Abstract
NecroX-5, one of the derivatives of NecroX series compounds, is a mitochondrial reactive oxygen species and reactive nitrogen species scavenger that inhibits cell death against various kinds of oxidative stresses. The objective of the present study was to evaluate the effects of NecroX-5 on neomycin-induced ototoxicity in transgenic zebrafish (Brn3C: EGFP). Five days post-fertilization, zebrafish larvae were exposed to 125 μM neomycin and one of the following NecroX-5 concentrations for 1 h: 10, 25, 50, and 75 μM. Hair cells within the neuromasts of the supraorbital (SO1 and SO2), otic (O1), and occipital (OC1) lateral lines were analyzed using fluorescence microscopy (n = 10). The terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay and 2-[4-(dimethylamino) styryl]-N-ethylpyridiniumiodide (DASPEI) assay were performed for evaluation of apoptosis and mitochondrial damage. Ultrastructural changes were evaluated using scanning electron microscopy. NecroX-5 decreased neomycin-induced hair cell loss in the neuromasts (NecroX-5 50 μM: 13.4 ± 2.0 cells, 125 μM neomycin only: 8.1 ± 1.2 cells; n = 10, P < 0.05) and decreased the TUNEL reaction. The ultrastructural analysis showed that the structures of mitochondria and hair cells within the neuromasts were preserved in zebrafish exposed to 125 μM neomycin and 50 μM NecroX-5. NecroX-5 decreased apoptosis and mitochondrial damage. In conclusion, NecroX-5 attenuated neomycin-induced hair cell loss in zebrafish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aminoglycosides are used for treatment of Gram-negative bacterial infection (Rizzi and Hirose 2007; Selimoglu 2007; Warchol 2010). However, aminoglycosides are also ototoxic agents that cause drug-induced hearing impairment (cochleotoxicity) and vestibular loss (vestibulotoxicity) (Selimoglu 2007). It has long been known that the ototoxicity of aminoglycosides is irreversible. The ototoxic effects of these drugs manifest as permanent bilateral sensorineural hearing loss beginning at high frequencies (cochleotoxicity) and/or as disequilibrium, nystagmus, and ataxia (vestibulotoxicity) (Selimoglu 2007; Warchol 2010). The incidence of cochleotoxicity from aminoglycosides has been reported to occur in 2–25 % patients, and vestibulotoxicity occurs in up to 15 % patients. However, the ototoxic effects are underestimated because of the ambiguity of the symptoms (Huth et al. 2011; Park et al. 2012a). While the exact mechanisms of aminoglycoside ototoxicity have not yet been fully understood, it appears to be involved in both the formation of reactive oxygen species (ROS) and induction of apoptotic cell death pathways (Harris et al. 2003; Kim et al. 2009; Selimoglu 2007; Warchol 2010). In the inner ear, aminoglycosides appear to generate ROS, which causes permanent damage to hair cells that result in permanent sensorineural hearing loss (Huth et al. 2011; Selimoglu 2007). A recent study confirmed that the ROS generation is one of the crucial early steps in the mechanism of aminoglycoside ototoxicity. Thus, ROS is thought to play a key role in the initiation of hair cell death via oxidative stress (Warchol 2010; Xie et al. 2011). It has been reported that the outer hair cells are more vulnerable to ototoxic injury than the inner hair cells (Rizzi and Hirose 2007; Selimoglu 2007; Xie et al. 2011). Pathological findings of hair cell death resemble those of apoptosis, which is primarily modulated by the activation of caspases via either intrinsic or extrinsic pathways (Chang et al. 2011; Rybak and Kelly 2003; Selimoglu 2007). It has been documented that mitochondria release apoptogenic factors that activate caspases via the intrinsic pathway and that caspases are also activated via the extrinsic pathway, which is triggered by ligand binding to receptors such as Fas and TNFR1 (Huth et al. 2011; Rizzi and Hirose 2007; Rybak and Kelly 2003; Selimoglu 2007). Although several chemical agents that inhibit aminoglycosides-induced ototoxicity have been identified, none has been approved for use in clinical fields (Chang et al. 2011; Rizzi and Hirose 2007; Rybak and Whitworth 2005; Selimoglu 2007).
