Abstract
Despite six decades of extensive research in medical countermeasures against nerve agent poisoning, a broad spectrum acetylcholinesterase (AChE) reactivator is not yet available. One current approach is directed toward synthesizing oximes with high affinity and reactivatability toward butyrylcholinesterase (BChE) in plasma to generate an effective pseudocatalytic scavenger. An interim solution could be the administration of external AChE or BChE from blood products to augment pseudocatalytic scavenging with slower but clinically approved oximes to decrease nerve agent concentrations in the body. We here semiquantitatively investigate the ability of obidoxime and HI-6 to decrease the inhibitory activity of VX with human AChE and BChE from whole blood, erythrocyte membranes, erythrocytes, plasma, clinically available fresh frozen plasma and packed red blood cells. The main findings are that whole blood showed a VX concentration-dependent decrease in inhibitory activity with HI-6 being more potent than obidoxime. Using erythrocytes and erythrocyte membranes again, HI-6 was more potent compared to obidoxime. With freshly prepared plasma, obidoxime and HI-6 showed comparable results for the decrease in VX. The use of the clinically available blood products revealed that packed red blood cells showed similar kinetics as fresh erythrocytes. Fresh frozen plasma resulted in a slower and incomplete decrease in inhibitory plasma compared to freshly prepared plasma. In conclusion, the administration of blood products in combination with available oximes augments pseudocatalytic scavenging and might be useful to decrease the body load of persistent, highly toxic nerve agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recent attacks with the organophosphorus-based nerve agent sarin killed several hundred civilians in Syria and underline the need for effective medical countermeasures, especially if high numbers of patients or larger groups are affected (Dolgin 2013; Eisenkraft et al. 2014). The toxic mechanism of organophosphorus compounds (OP)—including nerve agents and pesticides—is based on a covalent binding with subsequent inhibition of the serine hydroxyl group in the active center of the pivotal enzyme acetylcholinesterase (AChE) (Aldridge and Reiner 1972). Failure of inhibited AChE to hydrolyze the neurotransmitter acetylcholine (ACh) results in an endogenous ACh overflow at muscarinic and nicotinic synapses resulting in cholinergic crisis. Finally, death may occur due to muscarinic receptor-mediated strong secretion in the respiratory tract, the nicotinic cholinoceptor-mediated paralysis of the respiratory muscles and central respiratory disturbance (Kwong 2002; Lee 2003). Approved standard antidotal therapies are based on the reactivation of inhibited AChE by the reactivators obidoxime, pralidoxime, HI-6 or trimedoxime in order to reactivate the inhibited enzyme by nucleophilic reactivation and restore the catalytic activity of AChE (Worek and Thiermann 2013). Atropine is administered to competitively antagonize muscarinic symptoms. This combined standard antidotal treatment is virtually unchanged for the past 60 years. New and experimental approaches are mainly based on the application of scavengers (Nachon et al. 2013). As stoichiometric scavengers, cyclodextrins and butyrylcholinesterase (BChE) are under research and have been evaluated in animal experiments (Mumford et al. 2011; Worek et al. 2014b). Yet, stoichiometric scavengers would necessitate the administration of an enormous amount in humans and would only prevent signs of poisoning if administered before the poison reaches target tissues (Mumford et al. 2013). Therefore, catalytic scavengers are being developed and show, in principal, feasibility for prophylaxis and therapy in nerve agent poisoning. However, substrate specificity with a small spectrum, low catalytic activity, low stability in vivo and potential immunogenicity are serious points that need to be addressed in future research to optimize catalytic scavengers and allow advanced development for clinical approval (Worek et al. 2016).
