Abstract
In this study, 20 endophytic actinobacteria were isolated from different parts of peanut plants growing in cropland with low and high salt in West Bengal, India. The endophytes underwent a rigorous morphological, biochemical, and genetic screening process to evaluate their effectiveness in enhancing plant growth. About 20% of these isolates were identified as potential plant growth-promoting endophytic actinobacteria, which showed high 16S rRNA gene sequence similarity (up to 99–100%) with different species of Micromonospora. Among these isolates, Micromonospora sp. ASENR15 produced the highest levels of indole acetic acid (IAA) and gibberellic acid (GA), while Micromonospora sp. ASENL2, Micromonospora sp. ANENR4, and Micromonospora sp. ASENR12 produced the highest level of siderophore. Among these leaf and root endophytic Micromonospora, strain ANENR4 was tested for its plant growth-promoting attributes. ANENR4 can be transmitted into the roots of a healthy peanut plant, enhances growth, and colonize the roots in abundance, suggesting the potential agricultural significance of the strain. Moreover, the study is the first report of endophytic Micromonospora in peanuts with PGP effects. The outcomes of this study open avenues for further research on harnessing the benefits of this endophytic Micromonospora for optimizing plant growth in agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endophytic microorganisms, residing within the internal tissues of plants, have gained increasing attention for their potential contributions to plant growth promotion and overall fitness (Jana et al. 2022). Over the past decades, research on endophytes has unveiled their diverse roles in plant–microbe interactions, including the enhancement of nutrient uptake, synthesis of growth-promoting substances, and modulation of host defense mechanisms. Numerous investigations have revealed that microorganisms associated with root systems are typically prevalent and exhibit advantageous plant growth-promoting rhizobacteria (PGPR), which promote plant growth, reduce pathogenicity, and mitigate abiotic stresses (Bacon and White 2016). These microorganisms are a component of the phytomicrobiome, which is the regulated microbial community associated with every plant (Smith et al. 2016). In agricultural systems, this community and the plant combine to form the holobiont, which is the entity that is subject to evolution and produces food and biomass (Smith et al. 2017). These days, efficient agricultural practices depend heavily on chemical fertilizers to boost plant development and yield. The main obstacles, however, are the expense, environmental issues, and health risks to humans that arise from the use of these chemical fertilizers in the food chain. Microorganisms have been shown to be a significant source of organic substances with agro-active properties. A major area of research in the fields of agriculture, microbiology, and biotechnology is the use of microbial consortia in the form of biofertilizers which in turn would reduce the use of chemical fertilizers, pesticides, and various other agrochemicals (Ahmad et al. 2008). As a result, identifying and screening PGPRs have received a lot of attention. The primary rhizobacteria being studied for their ability to promote plant growth are strains of root-associated bacterial genera, such as Azospirillum, Azotobacter, Arthrobacter, Burkholderia, Bacillus, Paenibacillus, Erwinia, Klebsiella, Serratia, Pantoea, Enterobacter, Xanthomonas, and Pseudomonas (Bhattacharyya and Jha 2012; Xing et al. 2016; Li et al. 2017). Some of these have already been used as biofertilizers (Bhardwaj et al. 2014).
Not only are bacteria present in the rhizosphere and on the root surface (rhizoplane), but they can also live endophytically (Sturz et al. 2000; Hardoim et al. 2015). Bacterial endophytes can exist within living plant tissues without significantly harming plants (Afzal et al. 2019). They can also be isolated from plant tissue that has been surface-sterilized or extracted from within the plant (Eevers et al. 2015). Numerous investigations have demonstrated the potential advantages for the host plant resulting from the interaction between endophytic bacteria and plants. Biological control against plant pathogens, plant growth promotion (PGP), induction of systemic resistance to plant pathogens, nitrogen (N) fixation, enhanced phytoremediation, and response to environmental stresses are a few examples of these advantages (Sturz et al. 2000; Rosenblueth and Martínez-Romero 2006; Trujillo et al. 2015; Khare et al. 2018). When it comes to rhizospheric bacteria, endophytic bacteria are less able to compete with their host for nutrients, have a higher ability to multiply inside plant tissue, and are better shielded from external stimuli (Rosenblueth and Martínez-Romero 2006; Khare et al. 2018). Thus, this relationship could contribute to the development of sustainable crop production (Khare et al. 2018).
A unique group of bacteria, actinobacteria live abundantly in soil (Streptomyces), as significant plant pathogen (Streptomyces scabies), and as N-fixing symbiont of non-leguminous plants (Frankia) (Stackebrandt 2000). Through the advancement of genomics, it has been established that species of Actinobacteria, Proteobacteria, Bacteroides, and Verrucomicrobia are the major taxa that chose their endophytic life (Manter et al. 2010). More than 40 genera of filamentous Actinobacteria from the roots of Triticum aestivum have been reported (Conn and Franco 2004). Actinobacteria, known for their diverse physiological and biochemical properties, have gained significant attention in recent years for their plant growth-promoting (PGP) properties. Plant growth-promoting endophytic bacteria (PGPB) have the potential to directly or indirectly promote plant growth and yield. Productions of siderophores that sequester iron (Fe) or substances with antimicrobial properties are examples of indirect mechanisms of growth promotion that help defend plants from soil-borne pathogens (Rosenblueth and Martínez-Romero 2006; Khare et al. 2018). The fixation of atmospheric nitrogen, the solubilization of minerals like phosphorus (P), or the synthesis of plant growth regulators (PGRs) like auxins, gibberellins, polyamines, and cytokinin, which directly influence plant growth, are examples of direct mechanisms (Rosenblueth and Martínez-Romero 2006; Glick 2015; Trujillo et al. 2015; Khare et al. 2018). The growth-promoting bacteria help to promote growth, reduction of numerous stresses, and aid in their adaptation and survival through the ACC deaminase present in them (Glick 2015).
