Abstract
The occurrence of idiosyncratic drug-induced liver injury (IDILI) is a leading cause of post-marketing safety warnings and withdrawals of drugs. Carbamazepine (CBZ), widely used as an antiepileptic agent, could cause rare but severe idiosyncratic liver injury in humans. Although recent studies have shown that inflammasome is implicated in CBZ-induced hepatocellular injury in vitro, the precise pathogenesis of hepatotoxicity remains largely unexplored. Here we report that CBZ causes idiosyncratic liver injury through promoting specific stimuli-induced NLRP3 inflammasome activation. CBZ (40 μM) enhances NLRP3 inflammasome activation triggered by adenosine triphosphate (ATP) or nigericin, rather than SiO2, monosodium urate crystal or intracellular lipopolysaccharide (LPS). In addition, CBZ has no effect on NLRC4 or AIM2 inflammasome activation. Mechanistically, synergistic induction of mitochondrial reactive oxygen species (mtROS) is a crucial event in the enhancement effect of CBZ on ATP- or nigericin-induced NLRP3 inflammasome activation. Moreover, the “C=C” on the seven-membered ring and “C=O” on the nitrogen of CBZ may be contribute to NLRP3 inflammasome hyperactivation and hepatotoxicity. Notably, in vivo data indicate that CBZ (50 mg/kg) causes liver injury in an LPS (2 mg/kg)-mediated susceptibility mouse model of IDILI, accompanied by an increase in caspase-1 activity and IL-1β production, whereas the combination of CBZ and LPS does not exhibit the effect in NLRP3-knockout mice. In conclusion, CBZ specifically promotes ATP- or nigericin-induced NLRP3 inflammasome activation and causes idiosyncratic liver injury. Our findings also suggest that CBZ may be avoided in patients with NLRP3 inflammasome activation-related diseases that are triggered by ATP or nigericin, which may be risk factors for IDILI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiosyncratic drug-induced liver injury (IDILI), which develops unpredictably and independently of drug dose, or route or duration of administration (Bjornsson 2015, 2016; Fontana 2014), is a leading cause of failures in drug development and the main reason for post-marketing safety warnings and withdrawals of drugs (Chalasani et al. 2014; Garcia-Cortes et al. 2018; Holt and Ju 2006). Identifying potential drugs and individuals at risk for IDILI remains challenging and increasing evidences have indicated that most cases of IDILI are immune mediated (Adams et al. 2010; Ju and Reilly 2012). Therefore, various immune-related hypotheses, such as hapten and danger hypotheses, have been developed to elucidate the mechanism of IDILI (Cho and Uetrecht 2017). Consistent with these hypotheses, immunosurveillance checkpoint inhibitors have also been successfully used to develop an animal model of IDILI, which has been used to reveal the mechanism of liver injury induced by amodiaquine, nevirapine and isoniazid (Mak and Uetrecht 2015; Metushi et al. 2015). Although it is still controversial, non-hepatotoxic doses of lipopolysaccharide (LPS) evoke mild concurrent inflammation in animals that may mimic the specific susceptibility factors or conditions under which human IDILI occurs. Numerous studies have demonstrated that IDILI can be replicated in animals through co-exposure to non-hepatotoxic doses of LPS and drugs with the ability to induce IDILI, such as trovafloxacin, amiodarone, monocrotaline and diclofenac (Deng et al. 2006; Hammad et al. 2011; Lu et al. 2013; Shaw et al. 2009).
Recent studies have shown that excessive activation of NLRP3 inflammasome may be an important mechanism to induce immune responses that lead to liver diseases in some patients (Mridha et al. 2017; Wree et al. 2014). NLRP3 inflammasome is a cytoplasmic multiprotein complex typically composed of three components (a sensor molecule NLRP3, an adaptor protein ASC, and an effector molecule pro-caspase-1) that can be activated by double activation signal: priming and activation. The recognition of LPS by toll-like receptor 4 will result in the activation of NF-κB and up-regulation of pro-IL-1β and NLRP3 protein expression, which is called the priming phase. In the step of activation, upon stimulation by a broad spectrum of stimuli, including adenosine triphosphate (ATP), nigericin, monosodium urate crystal (MSU), and SiO2, NLRP3 inflammasome complexes are assembled to process the cleavage of pro-caspase-1, and active caspase-1 subsequently functions to result in pyroptosis and cleavage of the proinflammatory cytokines pro-IL-1β and pro-IL-18 into their bioactive forms, IL-1β and IL-18, to amplify the inflammatory response via positive feedback (He et al. 2016).
NLRP3 inflammasome acts as an important sensor for DAMPs and PAMPs. Current studies suggest that THP-1 cells incubated with the supernatant of hepatocyte with amodiaquine, nevirapine and carbamazepine (CBZ), the drugs with the ability to induce IDILI, could lead to release of IL-1β and an increase in caspase-1 activity in vitro (Kato et al. 2019; Kato and Uetrecht 2017; Weston and Uetrecht 2014). However, whether NLRP3 inflammasome is involved in the occurrence of IDILI remains unclear. In this study, we demonstrated that CBZ facilitates specific stimulants induced NLRP3 inflammasome activation and causes idiosyncratic liver injury.
Materials and methods
Mice
Female C57BL/6 mice (6–8 weeks old) were purchased from SPF Biotechnology Co., Ltd (Beijing, China). NLRP3-knockout (NLRP3−/−) mice were kindly provided by Dr. Tao Li from National Center of Biomedical Analysis and backcrossed to C57BL/6 at least ten times for the current project. All animals were allowed unrestricted access to food and water for the duration of the experiment except during fasting tests and maintained under 12-h light/dark conditions at 22–24 °C. All experimental procedures in this study were performed according to the guidelines of laboratory animals care and use and approved by the animal ethics committee of the Fifth Medical Centre, Chinese PLA General Hospital (Beijing, China).
Cell culture
Bone-marrow-derived macrophages (BMDMs) were isolated from femoral bone marrow of 10-week-old female wild type or NLRP3−/− C57BL/6 mice and cultured in Dulbecco’s modified Eagle’s medium (DMEM) complemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 50 ng/mL murine macrophage colony-stimulating factor. Human THP-1 cells were grown in RPMI 1640 medium and stimulated by 100 nmol/L PMA overnight to differentiate into macrophages. DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin was used to culture HEK-293T. All cell lines were cultured under a humidified 5% (v/v) CO2 atmosphere at 37 °C.