Next to the stoichiometric and catalytic scavengers, the concept of so-called pseudocatalytic bioscavengers has been conceived by Maxwell and colleagues in the late 1990s (Maxwell et al. 1999). Basic component is an oxime that reactivates an inhibited AChE and/or BChE (both per se stoichiometric scavengers) and thus accelerates breakdown of circulating OP. This effect can be augmented by adding external ChE to increase the pool of enzyme in the circulation. At the moment, this concept is mainly based on BChE lacking an intrinsic mechanism of spontaneous reactivation, which must therefore be combined with an oxime. By now the reactivation of nerve agent-inhibited BChE using the approved (bis-)pyridinium oximes obidoxime or pralidoxime is rather weak (Aurbek et al. 2009; Elsinghorst et al. 2013). Current research is directed toward synthesizing oximes with new scaffolds combining high affinity and reactivatability toward BChE in plasma to generate an effective pseudocatalytic scavenger (Kovarik et al. 2010; Radić et al. 2013; Sit et al. 2014).
An interim solution would be the administration of native AChE and/or BChE from blood products which might work as a pseudocatalytic scavenger in combination with clinically approved oximes (Fig. 1). As AChE expressed on the membranes of red blood cells is coded by the same gene as the neuronal AChE, erythrocyte concentrates represent an available external AChE source in emergency medicine (Taylor and Radić 1994). Plasma preparations [e.g., fresh frozen plasma (FFP)] are also available in emergency departments and could be used as a treatment to elevate the concentration of BChE and proteins in the vascular compartment of a OP-poisoned patient (Wille et al. 2014; von der Wellen et al. 2016). Thus, we here set out to test human blood and erythrocytes as AChE source and plasma as BChE source in combination with the approved obidoxime and HI-6, an oxime in advanced development in several European countries, to evaluate the pseudocatalytic scavenging potency of blood components to decrease the highly stable nerve agent VX in vitro.
Concept of pseudocatalytic scavenging with VX-inhibited cholinesterases and oximes. In this concept, the administration of obidoxime or HI-6 allows the regeneration of the inhibited stoichiometric scavengers AChE and BChE. The ChE pool in the body can be elevated by addition of external AChE or BChE. This results in multiple reactivation-inhibition cycles of ChE and an increased detoxification of VX
Materials and methods
Materials
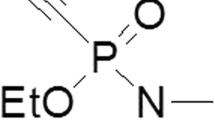
5,5′-Dithiobis-2-nitrobenzoic acid (DTNB), acetylthiocholine iodide (ATCh) and S-butyrylthiocholine iodide (BTCh) were supplied by Sigma-Aldrich (Taufkirchen, Germany). VX (purity >98 % by GC–MS, 1H NMR and 31P NMR; Fig. 1) was made available by the Ministry of Defence (Bonn, Germany) and HI-6 by Dr. Clement (Defence Research Establishment Suffield, Ralston, AB, Canada; Fig. 1). All other chemicals including obidoxime were purchased from Merck Eurolab GmbH (Darmstadt, Germany) at the purest grade available. Heparinized human whole blood was in part separated into plasma and erythrocytes by centrifugation (3000 rpm, 10 min). Packed red blood cells (PRBC) and FFP were supplied by the Bavarian Red Cross Blood Donation Service, Munich, Germany. They contained 86–88 % erythrocytes and 12–14 % CPD stabilizer solution (8.9 mM tri-natriumcitrate dihydrate, 1.6 mM citric acid, 13 mM glucose and 1.6 mM sodium dihydrogen phosphate dihydrate) or 80–84 % human plasma and 16–20 % CPD stabilizer solution.
Human erythrocyte ghosts
Hemoglobin-free erythrocyte ghosts were prepared as source of human erythrocyte AChE (HEG-AChE) (Worek et al. 2004). Prior to use, aliquots were homogenized on ice with a Sonoplus HD 2070 ultrasonic homogenator (Bandelin electronic, Berlin, Germany), three times for 5 s with 30-s intervals, to achieve a homogeneous matrix for the kinetic studies.
VX dilution
VX stock solutions for the biological assay (0.1 % in acetonitrile) were stored at ambient temperature. Working solutions were prepared in distilled water immediately before the experiment and kept on ice until use.