NecroX-5, one of the derivatives of NecroX series compounds, is a mitochondrial ROS and RNS scavenger that inhibits cell death induced by various kinds of oxidative stresses including oxidative stress induced by tBHP, DOX, CCl4, and hypoxic injury (Choi et al. 2010; Kim et al. 2010; Park et al. 2012a; Thu et al. 2012). NecroX-5 has also exhibited cytoprotective activity against various types of cell damage (Choi et al. 2010; Park et al. 2012a). Furthermore, NecroX-5 has been shown to attenuate myocardial damage resulting from reoxygenation injury in rat (Park et al. 2012a). Although the protective effect of NecroX-5 against cell damage has been documented in various models, the mechanisms underlying the protective effect of this compound on hair cells is poorly understood (Park et al. 2012a, b; Thu et al. 2012).
Zebrafish (Danio rerio) have been used as a model for general toxicology studies. Recently, several research groups have recognized the potential use of the zebrafish lateral line to perform toxicity studies on hair cells. The lateral line, a series of sensory organs aligned along the head and the body, consists of neuromasts comprising several sensory hair cells and adjacent supporting cells. The hair cells of neuromasts in the zebrafish lateral line are useful for studying ototoxicity (hair cell damage) after exposure to ototoxic agents (Barros et al. 2008; Bowman and Zon 2010; Chakraborty et al. 2009; Chiu et al. 2008; Coffin et al. 2010; Delvecchio et al. 2011; Harris et al. 2003; Kari et al. 2007; Kim et al. 2012; Lee et al. 2011; Zon and Peterson 2005).
Therefore, the purpose of the current study was to investigate the effects of NecroX-5 on neomycin-induced hair cell loss in zebrafish.
Materials and methods
Chemical reagents
Neomycin powder was purchased from Sigma Chemical Co. (CAS-No. 1405-10-3, St. Louis, MO, USA). The product included a minimum of 85 % neomycin B with the remainder as neomycin C. NecroX-5 was synthesized by LG Life Sciences (Korea).
Zebrafish preparation
The transgenic zebrafish larvae(Brn3C: EGFP) were produced by mating paired adult fish maintained at 28.5 °C in a zebrafish facility at Korea University Ansan Hospital. Larvae were maintained at a density of about 50 larvae per 100-mm2 Petri dish in embryo media (1 mM MgSO4, 120 μM KH2PO4, 74 μM Na2HPO4, 1 mM CaCl2, 500 μM KCl, 15 μM NaCl, and 500 μM NaHCO3 in dH2O). This study was approved by the Korea University Institutional Animal Care and Use Committee (No. of Approval: KUIACUC-2012-116). All protocols were performed in accordance with the guidelines of the Animal Care Ethics Committee of Korea University Medical Center and National Institutes of Health (NIH) guidelines.
Analysis of otoprotective effects in zebrafish
Neomycin solutions were prepared by adding the pure neomycin powder to the embryo medium. Neomycin has been shown to decrease the viability of neuromasts in a dose-dependent manner (Choi et al. 2013; Ou et al. 2009). In addition, previous results showed that neuromasts treated with 125 μM neomycin for 1 h show a viability rate of 50 % (data is not shown). The 125 μM neomycin was chosen as an adequate experimental condition for following methods. For this study, 125 μM neomycin was used. Ten fish were tested at each of the NecroX-5 concentrations specified below, and the experiments were repeated 3 times. The larvae of 5-day post-fertilization(dpf) zebrafish were exposed to 125 μM neomycin, and one of the following concentrations of NecroX-5 for 1 h: 10, 25, 50, 75, and 100 μM. After the zebrafish larvae were exposed to neomycin and NecroX-5, they were rinsed three times in embryo media and anesthetized with Tricane (3-aminobenzoic acid, 0.4 g/ethyl ester, 100 mL–pH 7, adjustments by Tris buffer) for 5 min.
