Abstract
We examined the effects of cadmium chloride (CdCl2) exposure on the phosphorylation and function of the forkhead box class O (FOXO) transcription factor FOXO3a in HK-2 human renal proximal tubular cells. Phosphorylation of FOXO3a (at Thr32 and Ser253) and its upstream kinase, Akt (at Thr308 and Ser473) were markedly increased following exposure to 10 or 20 μM CdCl2. Treatment with wortmannin (500 nM), an inhibitor of phosphoinositide-3-kinase (PI3K), suppressed CdCl2-induced phosphorylation of Akt and FOXO3a at their Akt phosphorylation sites. CdCl2-induced phosphorylation of FOXO3a was markedly suppressed by the epidermal growth factor receptor inhibitor, AG1478 (1 μM), the Ca2+/calmodulin-dependent kinase II inhibitor, KN-93 (10 μM), and the Src inhibitor, PP2 (10 μM), but only partially suppressed by the insulin-like growth factor-1 receptor inhibitor, PPP (2.5 μM). Furthermore, the p38 inhibitor, SB203580 (20 μM), suppressed CdCl2-induced phosphorylation of Akt and FOXO3a, suggesting possible cross-talk between p38 mitogen-activated protein kinase and Akt. Although phosphorylation of FOXO3a was associated with reduced levels of nuclear FOXO3a, this change in cellular localization was transient. Silencing of FOXO3a expression using short interfering RNA suppressed CdCl2-induced cellular damage and accumulation of cytoplasmic nucleosomes in HK-2 cells. These results show that cadmium exposure induces phosphorylation of FOXO3a through the PI3K/Akt signaling pathway and suggest that FOXO3a phosphorylation (inactivation) transiently promotes survival of HK-2 cells. Phosphorylation of FOXO3a by the PI3K/Akt pathway may regulate cell fate in proximal tubules exposed to cadmium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium is an occupational and environmental pollutant that damages various organs, especially renal proximal tubular cells (Nordberg et al. 2007). It has been reported that cadmium induces apoptotic cell death in the proximal tubules of experimental animals (Hamada et al. 1997) and in cultured LLC-PK1 porcine and HK-2 human proximal tubular epithelial cells (Matsuoka and Call 1995; Komoike et al. 2012). Conversely, cadmium exposure has also been shown to activate cell survival signals including phosphoinositide-3-kinase (PI3K) and its downstream serine/threonine kinase, Akt (also known as protein kinase B), in various cell types (Liu et al. 2008; Xiao et al. 2009; Chen et al. 2011; Son et al. 2012). Identification of the signaling molecules that promote cell survival and/or cell death in proximal tubules is important for designing strategies to prevent cadmium-induced damage to proximal tubules.
Downstream targets of the PI3K/Akt signaling pathway include the forkhead box, class O (FOXO) subfamily of forkhead transcription factors (Dansen and Burgering 2008). The four members of FOXO that have been identified and characterized in mammals are FOXO1 (FKHR), FOXO3a (FKHRL1), FOXO4 (AFX), and FOXO6 (Burgering 2008). FOXO1, FOXO3a, and FOXO4 are ubiquitously expressed in mammals. However, FOXO1 and FOXO4 are expressed at the highest levels in adipose tissue and skeletal muscle, respectively. FOXO3a is predominantly expressed in the heart, brain, kidney, and ovary. FOXO6 appears to be uniquely expressed in the brain (van der Vos and Coffer 2011; Zhang et al. 2011). FOXO transcription factors are involved in diverse cellular and physiological processes including cell proliferation, apoptosis, reactive oxygen species (ROS) response, longevity, cell cycle regulation, and metabolism (Tzivion et al. 2011). Akt phosphorylates FOXOs at three conserved serine/threonine residues and promotes their interaction with the 14-3-3 protein, which results in cytoplasmic retention and inactivation of FOXO function (Brunet et al. 1999). However, the phosphorylation status of FOXOs and Akt and the toxicological significance of phosphorylation of FOXOs in renal proximal tubular cells exposed to cadmium have not yet been reported.