Determination of concentration of binding sites for nerve agents
Human plasma was incubated with at least five concentrations of VX (10–60 nM) in phosphate buffer (0.1 mM, pH 7.4) for 10 min at 37 °C. Thereafter, residual BChE activity was determined. The residual BChE activity (% of control) was plotted versus the nerve agent concentration, and the concentration of the BChE binding sites was obtained from the X-intercept of the regression line. Concentrations resulting in zero activity were excluded to allow proper linear regression. FFP was adjusted to an identical BChE activity to gain a comparable amount of ChE binding sites. AChE binding sites were determined similarly for HEG-AChE with 2.5–20 nM VX final concentration. Blood dilutions and dilutions of PRBC were prediluted 1:1 with phosphate buffer (0.1 M, pH 7.4) containing 0.2 % gelatine and adjusted to an identical activity of HEG-AChE to achieve a comparable concentration of ChE binding sites.
Evaluation of pseudocatalytic activity
Heparinized blood, erythrocytes, HEG-AChE or plasma were mixed 1:1 with phosphate buffer (0.1 M, pH 7.4) containing 0.2 % gelatine. PRBC and FFP were tested as commercial external ChE sources and diluted comparably. In addition, equal parts of PRBC and FFP were premixed and then diluted 1:1 to mimic whole blood dilution. Subsequently, VX was added (100–400 nM final concentration) followed by 10 min incubation at 37 °C.
The sample was then incubated with HI-6 or obidoxime (50 µM, respectively) to start the oxime-mediated pseudocatalytic breakdown of VX (Fig. 2). As a control, an assay without oxime was performed. After various time intervals up to 240 min, 20 µL samples were taken from both of the two incubates and transferred to a prewarmed cuvette filled with 3000 µL phosphate buffer, 100 µL DTNB (0.3 mM final concentration). A preincubation of 5 min allowed saturation of unspecific sulfhydryl groups in the sample, and thereafter 50 µL ATCh or BTCh was added to determine AChE and/or BChE activity spectrophotometrically (Shimadzu UV 2600, Duisburg, Germany) with a modified Ellman assay (Worek et al. 1999) at 436 nm and 37 °C.
Scheme of the procedure to assess pseudocatalytic scavenging. Various sources of ChE were incubated with VX followed by addition of obidoxime or HI-6. ChE activity was either assessed immediately or assessed after incubation with uninhibited HEG-AChE to assess free VX in the early phase of the experiments
An additional sample of each incubate was taken and mixed with 20 µL non-inhibited HEG-AChE and was incubated for 15 min to allow inhibition of native HEG-AChE by free VX especially in the early phase of the experiment. Then, 20 µL was transferred to a prewarmed cuvette for determination of AChE activity. This assay allows the semiquantitative assessment of the inhibitory activity in the tested matrices.
Calculations
BChE or AChE activity of the previously VX-inhibited (fresh frozen) plasma, blood, erythrocytes, erythrocyte concentrate, and HEG-AChE was transferred into percent according to the control activity of the tested matrices. The inhibitory activity of incubates at a given time point was calculated as well.
Calculation of AChE and BChE activity
Calculation for inhibition assay plus ghost and reactivation assay plus ghost
with A (%) = activity in %, A S = activity of sample, A C = control activity, I (%) = inhibition in %, A Sg = activity of sample after incubation with ghost, A HEG = activity of HEG-AChE.
The VX concentration in the sample was calculated according to the following equation:
with [VX]t 0 = VX concentration at time zero, [VX] = initial VX concentration, [ChE] = calculated binding sites of BChE and/or AChE, [VX]t x = VX concentration at a time t x , [VX] t−1 = VX concentration calculated for a previous time point t x−1, t 1/2 = reactivation half-life.
The decrease in the VX concentration by the differently used matrices was calculated assuming a zero order kinetic in the presence of pharmacologically relevant oxime concentrations dependent on the determined ChE binding sites. The used kinetic constants for reactivation of VX-inhibited human AChE with obidoxime and HI-6 were taken from Worek et al. (2004).