Inflammasome activation
To induce inflammasomes activation, BMDMs at 5 × 106 cells/ml and PMA-primed THP-1 cells at 1.5 × 106 cells/ml were seeded in 24-well plates overnight. Then, the medium was replaced the following day, and the cells were stimulated with 50 ng/mL LPS or 1000 ng/ml Pam3CSK4 for 4 h. After that, the medium was changed to Opti-MEM containing CBZ (TargetMol, Shanghai, China) for 1 h and then were used for inflammasome stimulation as previously reported (Wang et al. 2019).
Western blotting
Protein extraction method of cell culture supernatant and immunoblot has been described previously (Wang et al. 2019).
Caspase-1 activity assay
The caspase-1 activity assay has been described previously (Wang et al. 2019).
Enzyme-linked immunosorbent assay
Supernatants from cell culture and mouse serum were assayed for mouse IL-1β, TNF-α, and human IL-1β, TNF-α according to manufacturer’s instructions (Dakewei, Beijing, China).
Alanine aminotransferase (ALT) and aspartate Transaminase (AST)
Serum and cultured supernatants ALT and AST were determined using the commercially available assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Lactate dehydrogenase (LDH) assay
LPS-primed BMDMs were treated with inflammasome stimulants in the presence of CBZ. The release of LDH into the culture supernatants was determined by LDH cytotoxicity assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions.
ASC oligomerization assay
The assay for ASC oligomerization has been described previously (Song et al. 2017).
Intracellular potassium detection
BMDMs were plated overnight in 6-well plates and then primed with 50 ng/ml LPS for 4 h. After that, cells were treated with CBZ for 1 h and then stimulated with different stimuli. The assay for intracellular potassium has been described previously (Huang et al. 2018).
Immunoprecipitation assay
For the exogenous NLRP3 interaction assay, HEK-293T cells (3 × 105 cells/ml) were transfected with plasmids (Flag-NLRP3, Myc-NLRP3) in 6-well plates via Lipofectamine 2000. 12 h later, the medium was changed to DMEM containing CBZ. After 24 h, cells were collected and lysed with NP-40 lysis buffer with protease inhibitor. Extracts were immunoprecipitated with anti-Flag antibody and beads and then were assessed by immunoblot analysis.
Confocal microscopy
Confocal microscopy analysis, which is carried out to test mitochondrial damage, has been described previously (He et al. 2018).
Mitochondrial reactive oxygen species assay
BMDMs were put onto 100 mm diameter culture dish tubes and primed with LPS (50 ng/ml) for 4 h. Then, cells were detached and transferred into 1.5 ml tubes for 1 h CBZ treatment. After that, cells were stimulated with ATP, nigericin or SiO2, after which the cells were washed twice with Hank’s balanced salt solution (HBSS). For mitochondrial reactive oxygen species (mtROS) measurement, BMDMs were loaded with 4 μM MitoSOX red mitochondrial superoxide indicator (Invitrogen) (Ex/Em: 510/580 nm) for 20 min and washed twice with HBSS. After staining and washing, cells were resuspended in HBSS and flow cytometry were conducted to test mtROS.
Assessment of the effects of LPS/CBZ cotreatment-induced DILI in vivo
Female mice might be a better model than male mice to study DILI (Chalasani and Bjornsson 2010). Therefore, female WT and NLRP3−/− C57BL/6 mice fasted for 24 h were given LPS (2 mg/kg) or its saline vehicle, iv via a tail vein. 2 h later, CBZ (50 mg/kg) or sterile phosphate buffered saline vehicle was administered through intraperitoneal injection. At 6 h after CBZ administration, mice serum and a fraction of liver samples were collected, and a portion of each excised liver was fixed in 10% formalin neutral buffer solution and used for immunohistochemical staining. The degree of liver injury was assessed by histopathological staining with hematoxylin and eosin (H&E), TUNEL and the serum IL-1β, TNF-α, ALT and AST levels. Moreover, liver homogenate was used to detect the activity of caspase-1 and secretion of IL-1β after normalization processing of BCA protein quantification kit (Solarbio, Beijing, China) according to the manufacturer’s instructions.
Statistical analyses
Statistical analysis was performed using the software Prism 6 (GraphPad Software, San Diego, CA, US). All experimental data were expressed as mean ± Standard Error of Mean (SEM). The significant differences were assessed using an unpaired Student’s t test in two groups and one-way ANOVA in multiple groups. The differences were considered statistically significant when P < 0.05.
Results
Numerous drugs with the ability to induce IDILI increase ATP-induced NLRP3 inflammasome activation
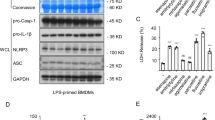
To elucidate the relationship between IDILI and NLRP3 inflammasome, seven drugs with the ability to induce IDILI (ticlopidine, flucloxacillin, amodiaquine, carbamazepine (CBZ), isoniazid, nevirapine and dimethyl fumarate) were chosen for testing (Nicoletti et al. 2017; Real et al. 2019). LPS pretreatment provides the first signal for NLRP3 inflammasome activation by promoting the expression of pro-IL-1β and NLRP3, and then NLRP3 inflammasome is activated in response to stimuli, such as ATP, nigericin or SiO2. Whether these drugs could serve as stimuli to provoke the activation of inflammasome is unknown. Hence, we treated LPS-primed BMDMs with the seven drugs for 6 h. The results showed that amodiaquine could induce NLRP3 inflammasome activation when LPS was involved in the priming phase (Fig. 1a, b), which is consistent with the results of Jack Uetrecht (Kato and Uetrecht 2017). Moreover, when LPS-primed BMDMs were pretreated with these drugs before ATP challenge, we found that amodiaquine, CBZ, isoniazid and nevirapine significantly enhanced ATP-induced caspase-1 activation and IL-1β production (Fig. 1c–e). On the other hand, the production of TNF-α, a cytokine that is inflammasome independent, was not affected by these drugs (Fig. 1f). Among them, CBZ showed the most potent effect on NLRP3 inflammasome activation, and we focused on investigating the influence of CBZ on NLRP3 inflammasome activation and its role in liver injury.