Therefore, we examined phosphorylation of FOXO3a at their Akt phosphorylation sites, Thr32 and Ser253 (Tzivion et al. 2011), in HK-2 cells exposed to cadmium chloride (CdCl2). Using wortmannin, an inhibitor of PI3K, we examined the involvement of PI3K/Akt in cadmium-induced phosphorylation of FOXO3a. Further, we examined the roles of epidermal growth factor receptor (EGFR), Ca2+/calmodulin-dependent kinase II (CaMKII), insulin-like growth factor-1 receptor (IGF-1R), and the Src family of non-receptor tyrosine kinases in HK-2 cells treated with CdCl2 to identify the upstream signaling pathways that regulate PI3K/Akt-mediated phosphorylation of FOXO3a. We also examined the effects of cross-talk between the PI3K/Akt pathway and mitogen-activated protein kinases (MAPKs) including extracellular signal-regulated protein kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38 on cadmium-induced phosphorylation of FOXO3a. Finally, we used short interfering RNA (siRNA) against the human FOXO3a gene to examine the effects of FOXO3a phosphorylation (inactivation) on cellular damage induced by long-time cadmium exposure.
Materials and methods
Chemicals
Cadmium chloride was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Wortmannin and KN-93 were obtained from MP Biomedicals, LLC (Solon, OH) and Cayman Chemical Company (Ann Arbor, MI), respectively. AG1478 and PPP were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). PP2 (AG1879), U0126, SP600125, and SB203580 were purchased from Calbiochem, Merck KGaA (Darmstadt, Germany). Antibodies against phospho-FoxO1 (Thr24)/FoxO3a (Thr32), phospho-FoxO3a (Ser253), total FoxO3a (75D8), phospho-Akt (Thr308) (C31E5E), phospho-Akt (Ser473) (D9E) XP®, and total Akt (pan) (C67E7) were obtained from Cell Signaling Technology, Inc. (Beverly, MA). Actin (I-19) and lamin A/C (14/LaminAC) antibodies were obtained from Santa Cruz Biotechnology, Inc. and BD Biosciences (Franklin Lakes, NJ), respectively. The siRNAs targeted against the human FOXO3a gene [Hs FOXO3 1 FlexiTube siRNA (SI04916366) and Hs FOXO3 3 FlexiTube siRNA (SI04916380)] and non-target siRNA (AllStars Negative Control siRNA) were purchased from Qiagen (Hilden, Germany).
Cell culture and treatments
HK-2 cells were obtained from the American Type Culture Collection (Manassas, VA) and grown in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 supplemented with 10 % heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO, Invitrogen Corp., Carlsbad, CA) in a humidified atmosphere of 5 % CO2 and 95 % air at 37 °C. Exponentially growing HK-2 cells were seeded at 4 × 105 cells/well in six-well culture plates or 1.4 × 104 cells/well in 96-well culture plates and cultured for 1 day before each experiment. CdCl2 was dissolved in water and sterilized by filtration. Cells were incubated in serum-free media containing the appropriate concentration of CdCl2 for 0.5–12 h at 37 °C. Wortmannin, AG1478, KN-93, PPP, U0126, SP600125, SB203580, and PP2 were dissolved in dimethyl sulfoxide (DMSO). After incubating cells in serum-free media with DMSO (0.1 %) or one of the inhibitors for 1 or 1.5 h, HK-2 cells were treated with 10 or 20 μM CdCl2 for an additional 4 h.
Preparation of whole cell lysates
At the end of the incubation, cells were washed with phosphate-buffered saline and lysed with sodium dodecyl sulfate–polyacrylamide gel Laemmli sample buffer. Cell lysates were collected, sonicated, and boiled for 5 min. Protein concentrations were determined using the RC DC Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA).
Preparation of cytoplasmic and nuclear fractions
Cytoplasmic and nuclear fractions were prepared using the Nuclear Extract Kit (Active Motif, Carlsbad, CA) following the manufacturer’s protocol.
