Abstract
Siberian ginseng (SG) has been widely and historically consumed as a health food product for the improvement of self well-being, but whether vascular relaxation may contribute to such a therapeutic health effect has not been studied. We therefore investigated the vasorelaxant effect of the aqueous extract of the roots of SG (Eleutherococcus senticosus Maxim) using several in vitro vascular rings prepared from dog carotid artery, rat aorta and rat mesenteric artery. SG extract (0.04–0.8 mg/ml) caused concentration-dependent relaxation in dog carotid arterial rings pre-contracted with 100 μM phenylephrine (PE), and the relaxation was primarily endothelium-dependent. Treatment with 100 μM L-NOARG (a nitric oxide synthase inhibitor) either prevented or totally reverted SG-induced relaxation, suggesting that the endothelium-dependent relaxation was mediated by NO. Similar endothelium-dependent vascular relaxant responses were also obtained with rat aortic and mesenteric arterial rings, except that it occurred over a relatively higher concentration range of SG (0.5–2.0 mg/ml). When tested in the presence of 300 μM L-NAME, the vasorelaxant effect of SG was inhibited totally in rat aorta but only partially in rat mesenteric artery. The relaxation to SG that was insensitive to L-NAME in rat mesenteric arterial rings was eliminated when the rings (both proximal and distal ends) were pre-treated with a combination of 300 μM L-NAME and 15 mM KCl indicating the involvement of endothelium-derived hyperpolarizing factor (EDHF). This vasorelaxant response of the SG extract was inhibited partially by atropine (1 μM), completely by TEA (5 mM), but not by indomethacin (1 μM) or propranolol (10 μM). SG up to 2 mg/ml had no effect on KCl-induced contraction in any of the vascular rings studied. When compared with carbachol-induced (CCh) relaxation, SG resembles CCh in that the sensitivity to L-NAME inhibition is dependent on vascular size, i.e. aorta >proximal end of mesenteric artery >distal end of mesenteric artery. However, SG exhibited different potencies to relaxation while CCh showed similar potency (EC50 of about 0.2 μM) in all three vascular segments. In conclusion, we have demonstrated that the vascular effect of SG is endothelium-dependent and mediated by NO and/or EDHF depending on the vessel size. Other vasorelaxation pathways, such as inhibition of K+-channels and activation of muscarinic receptors, may also be involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The medicinal herb, Siberian ginseng (Eleutherococcus senticosus Maxim or Acanthopanax senticosus Harms), is botanically different from the Korean ginseng (Panax ginseng) and North American ginseng (Panax quinquefolia), but the healing power of its roots is very similar to the root of the other two types of ginseng and has therefore been traditionally used in China as a ginseng-substitute for centuries because of its relatively lower cost. In fact, its medicinal value lies in its anti-fatigue effect following strenuous exercise and was claimed to be more superior to that of the ginseng (Hoffman 1994). Siberian ginseng (SG) was named so, because it was naturally grown in Siberia of Russia and the earliest studies (1955–1965) of its health effects were made extensively by Russian scientists (Baranov 1982). Acanthopanax senticosus is also widely grown in northern China and Hokkaido, Japan. In China it is commonly referred to as “Ciwujia” because of its thorny stem (Ci means thorny and wujia means five-pedal leaf; i.e. Acanthopanax in Chinese), whereas in Japan, it is referred to as “Ezoukogi” in Japanese (Nishibe and Chie 1998; Deyama et al. 2001).
According to ancient Chinese medical writings (reviewed in Hu 1996), Ciwujia root is traditionally used in China to fight against ailments due to stagnation of blood circulation and other body fluids and help clear excessive fluid cumulated in the body (as in edema). These ailments range from indigestion, urinary stagnation, general fatigue, arthritis, mild hyperglycemia to hypertension. Russian scientists reported that Siberian ginseng (SG) improves red blood cell production and perfusion of tissues, and thus efficient oxygen delivery and consumption (reviewed in Baranov 1982). These effects of SG on circulation noted by people in different cultures conceivably would contribute collectively to its well known anti-fatigue effect, which has also been confirmed in animal studies of physical endurance by scientists in Hokkaido (Nishibe and Chie 1998; Deyama et al. 2001). Accordingly, SG has been widely used by athletes and people involved in stressful activities. Despite its wide and huge global consumption of SG as a health food, reports on its pharmacologic profile on vascular system are scanty. PubMed search indicated that the general study of SG represents a very small portion (<1%) of the large body of studies on ginseng.