Effect of the drugs that cause idiosyncratic liver injury on NLRP3 inflammasome activation. a, b LPS-primed BMDMs were treated with drugs that cause idiosyncratic liver injury (40 μM) for 6 h. Caspase-1 activity (a) and western blot analysis of IL-1β (p17), caspase-1 (p20) (b) in culture supernatants (SN). c LPS-primed BMDMs were treated with these drugs (40 μM) and then stimulated with ATP. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in whole cell lysates (WCL). Caspase-1 activity (d), secretion of IL-1β (e), TNF-α (f) in SN described in (c). RLUs, the relative light units. Data are expressed as mean ± SEM (n = 3) from three independent experiments with biological duplicates in a, d–f. Statistics differences were analyzed using one-way ANOVA: ***P < 0.001 vs. the control group (a), *P < 0.05, **P < 0.01, ***P < 0.001 vs. the LPS plus ATP group (d–f)
CBZ specifically strengthens NLRP3 inflammasome activation triggered by ATP and nigericin
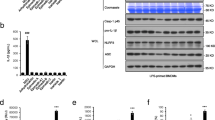
Next, we further evaluated the synergistic effect of CBZ, ATP and LPS on NLRP3 inflammasome activation. Results showed that BMDMs treated with ATP, CBZ or their combination did not release active caspase-1 and IL-1β in the absence of priming. CBZ could not induce NLRP3 inflammasome activation in LPS-primed BMDMs but did increase ATP-induced NLRP3 inflammasome activation in LPS-primed BMDMs (Fig. 2a, Supplementary Fig. 1a). In the following experiment, we assessed the dose–effect relationship of CBZ on ATP-induced NLRP3 inflammasome activation. Results showed that treatment of LPS-primed BMDMs with CBZ enhanced caspase-1 maturation and IL-1β secretion in response to ATP in a dose-dependent manner, but it had no effect on TNF-α production (Fig. 2b, Supplementary Fig. 1b). Moreover, CBZ increased ATP-induced caspase-1 cleavage and IL-1β release in wild-type (WT) BMDMs but not in NLRP3−/− BMDMs (Supplementary Fig. 1c–g). Meanwhile, caspase-1 activation can also directly induce a distinct form of programmed cell death called pyroptosis, accompanied by the release of lactate dehydrogenase (LDH), which is a widely used marker in cytotoxicity studies. In our study, CBZ enhanced ATP-induced LDH release in WT BMDMs (Supplementary Fig. 1f), which reflects caspase-1-dependent pyroptosis. To illuminate the direct hepatotoxicity of CBZ, we assessed the cell viability in BMDMs (Supplementary Fig. 2a), and the cultured supernatant ALT and AST levels in L02 cells (Supplementary Fig. 2b, c). Results showed that CBZ alone does not cause cell death in BMDMs and L02 cells.
Carbamazepine (CBZ) promotes NLRP3 inflammasome activation triggered by ATP and nigericin. a BMDMs or LPS-primed BMDMs were treated with ATP, CBZ, or ATP plus CBZ. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. Caspase-1 activity, secretion of IL-1β were detected in SN. b LPS-primed BMDMs were treated with various doses of CBZ (10, 20, 40 μM) and then stimulated with ATP. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. Caspase-1 activity, secretion of IL-1β were detected in SN. c LPS-primed BMDMs were treated with various doses of CBZ (10, 20, 40 μM) and then stimulated with nigericin. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. Caspase-1 activity, secretion of IL-1β were detected in SN. Data are expressed as mean ± SEM (n = 3) from three independent experiments with biological duplicates in a–c. Statistics differences were analyzed using one-way ANOVA: *P < 0.05, ***P < 0.001 vs. the LPS plus ATP or nigericin group (a–c)
In addition, we also evaluated whether CBZ enhances nigericin-induced NLRP3 inflammasome activation in BMDMs and THP-1 cells. Results showed that CBZ also dose-dependently enhanced caspase-1 maturation, IL-1β secretion and LDH release but had no effect on TNF-α production in response to nigericin in LPS-primed BMDMs (Fig. 2c, Supplementary Fig. 3a, b) and PMA-primed THP-1 cells (Supplementary Fig. 3c–g). In addition to ATP and nigericin, NLRP3 inflammasome can also be activated by MSU, SiO2 and cytosolic LPS. Unexpectedly, pretreatment with CBZ had no impact on cleaved caspase-1 and secreted IL-1β triggered by MSU, SiO2 and intracellular LPS (Fig. 3a–c, Supplementary Fig. 4a–c). Next, we also tested whether CBZ could strengthen AIM2 and NLRC4 inflammasomes activation triggered by poly(dA:dT) and Lfn-Flic, respectively. Results indicated that CBZ did not affect caspase-1 activation and IL-1β production in response to poly(dA:dT) and Lfn-Flic (Supplementary Fig. 4d–g). These results indicated that CBZ could specifically potentiate NLRP3 inflammasome activation triggered by ATP or nigericin.
CBZ has no effect on NLRP3 inflammasome activation induced by MSU, SiO2 and intracellular LPS. a LPS-primed BMDMs were treated with various doses of CBZ (10, 20, 40 μM) and then stimulated with MSU or ATP. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. Caspase-1 activity, secretion of IL-1β were detected in SN. b LPS-primed BMDMs were treated with CBZ (40 μM) and then stimulated with SiO2 or ATP. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. Caspase-1 activity, secretion of IL-1β were detected in SN. c Pam3CSK4-primed BMDMs were treated with various doses of CBZ (10, 20, 40 μM) and then stimulated with LPS, or LPS-primed BMDMs were treated with CBZ (40 μM) and then stimulated with ATP. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. Caspase-1 activity, secretion of IL-1β were detected in SN. Data are expressed as mean ± SEM (n = 3) from three independent experiments with biological duplicates in a–c. Statistics differences were analyzed using an unpaired Student’s t test (a–c): **P < 0.01, ***P < 0.001 vs. the LPS plus ATP group
CBZ has no effect on NLRP3-NLRP3 interaction and mitochondria damage, but facilitates NLRP3-dependent ASC oligomerization and ATP/nigericin-induced mtROS production. a LPS-primed BMDMs were treated with various doses of CBZ (10, 20, 40 μM) and then stimulated with ATP. Western blot analysis of WCL and cross-linked cytosolic pellets. b Western blot analysis of WCL and cross-linked cytosolic pellets of LPS-primed BMDMs treated with CBZ (40 μM) and then stimulated with ATP, poly (dA: dT), Lfn-Flic, or Pam3CSK4-primed BMDMs treated with CBZ (40 μM) and then stimulated with LPS transfection. c Immunoprecipitation (IP) and immunoblot analysis of the interaction of Flag-NLRP3 and Myc-NLRP3 in the lysates of HEK-293T cells. d Confocal microscopy analysis in LPS-primed BMDMs treated with CBZ (40 μM) and then left stimulated with ATP, followed by staining with Mitotracker red and DAPI. e LPS-primed BMDMs were treated with CBZ (20, 40 μM) before stimulated with ATP, nigericin or SiO2. BMDMs were loaded with MitoSOX red mitochondrial superoxide indicator (Ex/Em: 510/580 nm). After staining and washing, flow cytometry was conducted to test mtROS production. f LPS-primed BMDMs were treated with H2O2 and then stimulated with ATP, nigericin or SiO2. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN. Data are expressed as mean ± SEM (n = 3) from three independent experiments with biological duplicates in e. Statistics differences were analyzed using one-way ANOVA: #P < 0.05, ##P < 0.01 vs. the control. *P < 0.05 vs. the ATP or nigericin group
CBZ facilitates ATP-induced ASC oligomerization but has no effect on the NLRP3 oligomerization and potassium efflux
In the progression of NLRP3, NLRC4 and AIM2 inflammasomes activation, ASC oligomerization is the critical step for caspase-1 activation; therefore, the effect of CBZ on ASC oligomerization was assessed. LPS- or Pam3CSK4-primed BMDMs were treated with CBZ and stimulated with various stimuli. Then, we crosslinked ASC using disuccinimidyl suberate (DSS) and detected ASC oligomerization using immunoblotting. Consistent with the effects of CBZ on caspase-1 activation and IL-1β production, CBZ dose-dependently promoted ASC oligomerization induced by ATP (Fig. 4a). Nevertheless, CBZ had no impact on ASC oligomerization induced by poly(dA:dT), Lfn-Flic and cytosolic LPS (Fig. 4b). ASC oligomerization is necessary for all stimuli-induced NLRP3 inflammasome activation, so we speculated that CBZ acts upstream of ASC oligomerization to exacerbate ATP or nigericin-induced NLRP3 inflammasome activation.