Western blotting
Western blotting was carried out as described previously (Iwatsuki et al. 2011). Equal amounts of protein (20 μg) were subjected to sodium dodecyl sulfate-10 % polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Hybond-ECL, Amersham Pharmacia Biotech, Buckinghamshire, England). The membrane was blocked with 5 % non-fat milk in Tris-buffered saline containing 0.1 % Tween 20 for 1 h at room temperature. The membrane was then incubated overnight at 4 °C with the primary antibody, and protein was detected with a Phototope-HRP Western blot detection kit (Cell Signaling Technology, Inc.). The bands on the developed film were quantified with ImageJ 1.42 (National Institutes of Health, Bethesda, MD). The density of each band was normalized to that of actin or lamin A/C.
Gene knockdown of FOXO3a by siRNA
Transfection of siRNA against human FOXO3a and non-target siRNA into HK-2 cells was done using Lipofectamine RNAiMAX (Invitrogen Corp.) according to the manufacturer’s instructions with some adjustments. The siRNAs were dissolved in nuclease-free water and diluted to 0.2 μM with 250 μl Opti-MEM (Invitrogen Corp.). Five microliters of Lipofectamine RNAiMAX was also diluted 50-fold with Opti-MEM. Equal volumes of these two solutions were mixed (500 μl total) and immediately added to 2 ml culture media at the time of cell plating. After incubation for 24 h, cells were washed with media and used for the experiments.
Cell counts
Culture media containing floating cells were aspirated and reserved. Attached cells were trypsinized and suspended in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12. The numbers of attached cells and floating cells were counted using a TC10™ Automated Cell Counter (Bio-Rad Laboratories, Inc.).
Nucleosome assay
After preparing the cytoplasmic fraction, histone-associated DNA fragments (mono- and oligonucleosomes) were assayed with a Cell Death Detection ELISAPLUS (Roche Applied Science, Penzberg, Germany) according to the manufacturer’s instructions.
Statistical analysis
Results are expressed as the mean ± SD. Statistical significance was determined by Student’s t test. A value of P < 0.05 was considered to be statistically significant.
Results
Phosphorylation of FOXO3a and Akt following CdCl2 exposure
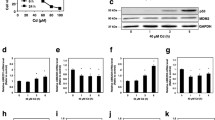
The levels of FOXO3a phosphorylated at Thr32 and Ser253 increased in HK-2 cells that were treated with 10 μM CdCl2 for 2 or 4 h (Fig. 1a). The level of FOXO3a also increased slightly after CdCl2 exposure. Phosphorylation of Akt at Thr308 and Ser473 increased after 1 h of exposure to 10 μM CdCl2. In contrast, the levels of Akt and actin did not change during the 6 h incubation period. When HK-2 cells were incubated with 2.5–40 μM CdCl2 for 4 h, concentrations higher than 2.5 or 5 μM induced phosphorylation of FOXO3a at Thr32 and Ser253 and increased the level of FOXO3a (Fig. 1b). In addition, Akt phosphorylation at Thr308 and Ser473 increased in a dose-dependent manner. On the other hand, the levels of Akt and actin were not changed after 4 h of incubation with any of the concentrations tested. While there was a slight increase in the level of FOXO4 phosphorylated at Thr28, phosphorylation of FOXO1 at Thr24 was not detectable in HK-2 cells exposed to 2.5–40 μM CdCl2 (data not shown). Thus, to examine the mechanisms that regulate phosphorylation of FOXO3a, HK-2 cells were treated with 10 or 20 μM CdCl2 for longer than 4 h.
Time course (a) and dose effects (b) of cadmium-induced phosphorylation of FOXO3a and Akt in HK-2 cells. Cells were incubated with 10 μM CdCl2 (Cd) for 0.5–6 h (a) or with 2.5–40 μM CdCl2 for 4 h (b). The untreated control in the time course experiment is labeled 0 h (a). Cell lysates were subjected to western blotting using antibodies against phospho-FoxO1 (Thr24)/FoxO3a (Thr32), phospho-FoxO3a (Ser253), total FoxO3a, phospho-Akt (Thr308), phospho-Akt (Ser473), total Akt, and actin. Results of densitometric analysis are also shown. Results are representative of at least three independent experiments
Effects of wortmannin on CdCl2-induced phosphorylation of FOXO3a
To reveal the involvement of PI3K/Akt in cadmium-induced phosphorylation of FOXO3a, HK-2 cells were pretreated with wortmannin, an inhibitor of PI3K (Fig. 2). Treatment with wortmannin (500 nM) suppressed CdCl2-induced phosphorylation of FOXO3a at Thr32 and Akt at Thr308 in HK-2 cells. Furthermore, the level of FOXO3a in CdCl2-treated HK-2 cells was suppressed by wortmannin. However, the levels of Akt and actin were not affected by wortmannin.