One of the top five important oil-seed crops is Arachis hypogaea, a member of the Fabaceae family and a dicotyledonous plant source of calories, minerals, vitamins, and protein (Wang et al. 2018). It serves as both an oil seed and a source of food for livestock, giving both farmers and commercial producers a sizable source of income. In comparison to the majority of other closely related plant species, peanuts are thought to be more drought-tolerant (Wan et al. 2014). Peanut plants are susceptible to a variety of bacterial and fungal diseases, including wilt (Ralstonia solanacearum), pod rot (Rhizoctonia solani), root rot (Fusarium moniliforme), leaf blight (Alternaria tenuissima), and seed rot and crown (Aspergillus niger) (Rojo et al. 2007; Haggag and Timmusk 2008; Wang and Liang 2014; Jiang et al. 2017). In agriculture, a number of endophytic actinobacteria—particularly root endophytic actinobacteria—are regarded as excellent biofertilizers and biocontrol agents. Numerous endophytic bacteria have been showed to stimulate peanut growth, including Enterobacter sp. J49, Methylobacterium sp., Sphingomonas sp., Bacillus sp., and Paenibacillus sp. (Madhaiyan et al. 2006; Haggag and Timmusk 2008; Liu et al. 2017; Ludueña et al. 2019; Chen et al. 2019). Peanuts are interacting with a wide range of microorganisms, most commonly those involved in biotic stress and N2 fixation (Chen et al. 2019).
This study aimed to isolate endophytic actinobacteria from the peanut (A. hypogaea), determine the morphological, physiological, and sequencing of the 16S rRNA genes of these isolates, and determine which actinobacteria are responsible for different plant growth-promoting activities (PGPAs). Axenic conditions were used to examine the PGP potential of a few chosen isolates, as well as their impact on peanut growth. Considering that the potential for PGPAs of the Micromonospora genus isolated from India is not widely known, this study offers new and unique views.
Materials and methods
Plant material, isolation of endophytes
In March 2017, plant samples were obtained from the cultivated land of peanuts (A. hypogaea) plants in West Bengal (District: Purba Medinipore, Egra; 21° 53′ 58.09″ N, 87° 32′ 16.58″ E) and Gosaba (22° 09′ 36.00″ N, 88° 47′ 60.00″ E) in India for the purpose of isolating actinobacteria. The surfaces of the peanut root and leaves samples were sterilized to isolate endophytic actinobacteria. This was accomplished by washing the dirt off of them with flowing sterile milli Q water and then dipping them in 0.1% HgCl2 for 1 min. After another rinse in a 0.9% (w/v) NaCl solution, the roots were submerged in 70% ethanol for a minute. Next, 0.9% (w/v) NaCl solution was used to wash the samples once more. Next, a sterile mortar and pestle were used to collect and crush roots measuring approximately 1 cm in length, in 10 mL of 0.9% (w/v) NaCl solution. The supernatant was then collected and serially diluted. 100 µL of each of the diluted solutions were sprayed to distinct plates made of different agar, such as Actinomycetes isolation agar (AIA) (for 1 L medium; glycerol 5 g, sodium propionate 4 g, sodium caseinate 2 g, l-asparagine 0.1 g, FeSO4, 7H2O 0.001 g, and agar 15 g, pH 8.1) starch casein 1 L: soluble starch 10 g, K2HPO4 2 g, KNO3 2 g, casein 0.3 g, MgSO4, H2O 0.05 g, CaCO3 0.02 g, FeSO4, 7H2O 0.01 g, and agar 15 g, pH 7.0) and yeast extract mannitol agar (YEMA) (1 L medium: 10.0 g mannitol, 1 g yeast extract, 0.5 g K2HPO4, 0.2 g MgSO4,·7H2O, 0.1 g NaCl, 0.025 g Congo red, and 20.0 g agar powder) and incubated in 30 °C.
Rapid test for actinobacterial screening
The effect of isolated actinobacteria on seedling growth in vitro was monitored. To screen for possible endophytic actinobacteria, about 20 newly isolated actinobacterial strains were used. International Streptomyces Project-2 (ISP-2) medium (for 1 L medium, malt extract 10 g, yeast extract 4 g, dextrose 4 g, and agar 20 g) was regularly used to maintain the isolated actinobacterial strains used for both initial and secondary screening. In ISP-2 broth, single colonies were inoculated and cultured aerobically at 28 °C with 150 rpm shaking for 3–5 days. Before inoculation, the bacterial cells were harvested using centrifugation (Eppendorf Centrifuge 5424, USA) (6000×g for 10 min) and the pellet was resuspended in 0.9% (w/v) NaCl to get density of 108 CFU/mL. The peanut seeds bacterization was done with the bacterial cell suspension. Briefly, bacterial cell suspension was incubated with sterilized peanut seeds for 1 h at room temperature. Seed treatment with sterile 0.9% (w/v) NaCl solution was treated as control experimental set. Actinobacterial cell suspension was incubated with sterilized peanut seeds for 1 h at room temperature. Subsequently, 8 plates were used for each treatment, with 6 seeds sown on ½ MS (Murashige-Skoog) medium plates. Compared to control plants, distinct biometric parameters were recorded at day 21 after seeding.
Actinobacterial secondary screening
Following a preliminary screening, potential actinobacterial isolates were chosen, and they underwent a second screening in vivo on sterile vermiculite. The peanut seeds were surface-sterilized and then incubated for 1 h at room temperature in 2 mL of actinobacterial cell suspension in 0.9% NaCl. The surface sterility was confirmed when no growth was seen after plating 100 µL of the final wash in YEMA and ISP-2 agar plates, and also after placing the sterilized seeds on the semi-solid MS medium. The seeds were then stored in sterile conditions on Whatman filter paper and placed in Petri plates, where they were kept in total darkness for 5 days. In sterile vermiculite (Soilrite, Keltech Energies), germinated seeds were transplanted, grown, and watered regularly. Each treatment was replicated five times, with three seedlings in each pot. The actinobacteria-inoculated seedlings and control seedlings were harvested at 21 days after seeding, and the biometric parameter was determined.
Phenotypic and biochemical characterization
For phenotypic observations, ISP-2 agar medium was used as the standard medium after 14 days of incubation at 28 ± 2 °C (Shirling and Gottlieb 1966). The color of the substrate and aerial mycelia, the presence of diffusible pigments, the growth pattern, and the formation of melanin pigments are some of these characteristics. After 14 days of growth on ISP-2 agar media at 28 ± 2 °C, the spore morphology was examined by scanning electron microscopy (SEM) (ZEISS-EVO-MA-10; Carl Zeiss Pvt. Ltd., Oberkochen, Germany). Samples were prepared for SEM following the standard protocol (Maiti and Mandal 2020).