We then investigated whether CBZ could affect the direct NLRP3-NLRP3 interaction. HEK-293T cells were transfected with Flag-NLRP3 and Myc-NLRP3 and treated with CBZ, and then, a co-immunoprecipitation assay was performed. The results showed that CBZ could not inhibit the direct NLRP3-NLRP3 interaction in HEK-293T cells (Fig. 4c), suggesting that CBZ does not directly affect stimuli-independent NLRP3 oligomerization in vitro. In addition, it was noted that potassium efflux is a specific requirement for the NLRP3 inflammasome activation (He et al. 2016), while it is dispensable for AIM2 and NLRC4 activation. We then examined whether CBZ could affect potassium efflux and results indicated that CBZ did not facilitate potassium efflux triggered by ATP, nigericin, MSU, SiO2 and poly(I:C) (Supplementary Fig. 5a, b). These data suggest that CBZ does not promote ATP- or nigericin-induced NLRP3 inflammasome activation by modulating potassium efflux.
CBZ promotes ATP/nigericin-induced NLRP3 inflammasome activation by synergistic induction of mitochondrial ROS production
Although the roles of oxidative stress and mitochondrial damage in the activation of NLRP3 inflammasome are still controversial, most studies have shown that they appear to be necessary for NLRP3 inflammasome activation (Lugrin et al. 2014; Mills et al. 2017). Therefore, the effect of CBZ on mitochondrial damage was evaluated in the following experiment. Results showed that mitochondrial damage was not induced after CBZ treatment alone, and ATP-induced mitochondrial damage was observed in BMDMs (Fig. 4d). mtROS is thought to be the important factors in NLRP3 inflammasome activation. The MitoSOX Red mitochondrial superoxide indicator assay was used to record the amount of mtROS production during the course of ATP, nigericin or SiO2 treatment in the presence or absence of CBZ. Results indicated that mtROS production was not induced after CBZ treatment alone. Interestingly, CBZ successfully potentiated mtROS production induced by ATP and nigericin, but not SiO2 (Fig. 4e, Supplementary Fig. 6) in LPS-primed BMDMs, suggesting that a synergistic increase in ROS production is a crucial event in the enhancement effect of CBZ on NLRP3 inflammasome triggered by ATP and nigericin. To determine whether the increase in ROS production could promote NLRP3 inflammasome activation by ATP or nigericin, LPS-primed BMDMs were pretreated with an oxidizing agent H2O2 before ATP, nigericin or SiO2 stimulation. Western blot analysis suggested that H2O2 enhanced caspase-1 cleavage and IL-1β maturation in a certain range of doses triggered by ATP or nigericin but not SiO2 (Supplementary Fig. 7a–c, Fig. 4f). These results indicated that CBZ promotes ATP/nigericin-induced NLRP3 inflammasome activation by synergistic induction of mtROS production.
Carbon–carbon double bond and carbonyl group in the structure of CBZ may contribute to NLRP3 inflammasome hyperactivation
To investigate the relationship between the structure of CBZ and NLRP3 inflammasome activation, and whether five CBZ metabolites or other antiepileptic drugs with structures similar to CBZ (Fig. 5a) have an enhancement effect on NLRP3 inflammasome activation, we chose two other antiepileptic drugs (oxcarbazepine and eslicarbazepine acetate), two synthetic CBZ intermediates (iminostilbene and iminostilbene carbonyl chloride) and an active CBZ metabolite (CBZ-10,11-epoxide) to explore the regulatory effect on NLRP3 inflammasome. LPS-primed BMDMs were treated with these drugs or ingredients before ATP stimulation. Caspase-1 activity assay and western blot analysis demonstrated that CBZ and iminostilbene carbonyl chloride amplified cleaved caspase-1 and mature IL-1β induced by ATP (Fig. 5b, c). The efficacy and safety of eslicarbazepine acetate, a third-generation antiepileptic drug, have been established in real-life settings (Gomez-Ibanez et al. 2017). Our study also confirmed the safety of eslicarbazepine acetate compared with that of CBZ from the perspective of activation of NLRP3 inflammasome. Moreover, these results suggested that the carbon–carbon double bond (C=C) on the seven-membered ring and the carbonyl group (C=O) on the nitrogen in the structure of CBZ may be conducive to strengthening NLRP3 inflammasome activation.