Although the health effect of SG, like that of the Panax ginseng or Panax notoginseng may be attributed to a general improvement of body circulation (reviewed in Hu 1996), probably via vasodilatory effect elicited by its active ingredients (Guan et al. 1988; Kang et al. 1995), direct in vitro studies of SG or its extracts on vascular function are so far not available. On the other hand, in both Panax ginseng and Panax notoginseng, the total glycosidic saponins (also referred to as ginsenosides) are the major active substances known to cause vascular relaxation by endothelium-dependent (Gillis et al. 1997; Kang et al., 1995) and endothelium–independent (Guan et al. 1988; Kwan 1999) mechanisms, respectively. Siberian ginseng, however, does not contain these vasodilatory ginsenosides (Deyama et al. 2001) and a direct vasodilatory effect of SG has not been experimentally demonstrated despite historical claims insinuating vasodilatation in exerting its health effect. Therefore, the primary purpose of this communication is to test the hypothesis that SG extract, the aqueous extract of Eleutherococcus senticosus Maxim can induce in vitro vascular relaxation in vessels precontracted with phenylephrine and that this relaxation is a generalized effect occurring in different vascular beds. Furthermore, we would like to determine the mechanistic nature of the vascular relaxation as to whether it is endothelium-dependent or acting directly on vascular smooth muscle and the possible relaxation factors responsible for the relaxation induced by the SG extract.
Materials and methods
Tissue preparation
Male Sprague-Dawley rats weighing 250–300 g were anesthetized with i.p. injection of 50 mg/kg sodium pentobarbital and killed by bleeding from the carotid artery. The thoracic aorta and the superior mesenteric artery were immediately isolated and cut into rings. In some studies, carotid arterial rings from 20–25 kg Mongrel dogs of either sex were also used. Dogs were sacrificed by over-dose of pentobarbital under the standard university guidelines for the use of experimental animals (Wilson et al. 2002). Each ring was about 3 mm wide and suspended in organ bath between two parallel stainless steel hooks. One hook was fixed, while the other was connected to a force transducer for the isometric tension. The organ bath contained 4 ml Krebs’ solution at 37°C, bubbled with 95% O2 and 5% CO2 to give a pH of 7.3–7.4. The Krebs’ solution contained the following composition (in mM): NaCl 115.5; KCl 4.6; MgSO4 1.2; NaH2PO4 1.2; CaCl2 2.5; NaHCO3 22.0; D-glucose 11.1. The rings were stretched progressively to an optimal basal tension of 1.5 g for rat mesenteric artery (Kwan et al. 2004), 2.0 g for rat aorta (Shen at al. 2003) and 3.0 g for dog carotid artery (Wilson et al. 2002) and then allowed to equilibrate for at least 90 min, during which time the bath solution was replaced with pre-warmed and oxygenated Krebs’ solution every 15 min. Each experiment began with repeated contraction of the rings induced by 60–80 mM KCl till the plateau of contractions remained within 15% between two consecutive contractions. After that, they were rinsed with pre-warmed and oxygenated Krebs’ solution several times until muscle tension returned to the basal level. Some of the rings were incubated with 200 μg/ml Sigma saponins for 5–6 min to remove the endothelium. The presence or lack of functional endothelium was examined by demonstrating the presence or absence of relaxation induced by carbachol (CCh; 3 μM) in vascular rings precontracted with phenylephrine (PE, 1 μM for rat aorta and mesenteric artery and 100 μM for dog carotid artery). Other specific experimental conditions are described in the corresponding figure legends.