The actinobacterial isolate was tested for biochemical characterization by incubation culture at 28 ± 2 °C for 14 days in ISP-2 agar as the basal medium. This allowed for evaluation of growth at different temperatures (10, 20, 30, and 40 °C), pH (4–10), and 1–7% NaCl (w/v). The use of carbon was evaluated using ISP-9 agar medium (each liter contains: (NH4)2SO4-2.64 g, KH2PO4-2.38 g, K2HPO4, 3H2O-5.65 g, MgSO4, 7H2O-1 g, CuSO4, 5H2O-0.0064 g, FeSO4, 7H2O-0.0011 g, MnCl2, 4H2O-0.0079 g, ZnSO4, 7H2O-0.0015 g, pH 7.0) with 1% (w/v) of the various carbon sources (Maiti and Mandal 2019; Shirling and Gottlieb 1966). Starch hydrolysis, hydrolysis of tributyrin, nitrate reduction, decomposition of cellulose, liquefaction of gelatin, and H2S, catalase and urease production were tested, according to Maiti and Mandal (2020). It has been found that these actinobacterial isolates could hydrolyze xanthine, hypoxanthine, xylan, tyrosine, casein, and arbutin (Maiti and Mandal 2020).
Genomic DNA isolation
The endophytic isolates were cultured individually for 4–7 days at 30 °C and 120 rpm in 5 mL of ISP-2 broth. For each isolate, the phenol–chloroform technique was used to prepare the genomic DNA (Marmur 1961).
Analysis of 16S rDNA sequence and phylogeny
The 16S rRNA genes were amplified using the universal primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-ACGGTTACCTTGTTACGACTT-3′) (Turner et al. 1999). Purification and sequencing of the PCR amplicons were done using the Sanger chain-termination method. The BioEdit program (http://bioedit.software.informer.com) was used to trim and align the raw sequences to obtain the almost full 16S rDNA sequences of these isolates. The 16S rDNA sequences of isolated strains were uploaded to the EZBioCloud (https://www.ezbiocloud.net/) website to determine the closest-type strain (Yoon et al. 2017). The ClustalW program of MEGA11 software was used to obtain multiple aligned sequences from the 16S rDNA sequence of the nearest type strains that were downloaded from the same site. The neighbor-joining (NJ) phylogenetic trees were created (Saitou and Nei 1987), and the Kimura 2 parameter model (Kimura 1980) was used to calculate the evolutionary distances. To validate each branch of the phylogenetic tree, bootstrap values were produced by resampling 1000 times.
In vitro analysis of plant growth promotion traits
Indole-3-acetic acid (IAA) production
The level of indole-3-acetic acid (IAA) produced by isolated actinobacteria from the broth cultures was estimated qualitatively and quantitatively using the colorimetric technique following Gordon and Weber (1951). Isolated actinobacteria were cultured in ISP-2 broth medium supplemented with 100 µg/mL to 500 µg/mL of l-tryptophan for 7 days at 30 °C. For estimating the level of produced IAA, Salkowski's reagent (1 mL of 0.5 M FeCl3 in 50 mL of 35% HClO4) was added to the centrifuged culture supernatant and incubated for 15 to 20 min. IAA production is predicted to be positive when a deep pink color appears. The intensity of the pink color was measured at 530 nm using UV–visible spectrophotometer (Jasco V-530, made in Japan). The absorbance was compared to a standard curve to determine the quantities of IAA produced by actinobacteria per mL of culture broth (Borah et al. 2021). After preparing the standard curve by adding 2 mL of Salkowski reagent with different concentrations of known IAA (100–500 g/mL) in ISP-2 broth, absorbance was measured at 530 nm after 15–20 min of incubation.
Gibberellic acid (GA) production
The selected actinobacterial culture was added to ISP-2 broth media and then incubated at 30 °C for 3–4 days to determine the amount of gibberellic acid (GA). For 10 min, the culture was centrifuged at 8000 rpm to obtain a cell-free supernatant. Then, 0.4 mL of zinc acetate (21.9 g zinc acetate with 1 mL glacial acetic acid and a volume built up to 1000 mL of ddH2O) was added to 5 mL of the cell-free supernatant. Potassium ferrocyanide (10.6 g in 100 mL ddH2O) was added to the mixture after it had been incubated for 2–3 min at room temperature. Thereafter, the mixture was centrifuged at 1000 rpm for 15 min. The collected supernatant was placed in a test tube with 3 mL of 30% HCl, and was kept at 20 °C for 75 min. On a UV–vis spectrophotometer (Jasco V-530, made in Japan), the absorbance of the sample was measured at 254 nm after the blank sample was treated with 5% HCl (Sharma et al. 2018). By preparing a standard curve with gibberellic acid (GA3, SRL-95110) as the standard (0–100 g/mL), the number of gibberellins was determined.
Phosphate solubilization
Using Pikovskaya's medium (Himedia-GM1719), phosphorus solubilization by an isolated strain was measured (Gaur 1990). Pikovskaya's plates were inoculated with various bacterial strains, which were then cultured at 30 °C for 5–7 days. The presence of a visible halo zone around the spot indicated the presence of the phosphatase enzyme.
Nitrogen fixation
The isolates were grown on bromothymol blue malate medium (NFBM) without a nitrogen source to assess their capacity to fix nitrogen (Döbereiner et al. 1989; Mir et al. 2021). Each liter medium contains 0.5 g of malic acid, 0.2 g of CaCl2, 0.1 g of NaCl, 0.002 g of Na2MoO4, 0.01 g of MnSO4ꞏH2O, 0.5 g of KH2PO4, 1.64%-4 mL of EDTA, 0.5%-3 mL of bromothymol blue, 0.1 mg of biotin, and 4.5 g of KOH (pH 6.8). The isolates were plated onto NFB broth medium and left to grow for 4–5 days at 30 °C. The development of thin pellicles is a sign of N2 fixers.