The effect of CBZ derivatives on NLRP3 inflammasome activation. a The structure of CBZ derivatives. b, c LPS-primed BMDMs were treated with CBZ derivatives (40 μM) and then stimulated with ATP. Caspase-1 activity (b) and western blot analysis of IL-1β (p17), caspase-1 (p20) in SN (c). Data are expressed as mean ± SEM (n = 3) from three independent experiments with biological duplicates in b. Statistics differences were analyzed using one-way ANOVA: ***P < 0.001 vs. the LPS plus ATP group
Combination of CBZ and LPS induces liver injury in WT mice but not in NLRP3−/− mice
CBZ specifically promoted ATP/nigericin-induced NLRP3 inflammasome activation in our study, however, whether the activation of NLRP3 inflammasome is the reason for hepatic injury induced by CBZ remains to be investigated. Coexisting inflammation factors, such as LPS, should be considered as a determinant of susceptibility to IDILI (Ganey et al. 2004). Therefore, we evaluated the effects of CBZ on NLRP3 inflammasome activation and its hepatotoxicity in an LPS-mediated susceptibility mouse model of IDILI. Results showed that co-treatment with LPS and CBZ significantly increased serum ALT, AST, IL-1β and TNF-α levels in WT mice but not in NLRP3−/− mice (Fig. 6a–d). Moreover, an evident increase in caspase-1 activity in the livers of WT mice following LPS/CBZ treatment, but not in the livers of NLRP3−/− mice (Fig. 6e), suggesting that NLRP3 inflammasome is necessary for CBZ-induced liver injury. Histological analysis of mouse liver tissues was performed by H&E staining assay. As illustrated in Fig. 6g, the livers had no histologic lesions in the control group, CBZ group, LPS group and all the NLRP3−/− groups. However, co-administration of LPS and CBZ in WT mice resulted in pathological changes, including inflammatory infiltration and hepatocellular necrosis. Similarly, TUNEL fluorescence staining showed that the apoptotic index increased significantly when WT mice co-exposed to LPS and CBZ compared with that in the other groups (Fig. 6f, g). Taken together, these results suggest that NLRP3 inflammasome indeed mediates CBZ-induced IDILI.
Early liver injury and inflammatory mediator production after LPS/CBZ cotreatment. a–g Wild type (WT) and NLRP3−/− female C57BL/6 mice were pretreated with LPS (2 mg/kg) through the tail vein. 2 h later, intraperitoneally CBZ (50 mg/kg, n = 6) injection was conducted. 6 h after CBZ injection, serum levels of ALT (a), AST (b), IL-1β (c), TNF-α (d) were measured by assay kit, caspase-1 activity (e) in the livers was detected after BCA protein quantification and normalization processing. H&E staining (g), TUNEL staining (g, f) were conducted to observe liver injury and apoptosis. Data are shown mean ± SEM. Statistics differences were analyzed using one-way ANOVA: #P < 0.05, ##P < 0.01, ###P < 0.001 vs. the WT control group. *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT LPS group. ▲P < 0.05, ▲▲▲P < 0.01 vs. the WT LPS plus CBZ group
Discussion
IDILI has become a major clinical challenge because of its high morbidity, mortality, unpredictable nature, frequent hospitalization, and need for liver transplantation. Some drugs with the ability to induce IDILI could induce the release of DAMPs from hepatocytes, leading to NLRP3 inflammasome activation in macrophages (Kato and Uetrecht 2017). However, whether NLRP3 inflammasome is involved in the occurrence of IDILI remains unclear. In this study, seven drugs with a relatively high incidence of IDILI were administered in the presence or absence of ATP, which is a stimulating factor for activating NLRP3 inflammasome, to evaluate the regulatory effect on NLRP3 inflammasome activation. Except for amodiaquine, none of them could directly activate NLRP3 inflammasome without ATP treatment, which indicated that these drugs could not directly serve as stimuli to activate NLRP3 inflammasome. Four drugs (amodiaquine, CBZ, isoniazid and nevirapine) could obviously promote NLRP3 inflammasome activation induced by ATP. These results suggest that the mechanism of liver injury induced by these four drugs may have something in common. Our research may provide a simple method to study the mechanism of IDILI and even predict which drug candidates are likely to cause such adverse reactions.
Among the four drugs that could promote the activation of NLRP3 inflammasome in the presence of ATP, CBZ had the most potent effect on NLRP3 inflammasome activation. Moreover, CBZ is widely used as an antiepileptic agent and could cause Stevens Johnson Syndrome/toxic epidermal necrolysis (SJS/TEN) with rare but severe liver injury in humans (Chalasani et al. 2015; Tangamornsuksan et al. 2013). A current research pointed out that the activation of inflammasomes may be an important step in the immune system activation by CBZ, which can lead to hypersensitivity reactions (Kato et al. 2019). In addition, a finding uncovered that CBZ-10,11-epoxide, the main metabolite of CBZ, induces NLRP3 inflammasome activation in SJS/TEN keratinocytes(Zhang et al. 2018a). So, it is apparent to us that NLRP3 inflammasome is involved in the occurrence of CBZ-induced idiosyncratic drug reactions. Therefore, the effect of CBZ on NLRP3 inflammasome activation and liver injury as well as the related mechanism was evaluated in this study. Surprisingly, we observed that CBZ could specifically reinforce NLRP3 inflammasome activation induced by ATP or nigericin but not MSU, SiO2, and intracellular LPS. Furthermore, CBZ did not promote the activation of AIM2 and NLRC4 inflammasomes triggered by poly(dA:dT) and Lfn-Flic, respectively. CBZ specifically amplified ATP or nigericin-induced the activation of NLRP3 inflammasome, suggesting that CBZ enhances the susceptibility of IDILI in patients with ATP or nigericin-related diseases, which are potential risk factors for IDILI. Nevertheless, underlying NLRP3 inflammasome-driven diseases, such as MSU-induced gout and SiO2-induced silicosis, may not be risk signals of CBZ-induced liver injury.
The non-hepatotoxic dose of LPS, which evokes mild concurrent inflammation in animals that may mimic the preconditions of human IDILI, has been successfully validated by a variety of drugs with the potential to induce IDILI (Buchweitz et al. 2002; Luyendyk et al. 2003, 2004). In addition, LPS can directly activate NLRP3 inflammasome in vivo (Kayagaki et al. 2011; Lamkanfi and Dixit 2014). To evaluate the hypothesis that CBZ induces liver injury by reinforcing specific stimuli-induced NLRP3 inflammasome activation, we analyzed the effects of CBZ on hepatotoxicity and NLRP3 inflammasome in an LPS-mediated susceptibility mouse model of IDILI. Our results demonstrated that LPS or CBZ alone did not lead to liver injury in WT and NLRP3−/− mice, while the combination of CBZ and LPS induced liver injury in WT mice but not NLRP3−/−mice. Moreover, CBZ significantly increased LPS-mediated NLRP3 inflammasome activation in WT mice, indicating that CBZ could induce hepatotoxicity via promoting NLRP3 inflammasome activation in vivo. Previous studies have also shown that the inherited variant of the HLA-B gene, HLA-B* 15:02, confers susceptibility to CBZ-induced SJS/TEN[26]. The evidence also suggests that CBZ may synergistically interact with other risk factors associated with the body, such as genetic polymorphisms and NLRP3 inflammasome activation-related underlying diseases, to contribute to influencing CBZ susceptibility to hepatotoxicity.