The zebrafish were mounted with methylcellulose on a depression slide for evaluation by using a fluorescence microscope. Fish viability was confirmed under a microscopy by visualization of heartbeat and blood flow. Hair cells within the neuromasts of the supraorbital (SO1 and SO2), otic (O1), and occipital (OC1) lateral lines on one side of each fish were analyzed (Choi et al. 2013; Ou et al. 2009, 2007; Raible and Kruse 2000).
The average numbers of hair cells of the SO1, SO2, O1, and OC1 neuromasts were counted for each zebrafish under all experimental and control conditions (n = 10) using a fluorescence microscope (Imager. M1; Carl Zeiss).
Assessment of apoptosis and mitochondria in zebrafish
Apoptosis in the neuromasts was determined using the Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) method with an in situ cell detection kit (Roche Molecular Biochemicals, Mannheim, Germany) following the manufacturer’s protocol. The zebrafish larvae were exposed to medium containing 125 μM neomycin and 50 μM NecroX-5 for 1 h. Thereafter, the larvae were washed with phosphate-buffered saline (PBS) and fixed in 4 % paraformaldehyde. The larvae were then incubated with 50 μL TUNEL reaction mixture (TdT and fluorescein-dUTP) at 37 °C for 60 min in a humid atmosphere. The zebrafish were then evaluated under a fluorescence microscope (AxioCam MRc5; Carl Zeiss). The fluorescent dye, 2-[4-(dimethylamino) styryl]-N-ethylpyridinium iodide (DASPEI; Invitrogen, Carlsbad, CA, USA), was used to stain mitochondria within the hair cells. Larvae were then incubated in the embryo medium containing 0.005 % DASPEI for 15 min and were analyzed as described above.
Scanning electron microscopy
For scanning electron microscopy (SEM), the larvae of 5-dpf zebrafish exposed to 125 μM neomycin and NecroX-5 for 1 h were prefixed by immersion in 2 % glutaraldehyde in 0.1 M phosphate buffer and post-fixed for 2 h in 1 % osmic acid dissolved in PBS. The larvae were treated in a graded series of ethanol and t-butyl alcohol, dried in a freeze dryer (Hitachi, ES-2030; Hitachi, Tokyo, Japan), platinum coated using an ion coater (Eiko, IB-5; Eiko), and observed under an field emission SEM (Hitachi, S-4700) (Kim TY et al. 2002).
Statistical analysis
All values are shown as mean ± standard deviation. For statistical comparisons, one-way analysis of variance was used for multiple comparisons using the SPSS software (ver. 20.0; SPSS, Chicago, IL, USA). A p value of <0.05 was considered to indicate statistical significance.
Results
Effect of NecroX-5 on hair cells in zebrafish
Hair cells within 4 neuromasts, SO1, SO2, O1, and OC1, were evaluated. Transgenic zebrafish (Brn3C: EGFP) had green-colored neuromasts that could be visualized under a fluorescent microscope without prior staining with fluorescent stains. Treatment of 125 μM neomycin for 1 h significantly decreased the number of hair cells in the neuromasts. Hair cells from the 4 neuromasts were analyzed to investigate the changes after treatment with neomycin and NecroX-5. Fluorescent microscopy was used to calculate hair cell survival as the average number of the hair cells present in the control group not exposed to neomycin. While 125 μM neomycin significantly reduced the number of hair cells, NecroX-5 alleviated neomycin-induced hair cell loss (control: 15.2 ± 1.5 cells, 125 μM neomycin only: 8.1 ± 1.2 cells, NecroX-5 10 μM: 6.7 ± 1.2 cells, 25 μM: 8.0 ± 1.2 cells, 50 μM: 13.4 ± 2.0 cells, 75 μM: 8.4 ± 0.9 cells; n = 10, p < 0.05; Figs.1, 2).