Effects of wortmannin on cadmium-induced phosphorylation of FOXO3a and Akt in HK-2 cells. Cells were incubated with 0.1 % DMSO or 500 nM wortmannin for 1 h and then incubated with or without CdCl2 (Cd) (10 or 20 μM) for 4 h. Cell lysates were subjected to western blotting using antibodies against phospho-FoxO1 (Thr24)/FoxO3a (Thr32), total FoxO3a, phospho-Akt (Thr308), total Akt, and actin. Results of densitometric analysis are also shown. Results are representative of at least three independent experiments
Effects of EGFR inhibitor, CaMKII inhibitor, or IGF-1R inhibitor on CdCl2-induced phosphorylation of FOXO3a
We next examined the upstream signaling pathways that regulate PI3K/Akt-mediated phosphorylation of FOXO3a in HK-2 cells exposed to cadmium (Fig. 3). Treatment with the EGFR inhibitor, AG1478 (1 μM), suppressed CdCl2-induced phosphorylation of FOXO3a at Thr32 and Akt at Thr308 in HK-2 cells (lane 6). Similarly, the CaMKII inhibitor, KN-93 (10 μM), suppressed CdCl2-induced phosphorylation of FOXO3a and Akt in HK-2 cells (lane 7). However, KN-93 induced a slight increase in phosphorylation of FOXO3a and Akt in the absence of CdCl2 (lane 3). The IGF-1R inhibitor, PPP (2.5 μM), increased phosphorylation of FOXO3a and Akt in HK-2 cells in the absence of CdCl2 (lane 4) and suppressed CdCl2-induced Akt phosphorylation (lane 8). In contrast, PPP caused a less marked suppression of CdCl2-induced FOXO3a phosphorylation (lane 8). Significant changes in the expression levels of FOXO3a, Akt, and actin were not observed (lanes 6, 7, and 8).
Effects of epidermal growth factor receptor (EGFR) inhibitor (AG1478), Ca2+/calmodulin-dependent kinase II (CaMKII) inhibitor (KN-93), or insulin-like growth factor-1 receptor (IGF-1R) inhibitor (PPP) on cadmium-induced phosphorylation of FOXO3a and Akt in HK-2 cells. Cells were incubated with 0.1 % DMSO, 1 μM AG1478 (AG), 10 μM KN-93 (KN), or 2.5 μM PPP (PP) for 1.5 h and then incubated with or without 10 μM CdCl2 (Cd) for 4 h. Cell lysates were subjected to western blotting using antibodies against phospho-FoxO1 (Thr24)/FoxO3a (Thr32), total FoxO3a, phospho-Akt (Thr308), total Akt, and actin. Results of densitometric analysis are also shown. Results are representative of at least three independent experiments
Effects of MAPK inhibitors or Src inhibitor on CdCl2-induced phosphorylation of FOXO3a
We then examined the possible involvement of MAPK family members (ERK, JNK, and p38) or the Src family kinases in cadmium-induced phosphorylation of FOXO3a in HK-2 cells (Fig. 4). Treatment with U0126 (20 μM), an inhibitor of MAPK/ERK kinases 1 and 2 (MEK1/2), or SP600125 (10 μM), an inhibitor of JNK, did not affect CdCl2-induced phosphorylation of FOXO3a at Thr32 or Akt at Thr308 in HK-2 cells (lanes 7 and 8). In contrast, the p38 inhibitor, SB203580 (20 μM), or the Src inhibitor, PP2 (10 μM), suppressed CdCl2-induced phosphorylation of FOXO3a and Akt (lanes 9 and 10). Furthermore, treatment with SB203580 or PP2 reduced the level of FOXO3a mildly in HK-2 cells exposed to CdCl2 (lanes 9 and 10). No significant changes in Akt and actin levels were found (lanes 7, 8, 9, and 10).