To elucidate the relationship between the structure of CBZ and NLRP3 inflammasome activation, two antiepileptic drugs (oxcarbazepine and eslicarbazepine acetate), two synthetic CBZ intermediates (iminostilbene and iminostilbene carbonyl chloride) and an active CBZ metabolite (CBZ-10,11-epoxide) were further evaluated in an ATP-induced NLRP3 inflammasome activation model. Oxcarbazepine and eslicarbazepine acetate could not promote caspase-1 cleavage and IL-1β maturation, which may explain why the second- or third-generation antiepileptic drugs have an advantage over CBZ. On the other hand, CBZ-10,11-epoxide, widely considered to be the active constituent involved in CBZ-induced liver injury, could not regulate NLRP3 inflammasome activation, suggesting that CBZ-10,11-epoxide-induced liver injury may have nothing to do with promoting activation of NLRP3 inflammasome. CBZ and iminostilbene carbonyl chloride could enhance the activation of NLRP3 inflammasome. Both of them contain the “C=C” on the seven-membered ring and “C=O” on the nitrogen, which hinted that CBZ analogues containing these functional groups may strengthen the activation of NLRP3 inflammasome.
It has been demonstrated that NLRP3 inflammasome activation leads to the increase of pro-inflammatory factors and infiltrating monocytes/macrophages and neutrophils (Kubes and Mehal 2012; You et al. 2006), which contributes to liver injury under certain circumstances (Honda et al. 2013; Oliveira et al. 2018; Petrasek et al. 2012; Tilg et al. 2016). Moreover, NLRP3 inflammasome activation leads to pyroptosis (He et al. 2016), this distinct form of programmed cell death mediates the development of liver injury (Chen et al. 2016; Geng et al. 2015; Zhang et al. 2018b). In addition, one of important upstream signaling of NLRP3 inflammasome activation is ROS production (Minutoli et al. 2016; Zhou et al. 2011), which can lead to mitochondrial dysfunction through an intracellular oxidant stress in hepatocytes leading mainly to oncotic necrosis and less apoptosis (Jaeschke 2011; Schwabe and Brenner 2006). Our study demonstrates that CBZ enhances NLRP3 inflammasome mediated IL-1β maturation, monocytes/macrophages or neutrophils infiltration and pyroptosis by synergistic induction of mitochondrial ROS production, and the combination of CBZ and LPS induces liver injury in WT mice but not in NLRP3−/− mice. Therefore, our results provided novel insights on CBZ-triggered idiosyncratic hepatotoxicity that CBZ induced liver inflammation and hepatocyte necrosis through enhancing NLRP3 inflammasome activation.
Abbreviations
- IDILI:
-
Idiosyncratic drug-induced liver injury
- ATP:
-
Adenosine triphosphate
- CBZ:
-
Carbamazepine
- MSU:
-
Monosodium urate crystal
- LPS:
-
Lipopolysaccharide
- mtROS:
-
Mitochondrial reactive oxygen species
- PAMPs:
-
Pathogen-associated molecular patterns
- DAMPs:
-
Damage-associated molecular patterns
- PMA:
-
Phorbol-12-myristate-13-acetate
- BMDMS :
-
Bone-marrow-derived macrophages
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate transaminase
- LDH:
-
Lactate dehydrogenase
- HBSS:
-
Hank’s balanced salt solution
References
Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H (2010) Mechanisms of immune-mediated liver injury. Toxicol Sci 115(2):307–321. https://doi.org/10.1093/toxsci/kfq009
Bjornsson ES (2015) Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol 89(3):327–334. https://doi.org/10.1007/s00204-015-1456-2
Bjornsson ES (2016) Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci 17(2):224. https://doi.org/10.3390/ijms17020224
Buchweitz JP, Ganey PE, Bursian SJ, Roth RA (2002) Underlying endotoxemia augments toxic responses to chlorpromazine: is there a relationship to drug idiosyncrasy? J Pharmacol Exp Ther 300(2):460–467. https://doi.org/10.1124/jpet.300.2.460
Chalasani N, Bjornsson E (2010) Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology 138(7):2246–2259. https://doi.org/10.1053/j.gastro.2010.04.001
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ (2014) ACG Clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 109(7):950–966. https://doi.org/10.1038/ajg.2014.131(quiz 967)
Chalasani N, Bonkovsky HL, Fontana R et al (2015) Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology 148(7):1340-52.e7. https://doi.org/10.1053/j.gastro.2015.03.006
Chen YL, Xu G, Liang X et al (2016) Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury. Am J Transl Res 8(12):5685–5695
Cho T, Uetrecht J (2017) How reactive metabolites induce an immune response that sometimes leads to an idiosyncratic drug reaction. Chem Res Toxicol 30(1):295–314. https://doi.org/10.1021/acs.chemrestox.6b00357
Deng X, Stachlewitz RF, Liguori MJ et al (2006) Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J Pharmacol Exp Ther 319(3):1191–1199. https://doi.org/10.1124/jpet.106.110247
Fontana RJ (2014) Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology 146(4):914–928. https://doi.org/10.1053/j.gastro.2013.12.032
Ganey PE, Luyendyk JP, Maddox JF, Roth RA (2004) Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem Biol Interact 150(1):35–51. https://doi.org/10.1016/j.cbi.2004.09.002
Garcia-Cortes M, Ortega-Alonso A, Lucena MI, Andrade RJ (2018) Drug-induced liver injury: a safety review. Expert Opin Drug Saf 17(8):795–804. https://doi.org/10.1080/14740338.2018.1505861
Geng Y, Ma Q, Liu YN et al (2015) Heatstroke induces liver injury via IL-1beta and HMGB1-induced pyroptosis. J Hepatol 63(3):622–633. https://doi.org/10.1016/j.jhep.2015.04.010
Gomez-Ibanez A, Serratosa JM, Guillamon E et al (2017) Efficacy and safety of eslicarbazepine-acetate in elderly patients with focal epilepsy: case series. Seizure 48:53–56. https://doi.org/10.1016/j.seizure.2017.04.003
Hammad MA, Abdel-Bakky MS, Walker LA, Ashfaq MK (2011) Oxidized low-density lipoprotein and tissue factor are involved in monocrotaline/lipopolysaccharide-induced hepatotoxicity. Arch Toxicol 85(9):1079–1089. https://doi.org/10.1007/s00204-011-0649-6