Results
AChE and BChE binding sites
The tested fresh plasma revealed 38.8 ± 0.3 nM binding sites for BChE. The concentration of binding sites of HEG-AChE was 6.5 ± 0.1 nM.
AChE and BChE inhibition in the absence of an oxime
Incubation of the different matrices with VX without adding an oxime resulted in a complete inhibition of AChE and BChE activity throughout the experimental period (>99 %, Fig. 3a). Determination of inhibitory activity showed complete inhibition of added HEG-AChE (<2 % activity) indicating the presence of free VX until end of the experiment.
Pseudocatalytic scavenging of VX with obidoxime and HI-6 in whole blood. The non-oxime control showed persistent inhibition during the whole experiment (a, red line red inverted triangle). The addition of native HEG-AChE to an aliquot of VX-treated whole blood resulted in a complete inhibition of the added HEG-AChE and proofed free VX during the experiment duration (a, blue line blue triangle). HI-6 was more effective than obidoxime in detoxification of VX at identical VX and oxime concentrations (b vs. c). The decrease in inhibitory activity depended on the VX concentration and was delayed with increasing VX concentrations (c–e, red line red inverted triangle). Subsequently the activity increased (b–e, blue line blue triangle). Data are given as ChE activity (%) or inhibitory activity (%) of n = 4 experiments ± SD (color figure online)
Pseudocatalytic scavenging of VX by obidoxime and HI-6
Incubation of VX-treated whole blood dilution with 50 µM oxime resulted in a rapid decrease in inhibitory activity, i.e., reduction in free VX, which was substantially faster with HI-6 compared to obidoxime (Fig. 3b, c). Use of higher VX concentrations in combination with 50 µM HI-6 in whole blood dilutions resulted in a concentration-dependent delay of half-maximal loss of inhibitory activity after 2.2 min for 100 nM VX, 13.3 min for 200 nM VX and 49.3 min for 400 nM VX (Fig. 3d, e). Half-maximal increase in ChE activity was achieved after 17.2 min for 100 nM VX, 43.4 min for 200 nM VX and 84.4 min for 400 nM VX. The initial VX concentration showed a linear relationship with both the loss of inhibitory activity and the increase in ChE activity (R 2 > 0.99).
Addition of 50 µM obidoxime or HI-6 to VX-treated HEG-AChE, erythrocytes and plasma again resulted in a rapid decrease in inhibitory activity (Fig. 4). HI-6 was more effective than obidoxime with HEG-AChE and erythrocytes, while both oximes achieved a comparable effect with plasma.
Pseudocatalytic scavenging of VX with obidoxime and HI-6 in HEG-AChE, erythrocytes and plasma. Obidoxime showed a slower decrease in inhibitory activity (red line red inverted triangle) and increase in activity (blue line blue triangle) than HI-6 with VX-incubated (100 nM) HEG-AChE (a vs. b) and erythrocytes (c vs. d). With plasma, the decrease in inhibitory activity was comparable with both oximes (e vs. f). The calculated VX concentration is given as dashed black line. Data are given as ChE activity in % or inhibitory activity in (%) of n = 4 experiments ± SD (color figure online)
Model calculations of VX detoxification in HEG-AChE, erythrocytes and plasma
The theoretical decrease in the VX concentration was calculated with Eqs. (3) and (4) for HEG-AChE, erythrocytes and plasma assuming a linear decrease dependent on the ChE binding sites in the respective samples (Fig. 4, dashed black line). With an oxime concentration of 50 µM and k obs of 0.58 and 0.20 min−1, calculated half-lives (t 1/2 = ln 2/k obs) were 1.2 min for the reactivation of VX-inhibited AChE with obidoxime or 3.5 min for HI-6. The calculation for the reactivation of VX-inhibited human BChE by obidoxime (50 µM) was achieved with a k r of 0.995 min−1 and a K D of 1007 µM resulting in k obs of 0.05 min−1 and a half-life of 14.7 min. For HI-6, a k r of 0.812 min−1 and a K D of 450 µM were determined resulting in k obs of 0.08 min−1 and a half-life of 8.5 min (unpublished data). The decrease in the VX concentration in HEG-AChE, erythrocytes and plasma was in good agreement with the measured data for HI-6. However, with obidoxime, there was a delay between the determined decrease in inhibitory capacity and the calculated decrease in VX concentration with HEG-AChE and erythrocytes (Fig. 4a, c).
