47:321–325
Kim JS, He L, Lemasters JJ (2003) Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304:463–470
Kluza J, Jendoubi M, Ballot C, Dammak A, Jonneaux A, Idziorek T, Joha S, Dauphin V, Malet-Martino M, Balayssac S, Maboudou P, Briand G, Formstecher P, Quesnel B, Marchetti P (2011) Exploiting mitochondrial dysfunction for effective elimination of imatinib-resistant leukemic cells. PLoS One 6:e21924
Kongpetch S, Kukongviriyapan V, Prawan A, Senggunprai L, Kukongviriyapan U, Buranrat B (2012) Crucial role of heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. PLoS One 7:e34994
Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A (1996) Do nitroxide antioxidants act as scavengers of O2-. or as SOD mimics? J Biol Chem 271:26026–26031
Lee S, Kim WH, Jung HY, Yang MH, Kang GH (2002) Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol 161:1015–1022
Lee Y, Kim YJ, Choi YJ, Lee JW, Lee S, Chung HW (2012) Enhancement of cisplatin cytotoxicity by benzyl isothiocyanate in HL-60 cells. Food Chem Toxicol 50:2397–2406
Mi L, Gan N, Chung FL (2011a) Isothiocyanates inhibit proteasome activity and proliferation of multiple myeloma cells. Carcinogenesis 32:216–223
Mi L, Hood BL, Stewart NA, Xiao Z, Govind S, Wang X, Conrads TP, Veenstra TD, Chung FL (2011b) Identification of potential protein targets of isothiocyanates by proteomics. Chem Res Toxicol 24:1735–1743
Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung FL (2007) The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res 67:6409–6416
Mukherjee S, Bhattacharya RK, Roy M (2009) Targeting protein kinase C (PKC) and telomerase by phenethyl isothiocyanate (PEITC) sensitizes PC-3 cells towards chemotherapeutic drug-induced apoptosis. J Environ Pathol Toxicol Oncol 28:269–282
Norberg E, Gogvadze V, Vakifahmetoglu H, Orrenius S, Zhivotovsky B (2010) Oxidative modification sensitizes mitochondrial apoptosis-inducing factor to calpain-mediated processing. Free Radic Biol Med 48:791–797
Parasassi T, Brunelli R, Costa G, De Spirito M, Krasnowska E, Lundeberg T, Pittaluga E, Ursini F (2010) Thiol redox transitions in cell signaling: a lesson from N-acetylcysteine. ScientificWorldJournal 10:1192–1202
Patel T (2011) Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol 8:189–200
Rose P, Whiteman M, Huang SH, Halliwell B, Ong CN (2003) beta-Phenylethyl isothiocyanate-mediated apoptosis in hepatoma HepG2 cells. Cell Mol Life Sci 60:1489–1503
Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A (2012) The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends Parasitol 28:395–407
Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, Sripa C, Miwa M (2005) Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol 11:3392–3397
Suphim B, Prawan A, Kukongviriyapan U, Kongpetch S, Buranrat B, Kukongviriyapan V (2010) Redox modulation and human bile duct cancer inhibition by curcumin. Food Chem Toxicol 48:2265–2272
Trachootham D, Zhang H, Zhang W, Feng L, Du M, Zhou Y, Chen Z, Pelicano H, Plunkett W, Wierda WG, Keating MJ, Huang P (2008) Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood 112:1912–1922
Xiao D, Powolny AA, Moura MB, Kelley EE, Bommareddy A, Kim SH, Hahm ER, Normolle D, Van Houten B, Singh SV (2010) Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem 285:26558–26569
Acknowledgments
This work was supported by the Thailand Research Fund (BRG5480011), the Office of the Higher Education Commission through SHeP-GMS of Khon Kaen University, grant-in-aid from Faculty of Medicine, Khon Kaen University, and a scholarship from the Office of the Higher Education Commission to Ornanong Tusskorn. Valuable suggestions on the manuscript of Prof. Yukifumi Nawa of Faculty of Medicine, Khon Kaen University are gratefully acknowledged.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tusskorn, O., Prawan, A., Senggunprai, L. et al. Phenethyl isothiocyanate induces apoptosis of cholangiocarcinoma cells through interruption of glutathione and mitochondrial pathway. Naunyn-Schmiedeberg's Arch Pharmacol 386, 1009–1016 (2013). https://doi.org/10.1007/s00210-013-0906-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-013-0906-8