Quantitative assay for hair cell damage in the 5-dpf transgenic zebrafish(Brn3C: EGFP). Hair cells from 4 neuromasts (SO1, SO2, O1, and OC1) were analyzed. Treatment of the 5-dpf transgenic zebrafish with 125 μM of neomycin for 1 h significantly decreased the number of hair cells in the neuromasts. NecroX-5 protected against neomycin-induced hair cell loss in neuromasts (control: 15.2 ± 1.5 cells, 125 μM neomycin only: 8.1 ± 1.2 cells, NecroX-5 10 μM: 6.7 ± 1.2 cells, 25 μM: 8.0 ± 1.2 cells, 50 μM: 13.4 ± 2.0 cells, 75 μM: 8.4 ± 0.9 cells; n = 10, p < 0.05). (Neo: neomycin)
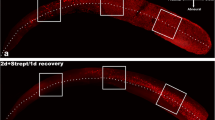
Fluorescent microscopy (SO2, ×40). The 5-dpf transgenic zebrafish were treated simultaneously with 125 μM of neomycin and 50 μM NecroX-5 for 1 h. Treatment with 125 μM neomycin resulted in a significant decrease in the number of hair cells, and 50 μM NecroX-5 attenuated neomycin-induced hair cell damage. Scale bar 10 μm
Effect of NecroX-5 on apoptosis and mitochondria in zebrafish
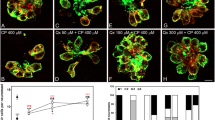
NecroX-5 was used at the concentration of 50 μM in the TUNEL assays for apoptosis and DASPEI for mitochondria because that concentration was the most effective dose. The TUNEL reaction was used to evaluate whether neomycin induced apoptosis of neuromasts and whether hair cell death could be attenuated by NecroX-5. Apoptotic cells were marked as light red dots by the TUNEL assay. As shown in Fig. 3, neomycin increased TUNEL-positive cells, and NecroX-5 treatment decreased TUNEL-positive cells. These results indicate that neomycin induces apoptotic cell death, which can be attenuated by NecroX-5 treatment. DASPEI staining was performed to analyze mitochondrial damage, and the staining results showed that mitochondria within hair cells were protected by NecroX-5 (Fig. 4).
Analysis of mitochondrial damage with DASPEI (SO2, ×40). The 5-dpf transgenic zebrafish were treated with 125 μM neomycin and 50 μM NecroX-5 for 1 h. Treatment with 125 μM neomycin resulted in a significant decrease in mitochondrial staining with DASPEI, and this overall mitochondrial damage was attenuated by NecroX-5. Scale bar 10 μm
Evaluation of TUNEL assay. TUNEL assay was used to identify neomycin-induced apoptotic cells. Apoptotic cells are marked as light red dots in red-colored fish after the TUNEL reaction under a fluorescent microscope (arrow indicates TUNEL-positive cells). No light red dots were identified in the negative control zebrafish. A comparison of the color intensity between the group treated with 125 μM neomycin and the group treated with 50 μM NecroX-5 for 1 h showed that the number of TUNEL-positive cells significantly decreased in the 5-dpf transgenic zebrafish with NecroX-5, and apoptotic cell death of the neuromasts hair cells was attenuated by NecroX-5 (green-colored fish of right column, control for TUNEL reaction; red-colored fish of middle column, TUNEL reaction; green- and red-colored fish of left column, combination of control and TUNEL reaction). Scale bar 200 μm
Ultrastructural changes in zebrafish treated with neomycin and NecroX-5
Figure 5 shows scanning electron micrographs of stereocilia and kinocilium from neomycin—and NecroX-5—treated zebrafish. Compared to control zebrafish, the zebrafish exposed to 125 μM neomycin for 1 h showed severe morphological damage to hair cells, including loss or fusion of stereocilia and kinocilium. However, NecroX-5-treated zebrafish showed lower neomycin-induced hair cell damage as compared to that of zebrafish treated with neomycin alone.