Suitability of fresh frozen plasma and packed red blood cells as clinically available source of ChE
To assess a potential clinical application of the concept of pseudocatalytic scavenging FFP, PRBC and a 1:1 mixture of both were tested (Fig. 5). Again, HI-6 was more effective in reducing inhibitory activity in the presence of PRBC than obidoxime; the effect was comparable to the results obtained with freshly drawn erythrocytes (Figs. 4, 5). With obidoxime, the loss of inhibitory activity was slower with PRBC compared to erythrocytes. Interestingly, the use of FFP resulted in a substantially slower and incomplete decrease in inhibitory activity compared to plasma. Finally, the detoxifying effect of oximes in combination with PRBC plus FFP was slightly slower compared to whole blood.
Pseudocatalytic scavenging of VX with obidoxime and HI-6 in packed red blood cells (PRBC), fresh frozen plasma (FFP) and combination of both. Obidoxime showed a slower decrease in inhibitory activity (red line red inverted triangle) and increase in activity (blue line blue triangle) than HI-6 with VX-incubated (100 nM) PRBC (a vs. b). With VX-incubated (100 nM) FFP the decrease in inhibitory activity was comparable with both oximes (c vs. d). Combination of PRBC and FFP resulted in a more rapid decrease in inhibitory activity and activity increase with HI-6 than obidoxime (e vs. f). Data are given as ChE activity in % or inhibitory activity in (%) of n = 4 experiments ± SD (color figure online)
Discussion
Current research is directed to develop primarily new BChE-specific reactivators to turn plasma BChE into a pseudocatalytic scavenger or to even augment this process by administration of recombinant BChE (Kovarik et al. 2010; Radić et al. 2013; Elsinghorst et al. 2013; Nachon et al. 2013; Sit et al. 2014). As a short-term solution, administration of the licensed obidoxime or HI-6 as oxime in advanced development together with additional—by itself stoichiometric—AChE and/or BChE from blood products is a practical approach to elevate the oxime-mediated pseudocatalytic breakdown of VX in the vascular compartment to decrease the toxic body load and improve physical recovery. We used 100 nM VX as multiple LD50 and a possible blood concentration after extensive inhalational uptake (Reiter et al. 2011). This represents a concentration overwhelming the endogenous scavenging capacity and mechanisms by a simple binding to free ChEs and proteins and provided complete and stable inhibition during the experiment duration (Fig. 3a).
We added the oximes obidoxime and HI-6 in concentrations of 50 µM to our model—a concentration comparable to the C max after intramuscular injection of 250 mg obidoxime and 500 mg HI-6 in humans (Sidell and Groff 1970; Clement et al. 1995). Both oximes are supposed to turn ChE into a pseudocatalytic scavenger thus elevating the endogenous scavenging capacity to steadily decrease the VX load in the body. Accordingly, the degradation of higher VX concentrations with whole blood resulted in a linear relationship of the VX concentrations and the half-maximal reactivation time (Fig. 3c–e). The results of this semiquantitative study show that the concept of pseudocatalytic scavenging does not necessarily depend on the reactivation of OP-inhibited BChE by specially designed oximes but that additional AChE and BChE in combination with established oximes might be a possible short-term solution too (Figs. 4, 5).