Effects of MAPK inhibitors (U0126, SP600125, and SB203580) or a Src inhibitor (PP2) on cadmium-induced phosphorylation of FOXO3a and Akt in HK-2 cells. Cells were incubated with 0.1 % DMSO, 20 μM U0126 (U), 10 μM SP600125 (SP), 20 μM SB203580 (SB), or 10 μM PP2 (P) for 1 h and incubated with or without 10 μM CdCl2 (Cd) for 4 h. Cell lysates were subjected to western blotting using antibodies against phospho-FoxO1 (Thr24)/FoxO3a (Thr32), total FoxO3a, phospho-Akt (Thr308), total Akt, and actin. Results of densitometric analysis are also shown. Results are representative of at least three independent experiments
Intracellular localization of FOXO3a following CdCl2 exposure
We examined the intracellular localization of FOXO3a in HK-2 cells exposed to cadmium (Fig. 5). Following exposure to 10 μM CdCl2, phosphorylation of FOXO3a at Thr32 was markedly increased in a time-dependent manner in both nuclear and cytoplasmic extracts. However, expression of FOXO3a was decreased in nuclear extracts at 4 h but returned to baseline after 9 h of exposure to CdCl2. In contrast, FOXO3a expression in cytoplasmic extracts was increased depending on the duration of exposure.
Intracellular localization of FOXO3a in HK-2 cells following exposure to cadmium. Cells were incubated with 10 μM CdCl2 (Cd) for 0, 4, or 9 h. Equal amounts of protein (20 μg) in nuclear and cytoplasmic extracts were subjected to western blotting using antibodies against phospho-FoxO1 (Thr24)/FoxO3a (Thr32), total FoxO3a, actin, and lamin A/C. Actin and lamin A/C served as a loading control for cytoplasmic and nuclear extracts, respectively. Results of densitometric analysis are also shown. Results are representative of at least three independent experiments
Effects of FOXO3a knockdown on CdCl2-induced cellular damage
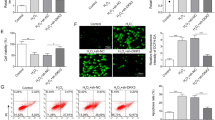
To clarify the toxicological significance of FOXO3a phosphorylation (inactivation), we compared cellular damage in normal and FOXO3a-deficient HK-2 cells following long-time exposure to cadmium. In comparison with cells transfected with negative control siRNA, transfection with siRNAs targeted against the human FOXO3a gene (siRNA 1 and siRNA 2) almost completely abolished FOXO3a protein expression in HK-2 cells without CdCl2 exposure (Fig. 6a). Expression of FOXO3a remained undetectable in cells treated with 20 μM CdCl2 for 10 h after FOXO3a knockdown (data not shown). Exposure to 20 μM CdCl2 for 10 h caused severe damage to HK-2 cells; many cells were floating (Fig. 6b, left panel). However, knockdown of FOXO3a with either siRNA 1 or siRNA 2 suppressed CdCl2-induced cellular damage (Fig. 6b, middle and right panels). The numbers of attached and floating cells were counted following exposure to 20 μM CdCl2 for 12 h (Fig. 6c). In comparison with cells transfected with control siRNA, knockdown of FOXO3a increased the number of attached cells and decreased the number of floating cells by approximately 10 % (P < 0.01). In addition, exposure to 20 μM CdCl2 for 12 h increased the amount of cytoplasmic nucleosomes by 1.48-fold in cells transfected with control siRNA, indicating apoptosis of HK-2 cells (Fig. 6d). Knockdown of FOXO3a markedly suppressed the CdCl2-induced increase in cytoplasmic nucleosomes in comparison with cells transfected with control siRNA (P < 0.01).