Scanning electron microscopy (SEM, OC1). Compared to the hair cells of negative control, the hair cells of the 5-dpf transgenic zebrafish treated with 125 μM neomycin for 1 h showed severe morphological damage to stereocilia and kinocilium under SEM. However, the damage was prevented by NecroX-5. Scale bar (at the bottom of each figure, one space) 10 μm
Discussion
To our knowledge, this study is the first to investigate the protective effects of NecroX-5 against neomycin-induced ototoxicity, using the transgenic zebrafish (Brn3C: EGFP), an excellent experimental model for ototoxicity. In the current study, NecroX-5 significantly attenuated neomycin-induced hair cell damage in zebrafish with anti-apoptotic activities.
Aminoglycosides are commonly used for the treatment of various diseases such as sepsis in infants and adults, urinary tract infections, and cystic fibrosis (Selimoglu 2007). Moreover, aminoglycosides are used in combination therapies for multi-drug-resistant tuberculosis (Park et al. 2012b; Rizzi and Hirose 2007). It has been demonstrated that aminoglycosides generate ROS within hair cells. The ROS formation subsequently causes either direct or indirect hair cell damage by ROS-induced disarray of stereocilia, which ultimately leads to apoptotic cell death and permanent sensorineural hearing loss (Huth et al. 2011; Selimoglu 2007). It has been reported that ROS is thought to occur because of aminoglycoside affinity for iron (Huth et al. 2011; Selimoglu 2007; Warchol 2010). Aminoglycosides interact with iron to form aminoglycoside-iron complexes that enhance iron-catalyzed oxidation and thereby directly promote ROS formation. It has been documented that ROS can also affect proteins and nucleic acids, thereby disrupting the activity of enzymes, ion channels, and receptors via lipid peroxidation (Huth et al. 2011; Xie et al. 2011). It has been shown that ROS can attack a variety of cell components such as cell membranes and DNA, causing irreversible damage and programed cell death (apoptosis) (Huth et al. 2011; Rizzi and Hirose 2007; Rybak and Whitworth 2005; Selimoglu 2007; Xie et al. 2011). The morphological features of apoptosis are condensed chromatin, nuclear pyknosis, and apoptotic body formation. Numerous studies have attempted to identify chemical agents that protect the inner ear from aminoglycoside-induced ototoxicity.
Many specific categories of toxicity can also be assessed in the zebrafish, including developmental toxicity, cardiotoxicity, and neurotoxicity including ototoxicity. The majority of the zebrafish toxicology studies have focused on acute toxicity to chemicals, pesticides, and pharmaceuticals (Coffin et al. 2010; Delvecchio et al. 2011).
More recently, the zebrafish has become a common model system in biomedical research studies such as hearing and vestibular function studies (Barros et al. 2008; Bowman and Zon 2010; Chiu et al. 2008; Kari et al. 2007; Ou et al. 2012, 2009; Owens et al. 2007; Raible and Kruse 2000; Scholz 2013; Ton and Parng 2005; Warchol 2010). All aquatic vertebrates, including zebrafish, have a sensory system, called lateral line, that is composed of hair cells on the body surface. It has been reported that hair cells of zebrafish are functionally and structurally similar to mammalian inner ear hair cells. These hair cells are organized into clusters of 10-15 cells, called neuromasts, and are located on the head and along the body (Harris et al. 2003; Lee et al. 2011; Ou et al. 2007; Ton and Parng 2005). The superficial location of hair cells, combined with the optical transparency, allows easy visualization and manipulation of hair cells in living animals (Chiu et al. 2008; Ou et al. 2007; Warchol 2010). Hair cells of the lateral line provide for rapid in vivo assessment of hair cell death and evaluation of ototoxic compounds and compounds that could prevent hair cell damage (Chakraborty et al. 2009; Choi et al. 2013; Kari et al. 2007; Kim et al. 2012; Zon and Peterson 2005).