The decrease in inhibitory activity with HI-6 for VX-inhibited HEG-AChE, erythrocyte and whole blood was considerably faster than the respective kinetics of obidoxime (Figs. 3b vs. c, 4, 5). In addition, there was a delay of the measured decrease in the VX concentration compared to the calculated one with obidoxime for HEG-AChE and erythrocytes (Fig. 4). This might be due to the effect of a formation of stable phosphyl oximes with the 4,4′-oxime obidoxime whose inhibitory activity toward AChE is of impact here in a cuvette-based system and delays pseudocatalytic scavenging with obidoxime compared to the 2-oxime HI-6 (Herkenhoff et al. 2004). Although AChE binding sites of erythrocytes were adjusted to that of HEG-AChE, the decrease in inhibitory activity was faster with erythrocytes and might be due to unspecific side reactions with the intact membrane of erythrocytes.
For plasma, the velocity of decrease in inhibitory activity showed similar kinetics as HEG-AChE and erythrocytes (Fig. 4). This is a little surprising if comparing the lower reactivation constants of BChE with obidoxime and HI-6 with the respective AChE data. However, as the BChE binding sites (38.8 ± 0.3 nM) were sixfold higher than the AChE binding sites (6.5 ± 0.1 nM), this effect is partly outweighed here (Figs. 4, 5).
Klose and Gutensohn (1976) described a case report of deliberate intramuscular demeton-O-methyl poisoning where the administration of 500 mg obidoxime showed no immediate beneficial effect, and thus it was decided to infuse isolated human BChE. The small amount of lyophilisated BChE (90 mg) does not necessarily explain the early improvement of the patient’s symptoms alone if one assumes a stoichiometric mechanism only. Yet, the combination of the previously administered oxime followed by the intravenous BChE application was possibly the first—although unwittingly—use of pseudocatalytic scavenging in a clinical case report. However, the isolation of human plasma BChE or production of recombinant BChE is time-consuming and costly, yet. We therefore tested PRBC, FFP and a mixture of both as external, clinically available ChE source to proof feasibility as therapeutic approach (Fig. 5).
The PRBC showed a decrease in inhibitory activity with HI-6 and obidoxime similar to the native erythrocytes (Figs. 4c vs. 5a, 4d vs. 5b), the decrease in the VX concentration being faster with HI-6. In contrast, the kinetics of inhibitory activity with FFP and freshly prepared plasma were comparable with both tested oximes.
Finally, the possible benefit of the emergency treatment options PRBC and FFP was calculated. Both were adjusted to the ChE activities of the fresh HEG-AChE and freshly drawn plasma due to the correlation between ChE activity and binding sites (Wille et al. 2014). Assuming 6.5 ± 0.1 nM AChE binding sites, 6 l blood and a haematocrit of 50 %, this would result in 19.5 nmol AChE binding sites in the blood compartment. With a PRBC volume of 450 mL, this pool is elevated by additional 3 nmol binding sites in the vascular compartment, i.e., 15 %. Accordingly, administration of 250 mL FFP with 38.8 ± 0.3 nM BChE binding sites would result in additional 10 nmol binding sites in the blood compartment and an increase of 9 %. Both erythrocyte and plasma administration would increase the ChE level in the body to allow a somewhat faster scavenging of highly toxic nerve agents in a nanomolar concentration range [2LD50 VX s.c., i.e., 18 µg kg−1 resulted in roughly 10 nM VX in whole blood samples (Worek et al. 2014a)]. From a practical point of view, the concept of pseudocatalytic scavenging would start with the first and even preclinical-administered oxime dose. Administration of external ChE sources should be considered as supportive treatment in the hospital if percutaneous or oral uptake by persistent OP has to be taken into account to counteract prolonged absorption and decrease or even inhibit OP-transfer into target tissue. For an acute inhalation type exposure with volatile nerve agents and rapid distribution for target tissues, this approach does not seem to be useful. For OP pesticide poisoning, the estimated body load would be much higher and concentrations of almost 1 mM of omethoate have been detected in blood (Pavlic et al. 2002). In such a case of mega-dose-poisoning, oxime-mediated breakdown of the OP pesticide could be accelerated and shorten the time of artificial ventilation. One has to keep in mind that AChE and BChE activities show inter-individual variability and are not routinely tested in blood products but will influence rate of pseudocatalytic scavenging (Augustinsson 1955).