Siderophore production
The Chrome Azural S (CAS) agar plate assay was used to qualitatively evaluate the production of siderophore by the selected bacteria (Louden et al. 2011). In CAS agar plates, bacterial isolates were spotted and incubated for 5–7 days at 30 °C. After the incubation, the siderophore production was observed as appearance of yellow–orange halo zone around the colony. Using a CAS shuttle test, siderophores were quantified. Bacterial isolates were added to an iron-free succinic acid broth medium (1 L contains: K2HPO4, 6 g; KH2PO4, 3 g; MgSO4.7H2O, 0.2 g; and succinic acid, 4 g, pH 7.0) and cultured for 4–6 days at 30 °C with constant shaking at 120 rpm. The broth was centrifuged at 10,000 rpm for 15 min at 4 °C following incubation. At 630 nm, the absorbance of the supernatant (1 mL) and CAS reagent (1 mL) was measured (Louden et al. 2011). The blank set consisted of 1 mL of succinic acid broth and 1 mL of CAS reagent. The extent of siderophore production was calculated using the formula [(AR−AS)/AR] × 100, where “AS” stands for the absorbance of the inoculated sample (CAS reagent liquid + supernatant) at 630 nm, and “AR” for the absorbance of the control sample (CAS reagent solution + uninoculated media) at 630 nm (Borah et al. 2021).
Hydrogen cyanide production
King's B medium supplemented with 0.44% (w/v) glycine was used to screen for HCN synthesis. Sterile Whatman filter paper was soaked in a picric acid solution (1.25% Na2CO3 in 0.2% picric acid) and placed on the top lids of Petri plates and further incubated for 7 days. The change of yellow color of the filter paper to reddish-brown color indicates the production of HCN (Devi and Thakur 2018).
Ammonia production
The qualitative estimation of ammonia-producing bacteria was done by adding 250 µL of Nessler's reagent (HgCl2 10 g; KI 7 g; NaOH 16 g; ddH2O up to 100 mL; pH-13.2 ± 0.5) in each peptone water broth test tube (peptone 10 g/L; NaCl 5 g/L), after 4–7 days of incubation of actinobacterial culture and the development of light yellow (Gupta and Pandey 2019).
Extracellular enzyme production
The synthesis of extracellular enzymes was assessed in vitro. Selected isolates were tested for their ability to produce high-quality exoenzymes. By streaking the colonies over the appropriate modified medium (1% CM cellulose for detection of cellulase), it was possible to detect the degradation of cellulose using the Congo-red overlay method (Anand et al. 2010). To carry out the experiment, plates were first overlayed with 0.1% aqueous Congo red for 10 min and then rinsed with 1 M NaCl. Plates were flooded with 1% CTAB, and Hankin medium was used for the pectinase assay (Hankin and Anagnostakis 1975). By removing the area around the colony on the appropriate medium, it was possible to determine the degradation of such components (cellulose, pectin, and lipase). The gelatinase assay was done on nutrient gelatin agar (Himedia, India). Amylase detection was confirmed with Gram's iodine solution after starch breakdown was carried out in modified starch agar media (1% starch, 0.3% beef extract, and 2% agar). The presence of urease was determined using urea broth. Skim-milk containing agar medium has been used to study caseinase secretion (Williams et al. 1983). The tests for H2S production were conducted as previously mentioned (Maiti and Mandal 2020).
Plant growth promotion experiment
Germination bioassay
The selected bacterial strain treated with peanut seeds was used to conduct a germination assay. Surface-sterilized peanut seeds were treated with 2 mL of bacterial culture in 0.9% NaCl for the seed treatment, and they were then incubated for an hour at room temperature. Sterile 0.9% NaCl was used as the control treatment. Subsequently, 10 mL of sterile distilled water were used to moisten the seeds (three duplicates and three seeds/phyta jar for peanuts) in a phyta jars that had been sterilized and covered with two sheets of filter paper. The plant growth chamber was used to incubate all of the phyta jar for 16 h each day at a temperature of 28 °C under 2000 lux of light. After 2 weeks of inoculation, the effect of actinobacteria on the percentage of seed germination and root-length of the seedling were determined.
Gnotobiotic experiment
Experiments with gnotobiotic inoculation were carried out on peanut plants. To accomplish this, peanut JL286 cultivar seeds were surface-sterilized, germinated in sterile conditions on Whatman filter paper, and then placed in Petri plate for 3 to 5 days in full darkness. After that, each seed was put into a phyta jar using 80 mL of partially solid Hoagland medium supplemented with 50 µg/mL of cycloheximide that had been previously inoculated with 103 CFU/mL of the ANENR4 strain. To do this, the strain was cultivated in ISP-2 media, and then, cells were introduced to the semi-solid medium after being suspended in sterile saline solution at a final concentration of 103 CFU/mL. The phyta jars with the seedlings were kept in a plant growth chamber with natural light, 28 °C temperature, a relative humidity of 70% ± 5.
Vermiculite inoculation experiment on peanut plants
Pregerminated seeds from cultivar JL286 were used in the inoculation studies, which were carried out in sterile vermiculite. Peanut seeds were surface-sterilized, germinated in a sterile environment on Whatman filter paper for 5 days in complete darkness, and then placed on Petri plates. 10 mL of a 108 CFU/mL inoculum was added with 250 g of sterilized vermiculite to produce a concentration of 105 CFU/g to conduct these investigations. Germinated seeds were then placed on pot containing inoculated vermiculite, and the seedlings were maintained for 41 days at 25 °C in the greenhouse (average temperature: 28 °C ± 2 °C, natural light; relative humidity: 70% ± 5). Bradyrhizobium japonicum strains were used as a positive control, and 0.9% NaCl was used as negative control. For every treatment, three biological replicates of each bacterial strain were used. Three different times following the inoculation, biometric characteristics were measured. After the plant material was dried at 50 °C, the dry weight of each plant was determined.