Measurement of vascular relaxation
We followed previously reported experimental protocol (Zheng et al. 1995; Kwan et al. 2003). After PE induced a sustained contraction at its optimal concentration (1 μM for rat aorta; 100 μM for dog carotid artery), Siberian ginseng extract (SG) was added cumulatively to induce a concentration-dependent relaxation in rings with or without functional endothelium. Some tissues were exposed to either 100 μM L-Nitro-N-arginine (L-NOARG) or 300 μM Nw-nitro-L-arginine methyl ester (L-NAME) for 20 min prior to application of PE in order to blunt the endothelial function by inhibiting the nitric oxide synthase (NOS). The reversibility of the relaxant effect of SG was tested by measuring the response to PE after repeated wash-out and subsequent equilibration for about 45 min. The relaxation to SG or CCh could be repeated indicating excellent reversibility and that the endothelial function was not compromised by SG. When investigating endothelium-dependent events, 3 μM CCh-induced relaxation were performed on PE-contracted vascular rings prior to the study with SG extracts. Those with relaxation >60% (in >80% of the tissues) were selected as endothelium-intact tissues. When required, some of these tissues, together with those showing <60% relaxation were treated with saponins to render the tissues completely denuded of endothelium (and subsequently confirmed by the lack of CCh-induced relaxation). In experiments using only endothelium-intact tissues, those tissues with <60% relaxation were excluded from the experiment.
Data analysis
Relaxant responses are given as percentage relaxation relative to the plateau-contraction induced by PE. Data were shown as mean ± SEM of n experimental animals. Statistical significance was estimated by Student’s t-test for unpaired observation or ANOVA test for concentration-response curve. A p-value of less than 0.05 was regarded to be significant.
Materials
The crude aqueous extract and purified components of Siberian ginseng were generous gifts from Mr. Hidetoshi Takashima, President of Yakuhan Pharmaceutical Co. Ltd. (Hokkido, Japan). The extract of Siberian Ginseng was produced from wild plants grown in Hokkaido, Japan, which was taxonomically authenticated by experts in Yakuhan Pharmaceutical Co., Hokkaido, Japan. The bark of the stem 2–3 cm in diameter was collected in September/October at Rikubetsu, Hokkaido. Dry powder of the stem bark was made by first extracted with hot water and the condensed water extract was spray-dried through flows of hot air (a spray-dry process similar to manufacturing instant coffee powder). The content of known main compounds in extract is as follows (analyzed by HPLC method; per 100 g powder): isofraxidin, 56.28 mg; chlorogenic acid, 922.71 mg; isofraxidin monoglucoside, 197.11 mg; syringaresinol di-O-glucoside, 942.11 mg; syringin, 803.77 mg. isofraxidin and syringaresinol di-O-glucoside are used as marker compound for standardization of the extract (Deyama et al. 2001). Aqueous extracts were dissolved in distilled water.
L-phenylephrine hydrochloride, saponins, Nw-nitro-L-arginine methyl ester hydrochloride (L-NAME), L-Nitro-N-arginine (L-NOARG), carbachol (CCh), TEA, atropine and sodium nitroprusside (SNP), products of Aldrich-Sigma Chemical Co. (Oakville, ON, Canada) were dissolved in double-distilled water.
Results
Effect of SG on the PE contraction of dog carotid artery
Our first observation of SG-induced relaxation was made in dog carotid arterial rings when we were studying the vascular effect of the papaya seed extracts (Wilson et al. 2002). Figure 1 shows the experimental protocols to investigate the effect of cumulative addition of SG extract to the endothelium-intact (+E) dog carotid artery rings precontracted with 100 μM PE. A complete relaxation (to 10–15% PE contraction, comparable to the similar level of relaxation induced by 3 μM CCh) was achieved at about 0.8 mg/ml SG (with a ED50 value of 0.25 mM). Subsequent addition of 1 μM sodium nitroprusside (SNP, which causes non-endothelium-dependent relaxation) did not cause further relaxation. In the absence of intact endothelium (−E), SG practically did not induce significant relaxation in PE-contracted rings while subsequent addition of SNP (1 μM) caused 85–90% relaxation. Thus, the relaxation induced by SG is practically entirely endothelium-dependent. Following the relaxation of the +E carotid arterial rings with 0.2 mg/ml SG, the subsequent addition of 100 μM L-NOARG, a nitric oxide synthase (NOS) inhibitor, inhibited the relaxation and reverted it to full contraction in the presence of PE. The inhibitory action of L-NOARG on endothelial NOS is also confirmed in 3 μM CCh-induced relaxation in separate +E rings. Similarly, preincubation of the +E vascular rings with 100 μM L-NOARG 20 min prior to the addition of PE totally prevented the relaxation upon subsequent introduction of SG. The quantitative data are shown in Fig. 2.