Effects of FOXO3a knockdown on cadmium-induced cellular damage in HK-2 cells. a Cells were transfected with siRNAs specific for the human FOXO3a gene (FOXO3a siRNA 1 [Hs FOXO3 1 FlexiTube siRNA] and siRNA 2 [Hs FOXO3 3 FlexiTube siRNA]) or negative control siRNA. Cell lysates were subjected to western blotting using antibodies against total FoxO3a and actin. Results of densitometric analysis are also shown. Results are representative of at least three independent experiments. b Cells transfected with control siRNA, FOXO3a siRNA1, or FOXO3a siRNA2 were incubated with or without 20 μM CdCl2 (Cd) for 10 h and phase-contrast micrographs were taken. Results are representative of at least three independent experiments. c Cells transfected with negative control siRNA or FOXO3a siRNA 2 were incubated with or without 20 μM CdCl2 (Cd) for 12 h, and the numbers of attached cells and floating cells were counted. Each value reflects the mean ± SD of three experiments with four samples per group in each experiment and represents the percentage of the total cell number (attached cells plus floating cells). **P < 0.01. d Cells transfected with negative control siRNA or FOXO3a siRNA 2 were incubated with or without 20 μM CdCl2 (Cd) for 12 h. The cytoplasmic fraction was used in an enzyme-linked immunosorbent assay to measure the levels of nucleosomes. Each value reflects the mean ± SD of three experiments with four samples per group in each experiment and represents the fold increase with respect to the untreated control (control siRNA without Cd). **P < 0.01
Discussion
The levels of FOXO3a phosphorylated at Thr32 and Ser253 increased in HK-2 cells following exposure to 10 μM CdCl2 for 2 or 4 h. On the other hand, Akt was phosphorylated at Thr308 and Ser473 as early as 1 h after exposure to 10 μM CdCl2. Furthermore, treatment with wortmannin suppressed cadmium-induced phosphorylation of Akt and inhibited phosphorylation of FOXO3a at Thr32 and Ser253 and FOXO4 at Thr28 (data not shown). These results indicate that activation of the PI3K/Akt pathway is responsible for the subsequent phosphorylation of FOXO3a and FOXO4 in HK-2 cells exposed to cadmium. We also found that wortmannin suppressed cadmium-induced expression of FOXO3a and FOXO4 (data not shown) in HK-2 cells. In contrast to our findings, stimulation of BJ-hTert human fibroblasts with platelet-derived growth factor suppressed expression of FOXO1 and FOXO4 mRNAs. This down-regulation was blocked with a PI3K inhibitor, LY294002 (Essaghir et al. 2009). Treatment with hydrogen peroxide (H2O2) or glucose oxidase induced expression and phosphorylation of FOXO1 and FOXO4 in human embryonic kidney 293 cells (Nakamura and Sakamoto 2008). Thus, cellular stresses induced by cadmium exposure or ROS stimuli may regulate the expression of FOXOs through Akt-dependent mechanisms that differ from those activated by growth factors such as platelet-derived growth factor.
We next examined the upstream signaling pathways that mediate PI3K/Akt-dependent phosphorylation of FOXO3a in HK-2 cells treated with cadmium. Cadmium exposure induced phosphorylation of EGFR, CaMKII, and Src in rat mesangial cells (Xiao et al. 2009) and IGF-1R in rat pheochromocytoma PC12 cells (Chen et al. 2011). In the present study, inhibitors of EGFR (AG1478), CaMKII (KN-93), IGF-1R (PPP), and Src (PP2) markedly suppressed cadmium-induced phosphorylation of Akt. Furthermore, cadmium-induced phosphorylation of FOXO3a at Thr32 was markedly suppressed by AG1478, KN-93, and PP2 but only partially suppressed by PPP. These results suggest that cadmium exposure activates the PI3K/Akt pathway through EGFR, CaMKII, IGF-1R, and Src and results in phosphorylation of FOXO3a in HK-2 cells, although the contribution of IGF-1R is less significant than other signaling pathways. Cadmium has been suggested to enhance the production of ROS including superoxide anion, H2O2, and hydroxyl radicals (Liu et al. 2009). We also found that treatment of HK-2 cells with the ROS scavengers, N-acetylcysteine (5 mM) or catalase (2500 U/ml), substantially reduced cadmium-induced phosphorylation of FOXO3a at Thr32 (data not shown). Further studies are needed to clarify whether ROS activate the cell survival signals that induce phosphorylation of FOXO3a in proximal tubules exposed to cadmium.