Plant colonization experiment
To evaluate the ability of the Micromonospora sp ANENR4 to colonize peanut plants, gnotobiotic and vermiculite inoculation experiments were carried out. Each experiment used three biological replicates of each strain. The inoculated peanut plants were surface-sterilized with 70% ethanol and 1% sodium hypochlorite separately for four minutes each and then repeatedly washed with sterile water to verify endophytic colonization. Subsequently, each plant was cut into three parts: the root, nodule, and leaves. These parts were subsequently macerated using a sterile pestle. By chopping and washing 1 cm pieces of the root, nodule, and leaves, bacterial strains were once again recovered from these plant parts. Following serial dilution, each suspension was plated on AIA and YEMA agar plate and incubated at 30 °C. To prevent the growth of other fungi, cycloheximide (50 g/mL) was added to the medium. Surface sterility was confirmed when no growth was seen, after plating 100 µL of the final wash, and after placing the sterilized plant parts on the AIA and YEMA agar plates. Phenotypic analysis and 16S rDNA amplification were used to confirm the identity of the colonies of re-isolated ANENR4 from the roots and nodules. The results were examined and reported as CFU/g of plant weight after 21 days of post-inoculation at 30 °C.
Microscopy study
Molecular biology-grade sterile water was used to generate a 10 mg/mL stock of 4′, 6-diamidino-2-phenylindole (DAPI) and the Live/Dead bacterial staining kit, which contains SYTO9 and PI. Thin free-hand longitudinal and transverse sections were prepared aseptically from fresh roots. These were stained with the fluorophores singly or in combination and examined under the fluorescence microscope (Olympus fluorescence microscope, Model no.-IX-73, made in Japan) after 10–20 min. The Olympus microscope with 20X lenses, a GFP filter for blue excitation, and a TRITC filter for green excitation were used to capture the images. The cellSens software was used to take the pictures.
Statistical analysis
To ensure statistical confidence, each experiment was carried out three times. Statistical analyses were conducted using unpaired t tests and were performed with GraphPad Software. The significance was mentioned as P value < 0.05 (*), P value < 0.01 (**), and P value < 0.001 (***) in comparison to the control. P values greater than 0.05 indicated that there was no significant difference and hence were presented as N.S. (no statistical difference).
Results
Peanut endophytic isolates
An in vitro investigation was performed to find out the plant growth-promoting properties of isolates by analyzing several physiological characteristics of peanut JL286 to identify prospective PGP endophytes from recently isolated Micromonospora (Fig. S1). For the initial screening on peanuts, a total of 20 isolates that were morphologically similar to actinobacteria were chosen. Only four isolates, identified as ANENR4, ASENL2, ASENR12, and ASENR15, were selected based on their 16S rDNA sequence homology, indicating that they belong to the Micromonospora group of actinobacteria.
Morphological and physiological characterization
Four Micromonospora were isolated from surface-sterilized roots and leaves. Isolate ANENR4, ASENR12, and ASENR15 were isolated from the root, whereas isolate ASENL2 was isolated from leaves. It was found that they were all filamentous, Gram-positive, and had substrate mycelium but no aerial mycelium (Fig. 1). In the tip of the substrate mycelium, they were all found to have spherical, non-motile spores. They are categorized as brown to black (ANENR4), chocolate to brown (ASENL2), brown (ASENR12), and black to brown (ASENR15) based on the color of the colony (Fig. 1). Isolated ANENR4 and ASENL2 produced diffusible pigment. The actinobacterial isolates were able to utilize different sugars as carbon sources like l-arabinose, raffinose, ribose, galactose, mannitol, fructose, and rhamnose were all used by these isolates (Table S1). The strains ANENR4 and ASENR12 were unable to utilize inositol, and likewise, ASENR12 and ASENR15 were unable to use xylose as their source of soul carbon (Table S1). The optimum growth parameters of these strains were observed at pH 7, 5% (w/v) NaCl, and 30 °C. All strains were able to grow at a temperature of 28–37 °C and a pH of 5–7 (Table S1). The optimum temperature and pH were found to be 28 °C, and 7, respectively.
Phylogenetic analysis
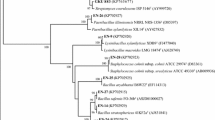
16S rRNA sequence-based phylogenetic analyses determined that all four strains are members of the genus Micromonospora (Fig. 2). The 16S rRNA genes were amplified, sequenced, and submitted to the NCBI data bank. The closest-type strains of each isolate showed sequence similarity ranging from 99.51 to 100%, based on EZBioCloud's sequence analysis (Table 1). Stain ANENR4 showed the highest closest to Micromonospora tulbaghiae DSM 45142 with similarity values of 99.51%. A similar analysis located strain ASENL2 showed highest similarity with Micromonospora maritima (100%), whereas strain ASENR12 and ASENR15 was related to Micromonospora siamensis (99.83%) and Micromonospora schwarzwaldensis (99.93%) similarity (Table 1). The 16S rDNA sequence-based phylogenetic tree were constructed by the neighbor-joining (NJ) method (Fig. 2). It has been found to be placed in separate clades and bootstrap value was greater than 50% considered in all cases. The accession numbers for 16S rDNA sequence of Micromonospora sp. ANENR4, Micromonospora sp. ASNEL2, Micromonospora sp. ASENR12, and Micromonospora sp. ASENR12 are MN737266, ON892542, ON892831 and ON892856, respectively (Table 1).