The responses to the aqueous extract of Siberian ginseng (SG; in μg/ml) of endothelium-intact (+E) dog carotid arterial rings precontracted with 100 μM phenylephrine (PE). Top: concentration-dependent relaxation to SG. Middle: reversal of SG-induced (left) or CCh-induced (3 μM; right) relaxation by 100 μM L-NOARG. Bottom: lack of concentration-dependent relaxation to SG after removal of endothelium, but sodium nitroprusside (SNP; 1 μM) still totally relaxed the vessel. Tracings are representative of four similar experiments
Data summary of results shown in Fig. 1. Concentration-dependent relaxation to Siberian Ginseng extract of endothelium-intact dog carotid arterial rings precontracted with 100 μM PE and its reversal by 100 μM L-NOARG. The level of plateau contraction at 100 μM PE before the edition of SG or vehicle was taken as 100%. *Significantly different from the vehicle control values (p<0.05). n number of dogs
Cumulative addition of SG up to 1 mg/ml had no effect at all on the basal resting tension in the absence of any stimulant. It also did not affect the contraction of carotid artery induced by 80 mM KCl (data not shown). We also showed unaltered contractile response to PE as well as a full relaxation to CCh in the same vascular rings, which were previously relaxed with SG and subsequently washed. These results further suggest that treatment of the dog carotid arterial rings with SG was fully reversible and had no damaging effects on the contractile function.
Effect of SG on the PE contraction of rat aorta
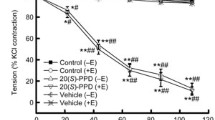
To examine whether the endothelium-dependent vasorelaxation induced by SG is a unique character for dog carotid artery or a general property applicable to other vascular tissues of different species or different vascular segments, we also performed similar experiments using rat arteries of different sizes, including aorta and mesenteric artery. Figure 3 shows that in rat aortic rings precontracted with 1 μM PE, SG caused a concentration-dependent relaxation and removal of endothelium following 5 min incubation of the vascular rings with 0.2 mg/ml saponins resulted in complete inhibition of SG-induced relaxation, suggesting that, in rat aorta like in the dog carotid artery, the relaxation induced by Siberian ginseng extract is primarily endothelium-dependent as well. In addition, inclusion of 300 μM L-NAME in the bath containing the endothelium-intact aortic rings totally inhibited SG-induced relaxation as in the case for dog carotid artery with L-NOARG, indicating that SG-induced relaxation can be accounted for mostly by the action of NO released from the endothelium. Different from dog carotid artery, rat aorta appeared to require higher concentration of SG to achieve the same degree of relaxation.
Concentration-dependent effect of Siberian Ginseng extract on 1 μM PE-induced contraction in rat aorta with or without (E−) intact endothelium. L-NAME (300 μM) practically abolished the entire endothelium-dependent relaxant effect of SG at all concentrations. *Significantly different from control values (p<0.05). n number of rats
Next, we explored the possible mechanisms of the SG extract responsible for the endothelium-dependent relaxant effect. Figure 4 shows that endothelium-dependent relaxation induced by SG is like that induced by CCh. Atropine at 1 μM strongly inhibited CCh-induced relaxation and almost completely restored PE contraction, but only partly and variably inhibited (15–40% in three separate experiments) relaxation induced by 2 mg/ml SG. On the other hand, in lieu of atropine, addition of a relatively nonselective K+-channel blocker TEA (5 mM) completely inhibited the relaxation induced by CCh or SG and restored the PE contraction. We have in addition observed that addition of 1 μM indomethacin or 10 μM propranolol did not affect the contraction induced by either 3 μM CCh or 2 mg/ml SG (Data not shown). This suggests that SG does not act through activating COX-1 pathway or β-adrenoceptors. Like in dog carotid artery, SG up to 2 mg/ml had no effect on KCl-induced contraction in rat aorta (data not shown).