He Y, Hara H, Nunez G (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41(12):1012–1021. https://doi.org/10.1016/j.tibs.2016.09.002
He H, Jiang H, Chen Y et al (2018) Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun 9(1):2550. https://doi.org/10.1038/s41467-018-04947-6
Holt MP, Ju C (2006) Mechanisms of drug-induced liver injury. AAPS J 8(1):E48–E54. https://doi.org/10.1208/aapsj080106
Honda M, Takeichi T, Asonuma K, Tanaka K, Kusunoki M, Inomata Y (2013) Intravital imaging of neutrophil recruitment in hepatic ischemia–reperfusion injury in mice. Transplantation 95(4):551–558. https://doi.org/10.1097/TP.0b013e31827d62b5
Huang Y, Jiang H, Chen Y et al (2018) Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. https://doi.org/10.15252/emmm.201708689
Jaeschke H (2011) Reactive oxygen and mechanisms of inflammatory liver injury: present concepts. J Gastroenterol Hepatol 26(Suppl 1):173–179. https://doi.org/10.1111/j.1440-1746.2010.06592.x
Ju C, Reilly T (2012) Role of immune reactions in drug-induced liver injury (DILI). Drug Metab Rev 44(1):107–115. https://doi.org/10.3109/03602532.2011.645579
Kato R, Uetrecht J (2017) Supernatant from hepatocyte cultures with drugs that cause idiosyncratic liver injury activates macrophage inflammasomes. Chem Res Toxicol 30(6):1327–1332. https://doi.org/10.1021/acs.chemrestox.7b00065
Kato R, Ijiri Y, Hayashi T, Uetrecht J (2019) The 2-hydroxyiminostilbene metabolite of carbamazepine or the supernatant from incubation of hepatocytes with carbamazepine activates inflammasomes; implications for carbamazepine-induced hypersensitivity reactions. Drug Metab Dispos. https://doi.org/10.1124/dmd.119.087981
Kayagaki N, Warming S, Lamkanfi M et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479(7371):117–121. https://doi.org/10.1038/nature10558
Kubes P, Mehal WZ (2012) Sterile inflammation in the liver. Gastroenterology 143(5):1158–1172. https://doi.org/10.1053/j.gastro.2012.09.008
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157(5):1013–1022. https://doi.org/10.1016/j.cell.2014.04.007
Lu J, Roth RA, Malle E, Ganey PE (2013) Roles of the hemostatic system and neutrophils in liver injury from co-exposure to amiodarone and lipopolysaccharide. Toxicol Sci 136(1):51–62. https://doi.org/10.1093/toxsci/kft170
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395(2):203–230. https://doi.org/10.1515/hsz-2013-0241
Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA (2003) Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J Pharmacol Exp Ther 307(1):9–16. https://doi.org/10.1124/jpet.103.054288
Luyendyk JP, Maddox JF, Green CD, Ganey PE, Roth RA (2004) Role of hepatic fibrin in idiosyncrasy-like liver injury from lipopolysaccharide-ranitidine coexposure in rats. Hepatology 40(6):1342–1351. https://doi.org/10.1002/hep.20492
Mak A, Uetrecht J (2015) The combination of anti-CTLA-4 and PD1−/− mice unmasks the potential of isoniazid and nevirapine to cause liver injury. Chem Res Toxicol 28(12):2287–2291. https://doi.org/10.1021/acs.chemrestox.5b00305
Metushi IG, Hayes MA, Uetrecht J (2015) Treatment of PD-1(−/−) mice with amodiaquine and anti-CTLA4 leads to liver injury similar to idiosyncratic liver injury in patients. Hepatology 61(4):1332–1342. https://doi.org/10.1002/hep.27549
Mills EL, Kelly B, O’Neill LAJ (2017) Mitochondria are the powerhouses of immunity. Nat Immunol 18(5):488–498. https://doi.org/10.1038/ni.3704
Minutoli L, Puzzolo D, Rinaldi M et al (2016) ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxidative Med Cell Longev 2016:2183026. https://doi.org/10.1155/2016/2183026
Mridha AR, Wree A, Robertson AAB et al (2017) NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 66(5):1037–1046. https://doi.org/10.1016/j.jhep.2017.01.022
Nicoletti P, Aithal GP, Bjornsson ES et al (2017) Association of liver injury from specific drugs, or groups of drugs, with polymorphisms in HLA and other genes in a genome-wide association study. Gastroenterology 152(5):1078–1089. https://doi.org/10.1053/j.gastro.2016.12.016
Oliveira THC, Marques PE, Proost P, Teixeira MMM (2018) Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Investig 98(1):51–62. https://doi.org/10.1038/labinvest.2017.90
Petrasek J, Bala S, Csak T et al (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Investig 122(10):3476–3489. https://doi.org/10.1172/jci60777
Real M, Barnhill MS, Higley C, Rosenberg J, Lewis JH (2019) Drug-induced liver injury: highlights of the recent literature. Drug Saf 42(3):365–387. https://doi.org/10.1007/s40264-018-0743-2
Schwabe RF, Brenner DA (2006) Mechanisms of liver injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290(4):G583–G589. https://doi.org/10.1152/ajpgi.00422.2005
Shaw PJ, Ditewig AC, Waring JF et al (2009) Coexposure of mice to trovafloxacin and lipopolysaccharide, a model of idiosyncratic hepatotoxicity, results in a unique gene expression profile and interferon gamma-dependent liver injury. Toxicol Sci 107(1):270–280. https://doi.org/10.1093/toxsci/kfn205
Song N, Liu ZS, Xue W et al (2017) NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell 68(1):185-197.e6. https://doi.org/10.1016/j.molcel.2017.08.017
Tangamornsuksan W, Chaiyakunapruk N, Somkrua R, Lohitnavy M, Tassaneeyakul W (2013) Relationship between the HLA-B*1502 allele and carbamazepine-induced Stevens–Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol 149(9):1025–1032. https://doi.org/10.1001/jamadermatol.2013.4114
Tilg H, Moschen AR, Szabo G (2016) Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 64(3):955–965. https://doi.org/10.1002/hep.28456
Wang Z, Xu G, Gao Y et al (2019) Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharm Sin B 9(4):734–744. https://doi.org/10.1016/j.apsb.2019.02.003
Weston JK, Uetrecht J (2014) Activation of inflammasomes by agents causing idiosyncratic skin reactions: a possible biomarker. Chem Res Toxicol 27(6):949–951. https://doi.org/10.1021/tx5001333