Neighbor-Joining (NJ) phylogenetic tree based on 16S rRNA gene sequences of Micromonospora species. Only > 50% bootstrap values (expressed as % of 1000 replications) are shown at the nodes. C. citrae IMSNU 22008 was used as an outgroup. “Actionobacterial isolates used in this study are in red” (color figure online)
Endophytic Micromonospora promotes plant growth
The potential of the isolated Micromonospora strains to promote plant growth was assessed, and the results are shown in Table 2. The results clearly showed that the growth-promoting activity of the four endophytes was not at the same level. Stains ASENL2 and ASENR12 were able to produce IAA and GA (Fig. S2, Table 2). The range of IAA production of ASENL2 and ASENR12 is 16.49 and 25.03 µg/mL, whereas GA production is 22.8 and 35.33 µg/mL, respectively (Fig. S2, Table 2). None of the isolates has the ability to phosphate solubilize activity. Following incubation, all four isolates exhibited a notable orange or yellow halo zone surrounding their colonies on blue chrome azural S (CAS) agar, suggesting that they were capable of producing siderophores (Fig. S2). The range of siderophore production is 60.2 to 83.83%. Siderophores were produced at a substantially higher rate (83.83%) by isolate ASENL2, whereas ASENR15 produced the least amount (Fig. S2, Table 2). The nitrogen fixation ability of the actinobacteria was observed qualitatively in N-free malate medium supplemented with an indicator bromothymol blue dye. The capacity of ANENR4 isolates to produce a thin pellicle and transition from bottle green to blue in NFB medium indicated that they were considered nitrogen fixers. This suggests that the isolates are capable of fixing nitrogen. A biochemical test reveals that strains ANENR4 and ASENL2 were able to produce ammonia and hydrogen cyanide, while strains ASENR12 and ASENR15 were not able to produce either of these substances (Table 2).
In planta Micromonospora sp. ANENR4 impact on the growth of peanut in the gnotobiotic experiment
Out of these four isolates, only ANENR4 increases shoot growth after the initial screening. ANENR4, which was found to have a significant impact on plant growth in two different experiments, such as the gnotobiotic and vermiculite inoculation experiments, will be the subject of further study. It has been found that, compared to controls and other isolates, ANENR4 (one of the four isolates that were chosen) consistently and significantly promoted growth (Fig. S1). In vitro seed germination bioassays showed that treatment of seeds of peanut with endophytic ANENR4 significantly stimulated germination percentage and root growth compared to controls, after 14 days of seedling growth (Fig. 3).
Effect of seed treatment with actinobacteria spore suspensions on plant growth promotion of A. hypogaea: a percentage of germination and b root length. Bar represents mean ± S.E. (standard error) of triplicate sample. P values calculated using unpaired t tests; the degree of significance was incorporated as P value < 0.05 (*) P value < 0.01 (**), P value < 0.001, and P value < 0.1 (not significant)
According to in vitro experiments, strains were able to produce various extracellular enzymes and at least one distinct PGP activity. In a gnotobiotic growth inoculation experiment, the strain Micromonospora sp. ANENR4 was further analyzed based on an in vitro preliminary experiment that examined its ability to promote plant development. Thus, in the gnotobiotic environment, peanut seeds inoculated with Micromonospora sp. ANENR4 exhibited a considerable increase in biometric parameters (Fig. S3, Table S3). It was also found that strain ANENR4 was able to increase fresh weight and dry weight of the inoculated seedling as well as shoot length of the JL286 peanut cultivar, indicating that it was able to promote peanut growth in comparison to non-inoculated plants.
In planta Micromonospora sp. ANENR4 impact on the growth of peanut in vermiculite
A study has been conducted to examine the impact of the Micromonospora sp. ANENR4 on peanut plants grown in vermiculite. Following an inoculation period of 41 days, peanut seedlings inoculated with Micromonospora sp. ANENR4 exhibited varying growth and yield parameters (Figs. 4, 5, Table 3). Peanut seedlings inoculated with strain Micromonospora sp. ANENR4 had significantly increased plant growth compared to the uninoculated control, with increases in plant root length, plant shoot length, length of leaf lamina, number of leaves, shoot fresh weight, shoot dry weight, and root fresh weight (Figs. 4, 5, Table 3).
Effect of endophytic strain ANENR4 spore suspensions on plant growth promotion of peanut (A. hypogaea) under vermiculite growth condition. a Root length, b shoot length, c leaves number, d length of leaf number, e shoot fresh weight, f shoot dry weight, g root fresh weight, and h root dry weight. Bar represents mean ± S.E. (standard error) of triplicate sample. P values calculated using unpaired t tests; the degree of significance was incorporated as P value < 0.05 (*) P value < 0.01 (**), P value < 0.001, and P value < 0.1 (not significant)
Experiments on plant growth promotion were conducted using sterile vermiculite and gnotobiotic conditions to assess how these strains functioned in different settings and predict how they would behave in the wild environment.
Endophytes can colonize peanut cultivars under gnotobiotic and vermiculite conditions
In gnotobiotic and vermiculite studies, the ANENR4 isolate, re-isolated from the endophytic roots and nodules even 21 days post-inoculation (dpi), suggesting that the isolate was able to colonize the roots (Fig. 6g, h). After being isolated, estimation of CFU/g root, and identified using morphology and 16S rDNA sequence-based analysis from surface-sterilized peanut JL286 plants maintained in a gnotobiotic environment, Micromonospora sp. ANENR4 were found to be able to colonize the root and nodule endosphere (Fig. 6g, h).
Population density of endophytic strain Micromonospora sp. ANENR4 inoculated to peanut under sterile vermiculite growth conditions. a Microscopic visualization of peanut root plants (JL286) colonized with Micromonospora sp. ANENR4. The transverse root sections (a–d) and longitudinal root sections (e and f) stained with PI (a and b), DAPI (c and d) and SYTO9 (e and f)) visualized under fluorescence microscope. a, c and e are the control set, whereas b, d and f are treated with Micromonospora sp. ANENR4. g, h Actinobacterial counts in colonization experiments
Transverse and longitudinal thin sections of roots stained with either DAPI, SYTO9, or PI were assessed for strain ANENR4 colonization. Our results indicated that DAPI, SYTO9, and PI staining of transverse and longitudinal thin sections of roots strongly suggested that ANENR4 actinobacteria colonize the internal region in the root (Fig. 6a–f). Staining of thin free-hand root tissue sections with DAPI showed the bacterial nuclei clearly under a fluorescence microscope. With SYTO9 and PI, staining of the thin root section yielded a clear fluorescence signal of bacterial colonization with FITC and TRITC.