The middle tracings show that SG-induced relaxation of PE-precontracted rat aorta (endothelium intact) was totally reversed by 5 mM tetraethylammonium chloride (TEA), but only partially reversed by 1 μM atropine (Atr). The top tracings are positive control experiment with the use of CCh-induced relaxation and the bottom tracings are the time control. Addition at the plateau PE contraction of 1 μM Atr had no effect, whereas addition of 5 mM TEA enhanced the plateau PE contraction by about 15–25%. These tracings are representative of three similar experiments
In view of the small inhibitory effect of atropine on SG-induced relaxation, we speculate that SG may elicit partial agonistic action at the muscarinic receptor as CCh. In so doing, SG may be acting, in part, at the same binding site as CCh on the endothelial cells and therefore a concentration-response curve against CCh was constructed in the presence and absence of 2 mg/ml SG. Figure 5 shows that although SG indeed inhibited the maximal relaxation to CCh, the apparent IC50 for CCh remained unaltered at 0.2–0.3 μM. This inhibitory profile of CCh-induced relaxation in the presence of 2 mg/ml SG was not in parallel with that of the control in the absence of SG, representing a lack of effect on the ED50 value for CCh. Since the concentration–relaxation curves for CCh in SG-treated and control vascular rings were carried out using different initial tension (SG at 2 mg/ml inhibits by 40–50% of the PE contraction), we also performed CCh-relaxation by inhibiting the PE contraction by about 40% with 0.1 or 0.3 µM nicardipine (acting via inhibition of smooth muscle L-type Ca2+-channels, which are not present in the endothelial cells). The CCh inhibition profile was identical to that of control vessels without nicardipine treatment and had an ED50 value of about 0.2 μM (result not shown in Fig. 5). Thus, the observed results with SG were independent from the initial tension and reaffirm that SG acts, at least in part, as a partial agonist at the muscarinic receptor site.
Effect of SG on the PE contraction of rat mesenteric artery end segments
While rat aorta is technically easier to study, smaller muscular artery such as mesenteric artery is physiologically more relevant in the regulation of blood pressure. We further extended the study to include the proximal and distal ends of the superior mesenteric artery of the rat. SG up to 2 mg/ml had no effect on the contraction induced by 80 mM KCl in these vascular rings whether or not the endothelium was intact (not shown). However, in these mesenteric arterial rings precontracted with 1 μM PE, SG caused a concentration-dependent relaxation, and removal of endothelium following 5 min incubation of the vascular rings with 200 μg/ml saponins resulted in almost complete inhibition of SG-induced relaxation (Fig. 6). These results confirms that, like in the dog carotid artery and rat aorta, the relaxation induced by SG extract in rat mesenteric artery is primarily endothelium-dependent as well.
Concentration-dependent effect of Siberian Ginseng extract on 1 μM PE-induced contraction in the proximal and distal segments of the rat superior mesenteric artery with (control) or without (E−) intact endothelium. L-NAME (300 μM) was used to inhibit the nitric oxide synthase whereas additional 15 mM KCl was included to block the release of endothelium-derived hyperpolarizing factor (EDHF). Note the more prominent L-NAME-insensitive component of the endothelium-dependent relaxation induced by SG in the distal segment (compared to the proximal segment) of the superior mesenteric artery. *Significantly different from the control values (p<0.05). n number of rats
Although prior incubation with 300 μM L-NAME of the distal and proximal segments of rat mesenteric artery with intact endothelium did inhibit the endothelium-dependent relaxation induced by SG in PE-precontracted rings, unlike in rat aorta the inhibition was incomplete despite the use of a very high L-NAME concentration (300 μM). We also noted that the degree of inhibition of SG-induced relaxation by L-NAME varied between these two end segments of the rat superior mesenteric artery; being less in the smaller distal end compared to the proximal end. We speculate that the relaxation to SG which is insensitive to L-NAME may involve other endothelium-derived relaxing factors (EDRF), such as endothelium-derived hyperpolarizing factor (EDHF), which appears to play a more prominent role in smaller muscular vessels (Shimokawa et al. 1996). Although the identification of EDHF still remains as a highly disputable issue (Busse et al. 2002), the hyperpolarizing effect of EDHF nevertheless can be antagonized by a small elevation of extracellular K+ concentration (Busse et al. 2002). We took advantage of such an intervention to test the presence of EDHF, which may be responsible for the L-NAME insensitive component of the relaxation. Figure 6 shows that as the blood vessel becomes smaller from thoracic aorta down to the proximal and distal segments of the superior mesenteric artery, the L-NAME sensitive component of the relaxation to SG becomes progressively smaller. The ED50 values are 0.75 mg/ml and 1.0 mg/ml for the proximal and distal segments of the mesenteric artery, respectively as compared to 1.75 mg/ml for aorta. Fig. 6 further shows that preincubation of the endothelium-intact vascular rings with a combination of 300 μM L-NAME and 15 mM KCl, which caused little or no contraction on its own, further eliminated the L-NAME-insensitive component of SG-induced relaxation resulting in almost full restoration of PE-induced contraction in both end segments in the presence of SG.