Mitogen-activated protein kinases are a family of serine/threonine protein kinases that are activated by mitogens or stress conditions and play essential roles in a diverse array of cellular functions including cell growth, differentiation, and apoptosis (Raman et al. 2007). We have previously found that cadmium exposure induces phosphorylation of MAPK family members, ERK, JNK, and p38, in HK-2 cells (Iwatsuki et al. 2011; Komoike et al. 2012). In the present study, cadmium-induced phosphorylation of Akt and FOXO3a was suppressed by the p38 inhibitor (SB203580) but not by the MEK1/2 inhibitor (U0126) or the JNK inhibitor (SP600125), suggesting that p38 signaling cross-talks with the Akt pathway. Consistent with these findings, activation of p38 by ROS has been reported to enhance Akt phosphorylation in human lung fibroblasts exposed to radiation (Park et al. 2010) and human hepatocytes treated with palmitic acid (Wang et al. 2011). An in vitro kinase assay showed that FOXO1 is phosphorylated by p38 (Asada et al. 2007). However, it remains to be determined whether p38 directly phosphorylates FOXO3a in HK-2 cells exposed to cadmium.
Akt-mediated phosphorylation of FOXO3a at Thr32 and Ser253 promotes interaction with the 14-3-3 protein and subsequent export into the cytoplasm (Brunet et al. 1999). Phosphorylation of FOXO3a at Thr32 was associated with reduced expression of FOXO3a in nuclear extracts of HK-2 cells exposed to 10 μM CdCl2 for 4 h. However, this change in FOXO3a localization was transient, as the levels of FOXO3a in nuclear and cytoplasmic extracts increased after 9 h of 10 μM CdCl2 exposure. These results suggest that additional mechanisms may regulate nuclear re-localization and up-regulation of FOXO3a in HK-2 cells exposed to cadmium. Phosphorylation of FOXO3a at Ser207 by the mammalian Ste20-like kinase-1 has been shown to reduce FOXO3a binding with the 14-3-3 protein, leading to increased nuclear localization and FOXO3a activity (Lehtinen et al. 2006). Similarly, JNK also promoted FOXO3a nuclear translocation through various mechanisms (Sunayama et al. 2005; Sunters et al. 2006; Wang et al. 2012). Therefore, delayed but prolonged phosphorylation of JNK in HK-2 cells exposed to cadmium (Iwatsuki et al. 2011; Komoike et al. 2012) might counteract PI3K/Akt-mediated nuclear exclusion of FOXO3a. Further studies are needed to understand the mechanisms by which cellular stresses including cadmium exposure regulate FOXO3a expression.
FOXOs actively promote apoptosis in mitochondria-independent and mitochondria-dependent manners by inducing expression of death receptor ligands and Bcl-2 family members, respectively (Fu and Tindall 2008). To clarify the toxicological significance of FOXO3a phosphorylation (inactivation), HK-2 cells were transfected with siRNA against the human FOXO3a gene and then exposed to 20 μM CdCl2 for 10 or 12 h. Knockdown of FOXO3a reduced cellular damage and accumulation of cytoplasmic nucleosomes in response to long-time cadmium exposure. These findings suggest that increased nuclear FOXO3a might play a role in cadmium-induced cell death of HK-2 cells. Therefore, cadmium-induced FOXO3a phosphorylation associated with nuclear export of FOXO3a appears to promote cell survival. On the other hand, FOXOs have also been shown to protect cells from oxidative damage by increasing transcription of manganese superoxide dismutase and catalase (van der Vos and Coffer 2011; Zhang et al. 2011). It has been reported that silencing FOXO3a expression by siRNA enhanced H2O2-induced apoptosis of HK-2 cells (Hasegawa et al. 2008). Thus, FOXO3a can regulate cell fate by modulating the expression of genes involved in apoptosis or oxidative stress depending on the type of cellular stress and its severity. Phosphorylation of FOXO3a may be an important process that regulates the balance between cell survival and cell death in proximal tubules exposed to cadmium. Further investigations into the mechanisms that regulate FOXO3a including additional phosphorylation events, acetylation, methylation, and ubiquitination are required to clarify the function of FOXO3a.