Discussion
The actinobacteria have been given lot of attention because of their ecological significance in cycling of nutrients and also plant growth promoters (Franco-Correa et al. 2010). Genilloud (2012) highlighted the significance of this microbe in this niche and mentioned that the earliest strains came from soil. The family Micromonosporaceae, of which this bacterium is a member, comprises mesophilic, filamentous, aerobic, and spore-producing microorganisms. Colonies of Micromonospora are often colored, with colors ranging from orange to red to brown. A brown-black, or black, mucous mass of spores is seen in many old cultures. The genus Micromonospora is primarily known for its single-spore production: however, it can also produce composite spores on the surface or completely embedded mycelium on substrate (Genilloud 2012; Trujillo et al. 2014a). Micromonospora has been documented in numerous locations across the globe. While soil is typically the primary source of isolation, this microbe is also found in marine and aquatic sediments as well as mangrove environments (Fu et al. 2020; Back et al. 2021). Micromonospora have been recognized as significant components of nitrogen-fixing root nodules in leguminous plants (Valdés et al. 2005; Trujillo et al. 2006; Carro et al. 2012, 2013).
The aim of this study is to extensively evaluate the Micromonospora strain to develop bioinoculants that may stimulate the growth and development of peanut plants. Our approach includes the screening of actinobacterial isolates (especially the Micromonospora group of actinobacteria) from different plant parts of peanuts, favoring the selection of actinobacteria from the same ecological niches; screening of plant growth-promoting bacteria in vitro and taxonomic identification of these isolates; assessment of plant growth promotion and colonization in vivo.
Twenty endophytic actinobacteria-like isolates were obtained from the peanut plant, and four isolates were selected for further studies. Taxonomic analysis based on 16S rDNA analysis confirmed that all four isolates belong to the genus Micromonospora (Fig. 2).
These selected isolates were investigated further to find out their ability to promote plant development and host-colonization. The response of these isolates within the host is unclear, particularly the impact they have on other beneficial and harmful bacteria present in the same niche (Rhizobia and Frankia). In recent past, few information available on the interaction of Micromonospora with the actinorhizal and legume plants (Trujillo et al. 2015). Studies on plant co-inoculation show that Micromonospora functions as a bacterium that promotes plant growth and has a beneficial impact on the plant (Martínez-Hidalgo et al. 2014). However, the present study confirmed that Micromonospora itself acts as a plant growth promoter in peanut plants. It has been found that the isolates ASENL2 and ASENR12 produced IAA and GA 16.49 ± 2.62, 25.03 ± 2.81 and 22.8 ± 4.90, 35.33 ± 2.33 µg/mL, respectively (Table 2), whereas another two isolates were unable to produce these phytohormone. It is noteworthy that the peanut endophytes ASENL2 and ASENR12 are capable of producing both GA and IAA. The ability of endophytes to produce phytohormones is the process by which plant growth is promoted and is most frequently studied (Verma et al. 2021). In particular, IAA is linked to cell cycle regulation, tissue differentiation, apical dominance, and other signals related to plant growth and development. It is the most prevalent phytohormone (Verma et al. 2021).
Two-thirds of the fixed nitrogen used in plant metabolism and growth come from biological N2 fixation (Shridhar 2012). The nitrogen fixation capacity of the isolates was examined using NFMM medium, or nitrogen-free malate medium. A change in the medium's color and the production of a thin pellicle due to a rise in pH were indicators of the generation of ammonia and nitrate by bacteria fixing N2. The strain ANENR4 is able to change the color and formed thin pellicles when culturing on NFB medium (Tan et al. 2014). Welbaum et al. (2004) suggest that endophytic microorganisms may serve as a substitute nitrogen fertilizer in the future due to their greater capacity to fix N2 than rhizospheric microbes.
Endophytic bacteria produce siderophores, which limit the growth of pathogens and sequester and deliver iron to plants, promoting plant growth (Yu et al. 2017). The production of siderophores was significantly higher in all four isolated Micromonospora groups of bacteria, ranging from 60.2 to 83.83% (Table 2). In contrast, strains ANENR4 and ASENL2 produced NH3 and HCN. The biocontrol of fungal phytopathogens is facilitated by the generation of both HCN and NH3, which restrict the proliferation of mycelial cells. Both directly and indirectly, the endophyte’s production of NH3 helps in the host's maintenance of fitness. Soil pH can increase to 9–9.5 when NH3 builds up in the soil. It is therefore difficult for some fungi to develop in such an alkaline environment, and different fungal spores are prevented from germinating (Swamy et al. 2016).
The present study showed that four isolated endophytes produce cellulase and pectinase, whereas ANENR4 and ASENL2 were unable to produce amylase, and also ASENR12 could not produce caseinase, and gelatinase (Table S2). It has been hypothesized that plant-polymer-degrading enzymes such as cellulases, xylanases, and pectinases contribute to internal plant colonization (Compant et al. 2005). Glycoside hydrolases, such as cellulases and endoglucanases, are used by plant pathogenic bacteria and fungi to aggressively break down plant cell wall components and obtain access (Trujillo et al. 2014a, b).
Endophytes are beneficial to plants, since they have co-evolved with them and aid in their transition from aquatic to terrestrial life. Crop plants have encountered a significant obstacle in the form of stunted development and decreased yield due to their incapacity to sustain growth and biomass production under stress conditions. In India, peanuts (A. hypogaea) are a significant oil-seed crop. There are few studies on biological inoculants that are used globally in peanut farming. The ability of plant growth-promoting microorganisms, which are isolated from the rhizosphere or plant tissues and have various capacities to support plant growth and development, to promote plant development, has, however, led to the generation of a great deal of knowledge (Basu et at. 2021). The use of plant growth-promoting microorganisms is the best way to address the significant challenges of peanut cultivation, such as reducing production costs, the dose of chemical fertilizers, and environmental impacts, without detriment to productivity (Escobar Diaz et al. 2021). The peanut endophyte B. velezensis LDO2 demonstrates potent antimicrobial activity against bacteria and pathogenic fungi in addition to growth-promoting characteristics (Chen et al. 2019).