Effects of CCh on PE contraction of rat aorta and rat mesenteric artery end segments
Stimulation of vascular endothelial muscarinic receptors with CCh caused not only the release of NO, but also the release of other EDRFs including EDHF, especially in smaller muscular vessels (Shimokawa et al. 1996). We therefore compared the endothelium-dependent relaxation induced by SG to that induced by CCh in order to determine the extent of similarity borne by rat aorta and the proximal end and distal end of the rat superior mesenteric artery. Figure 7 clearly shows that CCh caused almost full relaxation with a similar ED50 value of 0.2 μM. Also, like the SG-induced relaxation, CCh-induced relaxation showed different sensitivities to inhibition by L-NAME (component due to NO release) in rat aorta and mesenteric artery; and the L-NAME resistant relaxation could be further inhibited in the presence of 15 mM KCl (component due to EDHF). Although qualitatively similar to SG-induced relaxation shown in Fig. 6, the difference of relaxation in the presence of L-NAME between the proximal and distal segments of mesenteric artery as shown in Fig. 7 is relatively small, and reached statistical significance only at 0.1 μM CCh.
Concentration-dependent effect of CCH on 1 μM PE-induced contraction in the rat aorta and proximal and distal segments of the rat superior mesenteric artery with (control) or without (E−) intact endothelium. L-NAME (300 μM) was used to inhibit the nitric oxide synthase whereas additional 15 mM KCl was included to block the release of EDHF. Note the endothelium-dependent relaxation to CCh in rat aorta was totally blocked by L-NAME (e.g., dependent on NO). As the vessels become smaller from the proximal end to the distal end of the superior mesenteric artery, L-NAME-insensitive component (dependent on EDHF) of the endothelium-dependent relaxation induced by CCh became more prominent. *Significantly different from the control values (p<0.05). n number of rats
Discussion
To our knowledge, this work represents the first report on the vascular effects of Siberian ginseng (SG) extract. The present novel findings of the direct in vitro vasodilatory properties of SG on various vasculatures has confirmed our hypothesis and provided a sensible scientific basis for its historical health/medical use. Furthermore, we have determined the functional nature of the relaxation being of endothelial origin. In addition, we also provided novel information on the mechanistic nature of the endothelium-dependent relaxation induced by SG being NO-mediated in large arteries and EDHF as well as NO in smaller arteries.