Wree A, Eguchi A, McGeough MD et al (2014) NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59(3):898–910. https://doi.org/10.1002/hep.26592
You Q, Cheng L, Reilly TP, Wegmann D, Ju C (2006) Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology 44(6):1421–1431. https://doi.org/10.1002/hep.21425
Zhang C, Wang G, Fu M (2018a) 934 Carbamazepine-10,11-epoxide drives CD8+ T-cell skin trafficking through NLRP3 inflammasome upregulation and contributes to necroptosis of keratinocytes in severe cutaneous adverse drug reactions. J Investig Dermatol 138(5, Supplement):S159. https://doi.org/10.1016/j.jid.2018.03.946
Zhang X, Luan J, Chen W et al (2018b) Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale 10(19):9141–9152. https://doi.org/10.1039/c8nr00554k
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. https://doi.org/10.1038/nature09663
Acknowledgements
This work has been supported by grants from National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program” (2017ZX09301022, 2018ZX09101002-001-002), National Natural Science Foundation of China (81874368, 81630100, 81903891), Beijing Nova Program (Z181100006218001), Innovation Groups of the National Natural Science Foundation of China (81721002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
CBZ promotes the activation of NLRP3 inflammasome triggered by ATP, an effect that is not present in NLRP3-/- BMDMs. (a) BMDMs or LPS-primed BMDMs were treated with ATP, CBZ, or ATP plus CBZ. The secretion of TNF-α was detected in SN. (b) LPS-primed BMDMs were treated with various doses of CBZ and then stimulated with ATP. The secretion of TNF-α was detected in SN. (c-g) LPS-primed wild type (WT) BMDMs or NLRP3 knock out (NLRP3-/-) BMDMs were treated with ATP in the presence or absence of CBZ. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL (c). Caspase-1 activity (d), secretion of IL-1β (e), LDH (f), TNF-α (g) in SN from WT BMDMs and NLRP3-/- BMDMs described in (c). Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates in (a, b, d-g). Statistics differences were analyzed using an unpaired Student’s t-test: **P < 0.01, ***P < 0.001 vs. the WT ATP group. Supplementary Fig. 2 CBZ has no influence on the cell viability in BMDMs and cultured supernatant ALT and AST in L02 cells. (a) BMDMs were incubated at 37°C followed by treatment with CBZ for 24 h, then these cells were cultured with CCK-8 for 30 min. The optical density values at the wavelength of 450 nm were determined. (b, c) L02 cells were seeded in 96-well growth-medium plate overnight at 1×105 cells/well. Next, the cells were incubated by CBZ treatment for 24 h, then the cultured supernatant ALT (b) and AST (c) were determined. APAP, acetaminophen. Statistics differences were analyzed using one-way ANOVE: ***P < 0.001 vs. the control group. Supplementary Fig. 3 CBZ promotes NLRP3 inflammasome activation stimulated by nigericin in BMDMs and THP-1 cells. (a, b) LPS-primed BMDMs were treated with various doses of CBZ and then stimulated with nigericin. The secretion of LDH (a), TNF-α (b) were detected in SN. (c) PMA-primed THP-1 cells were stimulated with nigericin after CBZ treatment. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. (d-g) Caspase-1 activity (d), secretion of IL-1β (e), LDH (f), TNF-α (g) in SN from THP-1 cells described in (c). Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates in (a, b, d-g). Statistics differences were analyzed using one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001 vs. the LPS or PMA plus nigericin group. Supplementary Fig. 4 CBZ has no effect on the activation of NLRP3 inflammasome induced by MSU, SiO2 and intracellular LPS, as well as AIM2 and NLRC4 inflammasome. (a) LPS-primed BMDMs were treated with various doses of CBZ and then stimulated with MSU or ATP. The secretion of TNF-α was detected in SN. (b) LPS-primed BMDMs were treated with CBZ and then stimulated with SiO2 or ATP. The secretion of TNF-α was detected in SN. (c) Pam3CSK4-primed BMDMs were treated with various doses of CBZ and then stimulated with LPS, or LPS-primed BMDMs were treated with CBZ and then stimulated with ATP. The secretion of TNF-α was detected in SN. (d) LPS-primed BMDMs were treated with CBZ and then stimulated with ATP, poly(dA:dT) or Lfn-Flic. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. (e-g) Caspase-1 activity (e), secretion of IL-1β (f), TNF-α (g) in SN described in (d). Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates in (a-c, f-h). Statistics differences were analyzed using an unpaired Student’s t-test: **P < 0.01, ***P < 0.001 vs. the ATP group. Supplementary Fig. 5 CBZ has no effect on intracellular potassium. (a, b) Qualification of potassium efflux in LPS-primed BMDMs treated with CBZ and then stimulated with different stimuli. Supplementary Fig. 6 CBZ facilitates ATP/nigericin-induced mitochondrial reactive oxygen species (mtROS) production. LPS-primed BMDMs were treated with CBZ before stimulated by ATP, nigericin or SiO2. For mtROS measurement, BMDMs were loaded with MitoSOX red mitochondrial superoxide indicator (Ex/Em: 510/580 nm). After staining and washing, flow cytometry was conducted to test mtROS. Supplementary Fig. 7 The effect of H2O2 on NLRP3 inflammasome activation triggered by ATP, nigericin, and SiO2. (a-c) LPS-primed BMDMs were treated with H2O2 and then stimulated with ATP (a), nigericin (b) or SiO2(c). Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN. Data are expressed as mean ± SEM (n=3) from three independent experiments with biological duplicates. Statistics differences were analyzed using one-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001 vs. the ATP or nigericin group (PDF 1217 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Xu, G., Zhan, X. et al. Carbamazepine promotes specific stimuli-induced NLRP3 inflammasome activation and causes idiosyncratic liver injury in mice. Arch Toxicol 93, 3585–3599 (2019). https://doi.org/10.1007/s00204-019-02606-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02606-3