In conclusion, pseudocatalytic scavenging of VX can be achieved with commercially available AChE (PRBC)- and BChE (FFP)-containing blood products in combination with obidoxime or HI-6. Hereby, HI-6 proved to be more effective and the velocity of VX detoxification was more rapid with combined use of PRBC and FFP. This approach may be a useful supportive adjunct to standard atropine plus oxime treatment in case of poisoning with persistent nerve agents, e.g., VX and its analogues, in order to reduce the period of toxicologically relevant agent concentrations. Additional research is needed to evaluate the effectiveness of this concept in vivo. In the end, the use of commercial ChE products and established oximes may serve as an interim solution until more effective oximes and eventually AChE or BChE mutants with superior properties are available.
References
Aldridge WN, Reiner E (1972) Enzyme inhibitors as substrates: interactions of esterases with esters of organophosphorus and carbamic acids. North-Holland, Amsterdam
Augustinsson KB (1955) The normal variation of human blood cholinesterase activity. Acta Physiol Scand 35:40–52. doi:10.1111/j.1748-1716.1955.tb01262.x
Aurbek N, Thiermann H, Eyer F et al (2009) Suitability of human butyrylcholinesterase as therapeutic marker and pseudo catalytic scavenger in organophosphate poisoning: a kinetic analysis. Toxicology 259:133–139. doi:10.1016/j.tox.2009.02.014
Clement JG, Bailey DG, Madill HD et al (1995) The acetylcholinesterase oxime reactivator HI-6 in man: pharmacokinetics and tolerability in combination with atropine. Biopharm Drug Dispos 16:415–425
Dolgin E (2013) Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med 19:1194–1195. doi:10.1038/nm1013-1194
Eisenkraft A, Gilburd D, Kassirer M, Kreiss Y (2014) What can we learn on medical preparedness from the use of chemical agents against civilians in Syria? Am J Emerg Med 32:186. doi:10.1016/j.ajem.2013.11.005
Elsinghorst PW, Worek F, Thiermann H, Wille T (2013) Drug development for the management of organophosphorus poisoning. Expert Opin Drug Discov 8:1467–1477. doi:10.1517/17460441.2013.847920
Herkenhoff S, Szinicz L, Rastogi VK et al (2004) Effect of organophosphorus hydrolysing enzymes on obidoxime-induced reactivation of organophosphate-inhibited human acetylcholinesterase. Arch Toxicol 78:338–343. doi:10.1007/s00204-004-0547-2
Klose R, Gutensohn G (1976) Behandlung einer Alkylphosphatintoxikation mit gereinigter Serumcholinesterase. Prakt Anästh 11:1–7
Kovarik Z, Katalinić M, Sinko G et al (2010) Pseudo-catalytic scavenging: searching for a suitable reactivator of phosphorylated butyrylcholinesterase. Chem Biol Interact 187:167–171. doi:10.1016/j.cbi.2010.02.023
Kwong TC (2002) Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit 24:144–149
Lee EC (2003) Clinical manifestations of sarin nerve gas exposure. JAMA 290:659–662. doi:10.1001/jama.290.5.659
Maxwell DM, Saxena A, Gordon RK, Doctor BP (1999) Improvements in scavenger protection against organophosphorus agents by modification of cholinesterases. Chem Biol Interact 119–120:419–428
Mumford H, Price ME, Lenz DE, Cerasoli DM (2011) Post-exposure therapy with human butyrylcholinesterase following percutaneous VX challenge in guinea pigs. Clin Toxicol 49:287–297. doi:10.3109/15563650.2011.568944