Siberian ginseng’s vasodilatory effect is due to its direct action on endothelium, not on smooth muscle
Eleutherococcus senticosus (and also Acanthopanaxs senticosus) had long been referred to as Siberian ginseng, because they were used as health food or folk medicine in a similar way as ginseng roots. Interestingly, the endothelium-dependent NO-mediated relaxation property of SG has also been reported for Korean ginseng extract as well as the ginsenosides purified from it (Kang et al. 1995). However, not all ginsenosides caused endothelium-dependent vasorelaxation; for example, ginsenosides from Panax notoginseng can cause direct relaxation of the vascular smooth muscle (Guan et al. 1988) whereas ginsenosides of Panax quinquefolia do not and may even potentiate contraction (Kwan 1999). Furthermore, SG roots do not contain ginsenosides and the active ingredient(s) that caused endothelium-dependent vasorelaxation remains elusive. Nevertheless, SG root contains a large quantity of lignans (Deyama et al. 2001), a class of plant polyphenols present in high quantity in fruit, bark and vegetables, some of which have been reported to induce endothelium-dependent, NO-mediated vasorelaxation (reviewed in Achike and Kwan 2003). We and others have isolated some major lignans constituents, such as chlorogenic acid, syringin, (+)-syruinarensinol di-O-β-D-glucopyranoside, isofraxidin 7-O-β-D-glucopyranoside and isofraxidin, from Siberian ginseng (reviewed in Deyama et al. 2001). Our preliminary findings (not shown) indicated that at the same concentration, 2 mg/ml, these lignans showed no effects on the vascular contractile function, except for Isofraxidin, which yielded less than 30% of the relaxation produced by the SG extract. This suggests that either the active ingredient(s) in SG is not of lignan origin or it may be a minor unidentified constituent. Alternatively, the maximal relaxant effect of SG may result from synergism of multiple constituents rather than a single molecular entity. These possibilities would have to be taken into consideration in dealing with the identification of the active ingredients in any herbal medicine and represent a major undertaking in its own right.
Siberian ginseng’s action on vascular endothelium involves multiple mechanisms which vary in different vessels
Recently, we have also reported that rat aorta elicited endothelium-dependent, NO-mediated vasorelaxation in response to the aqueous extract of the leave and bark of eucommia ulmoides Oliv., an antihypertensive Chinese herbal medicine that is also highly enriched in lignans (Kwan et al. 2004). However, different from the SG extract which showed some muscarinic activity, the eucommia extract induced endothelium-dependent, NO-mediated relaxation that was not inhibited by 1 μM atropine, but, like the SG extract, the vasorelaxant effect was totally inhibited by the K+-channel blocker, TEA. Thus, different plant lignans may act via different pathways or mechanisms leading to the release of NO from endothelial cells (Achike and Kwan 2003). It is interesting to note that the relaxant responses to CCh in all three rat vascular segments showed a consistent EC50 value of 0.2 μM (Fig. 7), whereas those to the SG extract varied depending on the vascular preparations. The EC50 values ranged from 0.25 mg/ml for dog carotid artery (Fig. 2), and 0.75 mg/ml and 1.0 mg/ml for the proximal end of rat mesenteric artery (Fig. 6), respectively, to >1.75 mg/ml (Fig. 5) for rat aorta (Fig. 3). This supports the contention that the relaxation induced by the SG extract may involve multiple pathways including activation of K+-channels and, to a lesser degree, stimulation of the muscarinic receptor, more likely due to the multiple active ingredients (with respect to vascular relaxation) in the extract.
Another novel aspect of this study is that the SG extract can cause the release of not only endothelium-derived NO, but in addition EDHF from smaller muscular arteries. This may in part account for the greater relaxation caused by the Siberian ginseng extract seen in rat mesenteric arteries (80% at 2 mg/ml) compared to rat aorta (55% at 2 mg/ml). Since smaller muscular arteries play a more dominant role in the regulation of blood pressure and organ perfusion, the greater vasodilatory effect of SG at the site of small muscular arteries bears better relevance to its purported traditional use for increasing the blood flow as a result of reduced vascular resistance.
Cautions and limitations of this in vitro study
Owing to the limiting nature of the in vitro experimental approach employed in this work, one should exercise caution in extrapolating our experimental findings to in vivo clinical situations. First, this study is not performed on human vascular tissue, but on vascular tissues from experimental animals as research tools. In this connection, our preliminary study using radial arteries isolated from senior patients receiving coronary artery by-pass graft has also revealed endothelium-dependent relaxation by 2 mg/ml SG (data not shown). Second, as is usually the case in the study of therapeutically effective herbal extracts, the high concentration of SG required to produce a maximal acute in vitro relaxant effect may dampen its physiological significance. On the other hand, one should also realize that unlike the use of potent synthetic drugs for their acute therapeutic effects, many herbal medicinal drugs are said to take effect over a longer period of time; indeed, they are taken within their original cultural context for preventive measure rather than for therapeutic purpose. Therefore, on the basis of the finding from this acute in vitro effects, one should be open to the possibility that SG may require a relatively longer conditioning period to elicit its in vivo health effect as compared to its acute in vitro cellular effects over a very short exposure as seen in this work. Third, it is also unknown whether the SG administered (usually orally in the form of food or as powers in capsules) would reach the concentration needed for the in vitro study. Furthermore, it is possible that in vivo biotransformation could result in more potent vasoactive metabolites. However, resolution of the above limitations in terms of relevance of clinical application would require a totally different methodological approach, which is beyond the scope of our current study. Nonetheless, our findings in this study indeed provide a sound basis and inspiration towards those resolutions and warrant future investigations.