In the present study, we used transgenic zebrafish (Brn3C: EGFP) which have green neuromasts that are visible under a fluorescent microscope without staining, for determination of the potential ototoxicity of drugs and investigation of the protective activity of NecroX-5 with regard to neomycin-induced ototoxicity.
NecroX compounds were synthesized in 2006 by LG Life Sciences. These compounds are available as small molecules and show a good safety profile and low cellular toxicity. In recent studies, these compounds have shown protective effects against neurotoxicity, hypoxic injury, and oxidative stress (Kim et al. 2010; Park et al. 2012a, b; Thu et al. 2012). NecroX was known as a necrosis or necroptosis inhibitor that uses a RIP1 inhibitor. In addition, it was reported that NecroX-5 has antioxidant activity in the mitochondria and prevents reoxygenation injury by inhibiting the mitochondrial calcium uniporter (Thu et al. 2012). In the present study, the zebrafish were used to determine the potential ototoxicity of drugs and to investigate the protective activity of NecroX-5 with regard to neomycin-induced ototoxicity. NecroX-5 also inhibited neomycin-induced apoptosis of zebrafish hair cells in this experiment. Therefore, a necrosis inhibitor may be useful as a new treatment modality for drug-induced ototoxicity. The results of the present study corroborate those of an earlier study, which reported that NecroX shows a protective effect on hair cells in neonatal mouse cochlea cultures (Park et al. 2012b).
Although it has been documented that the zebrafish lateral line is a valuable system for evaluating drug-induced hair cell toxicity, it is important to know the critical differences between zebrafish hair cells and mammalian hair cells. Hair cells of the zebrafish lack stria vascularis and are morphologically and physiologically more similar to vestibular hair cells of the inner ear.
Therefore, the data obtained from zebrafish studies should be validated by mammalian studies (Chiu et al. 2008; Kim et al. 2012; Ou et al. 2007). Nevertheless, the zebrafish hair cell model is a sensitive and effective model for assessment of drug-induced ototoxicity and identification of otoprotective agents.
In conclusion, the results of the current study demonstrated that NecroX-5 attenuate neomycin-induced hair cell damage in zebrafish model. Further studies are currently ongoing to define the functional effects of NecroX-5 in vivo.
References
Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S (2008) Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol 154(7):1400–1413
Bowman TV, Zon LI (2010) Swimming into the future of drug discovery: in vivo chemical screens in zebrafish. ACS Chem Biol 5(2):159–161
Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G (2009) Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab 10(2):116–124
Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW (2011) Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res 282(1–2):92–96
Chiu LL, Cunningham LL, Raible DW, Rubel EW, Ou HC (2008) Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol 9(2):178–190
Choi JM, Park KM, Kim SH et al (2010) Effect of necrosis modulator necrox-7 on hepatic ischemia-reperfusion injury in beagle dogs. Transplant Proc 42(9):3414–3421
Choi J, Im GJ, Chang J et al (2013) Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol 33(2):125–133
Coffin AB, Ou H, Owens KN et al (2010) Chemical screening for hair cell loss and protection in the zebrafish lateral line. Zebrafish 7(1):3–11
Delvecchio C, Tiefenbach J, Krause HM (2011) The zebrafish: a powerful platform for in vivo, HTS drug discovery. Assay Drug Dev Technol 9(4):354–361
Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW (2003) Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol 4(2):219–234