Mumford H, Docx CJ, Price ME et al (2013) Human plasma-derived BuChE as a stoichiometric bioscavenger for treatment of nerve agent poisoning. Chem Biol Interact 203:160–166. doi:10.1016/j.cbi.2012.08.018
Nachon F, Brazzolotto X, Trovaslet M, Masson P (2013) Progress in the development of enzyme-based nerve agent bioscavengers. Chem Biol Interact 206:536–544. doi:10.1016/j.cbi.2013.06.012
Pavlic M, Haidekker A, Grubwieser P, Rabl W (2002) Fatal intoxication with omethoate. Int J Leg Med 116:238–241. doi:10.1007/s00414-002-0299-6
Radić Z, Dale T, Kovarik Z et al (2013) Catalytic detoxification of nerve agent and pesticide organophosphates by butyrylcholinesterase assisted with non-pyridinium oximes. Biochem J 450:231–242. doi:10.1042/BJ20121612
Reiter G, Mikler J, Hill I et al (2011) Simultaneous quantification of VX and its toxic metabolite in blood and plasma samples and its application for in vivo and in vitro toxicological studies. J Chromatogr B Anal Technol Biomed Life Sci 879:2704–2713. doi:10.1016/j.jchromb.2011.07.031
Sidell FR, Groff WA (1970) Toxogonin: blood levels and side effects after intramuscular administration in man. J Pharm Sci 59:793–797
Sit RK, Fokin VV, Amitai G et al (2014) Imidazole aldoximes effective in assisting butyrylcholinesterase catalysis of organophosphate detoxification. J Med Chem 57:1378–1389. doi:10.1021/jm401650z
Taylor P, Radić Z (1994) The cholinesterases: from genes to proteins. Annu Rev Pharmacol Toxicol 34:281–320. doi:10.1146/annurev.pa.34.040194.001433
von der Wellen J, Bierwisch A, Worek F et al (2016) Kinetics of pesticide degradation by human fresh frozen plasma (FFP) in vitro. Toxicol Lett 244:124–128. doi:10.1016/j.toxlet.2015.07.014
Wille T, Thiermann H, Worek F (2014) In vitro kinetics of nerve agent degradation by fresh frozen plasma (FFP). Arch Toxicol 88:301–307. doi:10.1007/s00204-013-1130-5
Worek F, Thiermann H (2013) The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol Ther 139:249–259. doi:10.1016/j.pharmthera.2013.04.009
Worek F, Mast U, Kiderlen D et al (1999) Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta 288:73–90
Worek F, Thiermann H, Szinicz L, Eyer P (2004) Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharmacol 68:2237–2248. doi:10.1016/j.bcp.2004.07.038
Worek F, Seeger T, Reiter G et al (2014a) Post-exposure treatment of VX poisoned guinea pigs with the engineered phosphotriesterase mutant C23: a proof-of-concept study. Toxicol Lett 231:45–54. doi:10.1016/j.toxlet.2014.09.003
Worek F, Seeger T, Zengerle M et al (2014b) Effectiveness of a substituted β-cyclodextrin to prevent cyclosarin toxicity in vivo. Toxicol Lett 226:222–227. doi:10.1016/j.toxlet.2014.02.010
Worek F, Thiermann H, Wille T (2016) Catalytic bioscavengers in nerve agent poisoning: a promising approach? Toxicol Lett 244:143–148. doi:10.1016/j.toxlet.2015.07.012
Acknowledgments
The study was funded by the German Ministry of Defence. However, the design, performance, data interpretation and manuscript writing were under the complete control of the authors and have never been influenced. The authors are grateful to G. Duman for her engaged technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wille, T., von der Wellen, J., Thiermann, H. et al. Pseudocatalytic scavenging of the nerve agent VX with human blood components and the oximes obidoxime and HI-6. Arch Toxicol 91, 1309–1318 (2017). https://doi.org/10.1007/s00204-016-1776-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1776-x