References
Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, Fukamizu A (2007) Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal 19:519–527
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868
Burgering BMT (2008) A brief introduction to FOXOlogy. Oncogene 27:2258–2262
Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD, Huang S (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic Biol Med 50:624–632
Dansen TB, Burgering BMT (2008) Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol 18:421–429
Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin J-B (2009) The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem 284:10334–10342
Fu Z, Tindall DJ (2008) FOXOs, cancer and regulation of apoptosis. Oncogene 27:2312–2319
Hamada T, Tanimoto A, Sasaguri Y (1997) Apoptosis induced by cadmium. Apoptosis 2:359–367
Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Washida N, Tokuyama H, Hayashi K, Itoh H (2008) Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun 372:51–56
Iwatsuki M, Inageda K, Matsuoka M (2011) Cadmium induces phosphorylation and stabilization of c-Fos in HK-2 renal proximal tubular cells. Toxicol Appl Pharmacol 251:209–216
Komoike Y, Inamura H, Matsuoka M (2012) Effects of salubrinal on cadmium-induced apoptosis in HK-2 human renal proximal tubular cells. Arch Toxicol 86:37–44
Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EBE, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A (2006) A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125:987–1001
Liu Z, Yu X, Shaikh ZA (2008) Rapid activation of ERK1/2 and AKT in human breast cancer cells by cadmium. Toxicol Appl Pharmacol 228:286–294
Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 238:209–214
Matsuoka M, Call KM (1995) Cadmium-induced expression of immediate early genes in LLC-PK1 cells. Kidney Int 48:383–389
Nakamura T, Sakamoto K (2008) Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol Cell Endocrinol 281:47–55
Nordberg GF, Nogawa K, Nordberg M, Friberg LT (2007) Cadmium. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT (eds) Handbook on the toxicology of metals, 3rd edn. Academic Press, Burlington, pp 445–486
Park S, Ahn J-Y, Lim M-J, Kim M-H, Yun Y-S, Jeong G, Song J-Y (2010) Sustained expression of NADPH oxidase 4 by p38 MAPK-Akt signaling potentiates radiation-induced differentiation of lung fibroblasts. J Mol Med 88:807–816
Raman M, Chen W, Cobb MH (2007) Differential regulation and properties of MAPKs. Oncogene 26:3100–3112
Son Y-O, Wang L, Poyil P, Budhraja A, Hitron JA, Zhang Z, Lee J-C, Shi X (2012) Cadmium induces carcinogenesis in BEAS-2B cells through ROS-dependent activation of PI3K/AKT/GSK-3β/β-catenin signaling. Toxicol Appl Pharmacol 264:153–160
Sunayama J, Tsuruta F, Masuyama N, Gotoh Y (2005) JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol 170:295–304
Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, Burgering BMT, Coombes RC, Lam EW-F (2006) Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res 66:212–220
Tzivion G, Dobson M, Ramakrishnan G (2011) FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta 1813:1938–1945
van der Vos KE, Coffer PJ (2011) The extending network of FOXO transcriptional target genes. Antioxid Redox Signal 14:579–592
Wang X, Liu JZ, Hu JX, Wu H, Li YL, Chen HL, Bai H, Hai CX (2011) ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radic Biol Med 51:539–551
Wang X, Chen WR, Xing D (2012) A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol 227:1168–1178
Xiao W, Liu Y, Templeton DM (2009) Pleiotropic effects of cadmium in mesangial cells. Toxicol Appl Pharmacol 238:315–326
Zhang X, Tang N, Hadden TJ, Rishi AK (2011) Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta 1813:1978–1986
Acknowledgments
This study was supported by the Department Fund of the Tokyo Women’s Medical University School of Medicine.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujiki, K., Inamura, H. & Matsuoka, M. Phosphorylation of FOXO3a by PI3K/Akt pathway in HK-2 renal proximal tubular epithelial cells exposed to cadmium. Arch Toxicol 87, 2119–2127 (2013). https://doi.org/10.1007/s00204-013-1077-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-013-1077-6