Huth ME, Ricci AJ, Cheng AG (2011) Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int j otolaryngol 2011:937861. doi:10.1155/2011/937861
Kari G, Rodeck U, Dicker AP (2007) Zebrafish: an emerging model system for human disease and drug discovery. Clin Pharmacol Ther 82(1):70–80
Kim TY, Choi B, Park CH (2002) A study of structure of the sucker of common freshwater goby (Rhinogobius brunneus) and Triden goby (Tridentiger brevispinis). Korean J Electron Microsc 32(1):57–66
Kim JB, Jung JY, Ahn JC, Rhee CK, Hwang HJ (2009) Antioxidant and anti-apoptotic effect of melatonin on the vestibular hair cells of rat utricles. Clin Exp Otorhinolaryngol 2(1):6–12
Kim HJ, Koo SY, Ahn BH et al (2010) NecroX as a novel class of mitochondrial reactive oxygen species and ONOO− scavenger. Arch Pharm Res 33(11):1813–1823
Kim HJ, Yoon KA, Lee MK, Kim SH, Lee IK, Kim SY (2012) A novel small molecule, NecroX-7, inhibits osteoclast differentiation by suppressing NF-kappaB activity and c-Fos expression. Life Sci 91(19–20):928–934
Lee CK, Shin JI, Cho YS (2011) Protective effect of minocycline against cisplatin-induced ototoxicity. Clin Exp Otorhinolaryngol 4(2):77–82
Ou HC, Raible DW, Rubel EW (2007) Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res 233(1–2):46–53
Ou HC, Cunningham LL, Francis SP et al (2009) Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J Assoc Res Otolaryngol 10(2):191–203
Ou H, Simon JA, Rubel EW, Raible DW (2012) Screening for chemicals that affect hair cell death and survival in the zebrafish lateral line. Hear Res 288(1–2):58–66
Owens KN, Cunningham DE, MacDonald G, Rubel EW, Raible DW, Pujol R (2007) Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol 502(4):522–543
Park J, Park E, Ahn BH et al (2012a) NecroX-7 prevents oxidative stress-induced cardiomyopathy by inhibition of NADPH oxidase activity in rats. Toxicol Appl Pharmacol 263(1):1–6
Park MK, Lee BD, Chae SW, Chi J, Kwon SK, Song JJ (2012b) Protective effect of NecroX, a novel necroptosis inhibitor, on gentamicin-induced ototoxicity. Int J Pediatr Otorhinolaryngol 76(9):1265–1269
Raible DW, Kruse GJ (2000) Organization of the lateral line system in embryonic zebrafish. J Comp Neurol 421(2):189–198
Rizzi MD, Hirose K (2007) Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg 15(5):352–357
Rybak LP, Kelly T (2003) Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg 11(5):328–333
Rybak LP, Whitworth CA (2005) Ototoxicity: therapeutic opportunities. Drug Discov Today 10(19):1313–1321
Scholz S (2013) Zebrafish embryos as an alternative model for screening of drug-induced organ toxicity. Arch Toxicol. doi:10.1007/s00204-013-1044-2
Selimoglu E (2007) Aminoglycoside-induced ototoxicity. Curr Pharm Des 13(1):119–126
Thu VT, Kim HK, le Long T et al (2012) NecroX-5 prevents hypoxia/reoxygenation injury by inhibiting the mitochondrial calcium uniporter. Cardiovasc Res 94(2):342–350
Ton C, Parng C (2005) The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res 208(1–2):79–88
Warchol ME (2010) Cellular mechanisms of aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg 18(5):454–458
Xie J, Talaska AE, Schacht J (2011) New developments in aminoglycoside therapy and ototoxicity. Hear Res 281(1–2):28–37
Zon LI, Peterson RT (2005) In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 4(1):35–44
Acknowledgments
This research was supported by a Korea University Grant, by the Soo ENT Clinic and the Communication Disorders Center, Korea University, and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0006038).
Conflict of interest
The authors do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, JJ., Chang, J., Choi, J. et al. Protective role of NecroX-5 against neomycin-induced hair cell damage in zebrafish. Arch Toxicol 88, 435–441 (2014). https://doi.org/10.1007/s00204-013-1124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1124-3