References
Achike FI, Kwan CY (2003) Nitric oxide, human diseases and the herbal products that affect the nitric oxide signaling pathway. Clin Exp Pharmacol Physiol 30:605–615
Baranov AL (1982) Medical uses of ginseng and related plants in the Soviet Union: recent trends in the Soviet literature. J Ethnopharmacol 6:339–353
Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH (2002) EDHF: bringing the concepts together. Trends Pharmacol Sci 23:374–380
Deyama T, Nishibe S, Nakazawa Y (2001) Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin 22:1057–1070
Gillis CN (1997) Ginseng pharmacology: a nitric oxide link? Biochem Pharmacol 54:1–8
Guan YY, Kwan CY, Daniel EE (1988) Inhibition of norepinephrine-induced contractile responses of canine mesenteric artery by plant total saponins. Blood Vessels 25:312–315
Hoffman D (1994) The herbalist. In Multimedia CD-Rom (version 2) Hopkins Technology, LLC Hopkins, MN
Hu XM (1996) National regulatory authority for Chinese medicinal drugs (Chinese). Ciwujia. In: Zhong Hua Ben Cao (Compendium of Chinese medicinal herbs), vol 1. Shanghai Science and Technology, Shanghai
Kang SY, Schini-Kerth VB, Kim ND (1995) Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in rat aorta. Life Sci 56:1577–1586
Kwan CY (1999) The effects of different ginseng extracts on vascular contraction in vitro: evidence for Yin-Yang principle. Acta Phytother 2:73–77
Kwan CY, Zhang WB, Kwan TK, Sakai Y (2003) In vitro relaxation of vascular smooth muscle by atropine: involvement of K+-channels and endothelium. Naunyn-Schmiedebergs Arch Pharmacol 368:1–9
Kwan CY, Zhang WB, Deyama T, Nishibe S (2004) Endothelium-dependent vascular relaxation induced by Eucommia ulmoides Oliv. bark extract is mediated by NO and EDHF in small blood vessels. Naunyn-Schmiedebergs Arch Pharmacol 369:206–211
Nishibe S, Chie FC (1998) The power of Siberian ginseng [in Japanese]. Mainiji Shinbon Sha, Tokyo
Shen JZ, Zheng XF, Wei EQ, Kwan CY (2003) Green tea catechins evoke a phasic contraction in rat aorta via H2O2-mediated multiple signaling pathways. Clin Exp Pharmacol Physiol 30:88–95
Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A (1996) The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxation in rat mesenteric circulation. J Cardiovasc Pharmacol 28:703–711
Wilson RK, Kwan TK, Kwan CY, Sorger GJ (2002) Effects of papaya seed extract and benzyl isothiocyanate on vascular contraction. Life Sci 71:497–507
Zheng XF, Kwan CY, Daniel EE (1993) Cyclopiazonic acid causes endothelium-dependent relaxation in rat aorta. Acta Pharmacol Sin 14:21–26
Acknowledgements
This work was supported by a seeding grant from McMaster University. S.M.S. is on sabbatical leave from the Department of Pharmacology, University of Malaya, Kuala Lumpur, Malaysia. We acknowledge the excellent technical assistance of Ms. J. Miller, who was supported by a summer research fellowship award from the Institute of Aboriginal People’s Health, CIHR, Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwan, CY., Zhang, WB., Sim, SM. et al. Vascular effects of Siberian ginseng (Eleutherococcus senticosus): endothelium-dependent NO- and EDHF-mediated relaxation depending on vessel size. Naunyn-Schmiedeberg's Arch Pharmacol 369, 473–480 (2004). https://doi.org/10.1007/s00210-004-0927-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-004-0927-4