Roles of four endophytic Micromonospora strains on the peanut seed germination and seedling growth were studied. After screening for plant growth-promoting ability, only isolate ANENR4 was selected for seed germination efficiency, growth promotion in gnotobiotic and sterile vermiculite growth condition, as well as colonization in vivo using the peanut JL286 cultivar. Strain ANENR4 carries some features that are related to plant growth promotion as well as biocontrol properties. Strain ANENR4 showed a high germination percentage as well as root length of seedlings compared to the uninoculated control plant. Our results show that peanut plants inoculated with ANENR4 in vermiculite soil showed significant increases in their biometric parameters, which was consistent with the actions observed in the gnotobiotic experiment. Which is similar to previously reported studies, such as plant biomass and the number of root nodules increased by the strains Bacillus sp. B.p.GL2 and Paenibacillus sp. P.g.YMR3, that enhanced peanut growth (Li et al. 2019). Moreover, it has been found that the tested strain of Micromonospora sp. ANENR4 was able to colonize the roots of peanut JL286 cultivar, even after 21 days post-inoculation. Interestingly, Micromonospora sp. ANENR4 was able to colonize the root endosphere, as it was recovered from surface-sterilized roots of a peanut plant under both gnotobiotic and vermiculite growth conditions based on CFU/g of root and morphology as well as 16S rDNA sequence analysis. Similar findings were reported for other Streptomyces strains (S. galilaeus, S. cyaneus, and S. lanatus) isolated from the endosphere of the plant roots (Tian et al. 2007). Actinomycetes have a positive impact on plants through their ability to suppress infection as well as promote plant growth. It has been reported that Streptomyces sp. RP1A-12 to promote peanut plant growth and control stem rot disease (Jacob et al. 2018). Field experiments with more extended growth periods may be required to determine if these strains can colonize the endosphere under natural conditions.
The endophytic strains were checked from the root section after staining them using DAPI, SYTO9, or PI. It has been found that the thin sections of roots contain ANENR4. Staining of thin free-hand root tissue sections with DAPI showed the bacterial nuclei clearly under a fluorescence microscope. With SYTO9 and PI, staining of the thin root section yielded a clear fluorescence signal with FITC and TRITC filters, indicates successful colonization. Rhizobia and Frankia are restricted to a specific host range of legumes and angiosperms, respectively, whereas Micromonospora is widely distributed among nitrogen-fixing plants (both legumes and actinorhizals). The ability of Micromonospora to infect plants and have a beneficial effect on them could be considered a benefit for future biotechnological applications (Trujillo et al. 2015).
Conclusion
Our findings indicate that the endophytic actinobacteria Micromonospora sp. ANENR4, Micromonospora sp. ASENL2, Micromonospora sp. ASENR12, and Micromonospora sp. ASENR15 that were isolated from peanut leaves and roots have the capacity to produce ammonia, siderophores, HCN, and IAA, which are traits that may promote plant growth. Further, the estimation of substantial in vivo plant growth-promoting activities and colonization abilities of root endophytic actinobacteria on peanut plants. The rarity of this endophytic strain in colonizing peanut roots adds a distinctive dimension to its agricultural significance. The findings suggest that this unique strain holds promise for future applications in enhancing growth attributes in crops, presenting a valuable addition to the ongoing efforts in harnessing the benefits of plant–microbe interactions, offering promising prospects for its application in agriculture.
Data availability
Not applicable.
References
Afzal I, Shinwari ZK, Sikandar S, Shahzad S (2019) Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res 221:36–49. https://doi.org/10.1016/j.micres.2019.02.001
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163(2):173–181. https://doi.org/10.1016/j.micres.2006.04.001
Anand AA, Vennison SJ, Sankar SG, Prabhu DI, Vasan PT, Raghuraman T, Geoffrey CJ, Vendan SE (2010) Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J Insect Sci 10:107. https://doi.org/10.1673/031.010.10701
Back CR, Stennett HL, Williams SE, Wang L, Ojeda Gomez J, Abdulle OM, Duffy T, Neal C, Mantell J, Jepson MA, Hendry KR, Powell D, Stach JEM, Essex-Lopresti AE, Willis CL, Curnow P, Race PR (2021) A new Micromonospora strain with antibiotic activity isolated from the microbiome of a Mid-Atlantic deep-sea sponge. Mar Drugs 19(2):105. https://doi.org/10.3390/md19020105
Bacon CW, White JF (2016) Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis 68:87–98. https://doi.org/10.1007/s13199-015-0350-2
Basu A, Prasad P, Das SN, Kalam S, Sayyed RZ, Reddy MS, El Enshasy H (2021) Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13(3):1140. https://doi.org/10.3390/su13031140
Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N (2014) Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact 8:13–66. https://doi.org/10.1186/1475-2859-13-66
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28(4):1327–1350. https://doi.org/10.1007/s11274-011-0979-9
Borah M, Das S, Bora SS, Boro RC, Barooah M (2021) Comparative assessment of multi-trait plant growth-promoting endophytes associated with cultivated and wild Oryza germplasm of Assam India. Arch Microbiol 203(5):2007–2028. https://doi.org/10.1007/s00203-020-02153-x
Carro L, Spröer C, Alonso P, Trujillo ME (2012) Diversity of Micromonospora strains isolated from nitrogen fixing nodules and rhizosphere of Pisum sativum analyzed by multilocus sequence analysis. Syst Appl Microbiol 35:73–80. https://doi.org/10.1016/j.syapm.2011.11.003
Carro L, Pujic P, Trujillo ME, Normand P (2013) Micromonospora is a normal inhabitant of actinorhizal nodules. J Biosci 38:685–693. https://doi.org/10.1007/s12038-013-9359-y
Chen L, Shi H, Heng J, Wang D, Bian K (2019) Antimicrobial, plant growth-promoting and genomic properties of the peanut endophyte Bacillus velezensis LDO2. Microbiol Res 218:41–48. https://doi.org/10.1016/j.micres.2018.10.002
Compant S, Duffy B, Nowak J, Cle´ment C, Barka EA, (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Conn VM, Franco CM (2004) Analysis of the endophytic actinobacterial population in the roots of wheat (Triticum aestivum L.) by terminal restriction fragment length polymorphism and sequencing of 16S rRNA clones. Appl Environ Microbiol 70:1787–1794. https://doi.org/10.1128/AEM.70.3.1787-1794.2004
Devi R, Thakur R (2018) Screening and identification of bacteria for plant growth promoting traits from termite mound soil. J Pharma Cogn Phytochem 7:1681–1686
Döbereiner J (1989) Isolation and identification of root associated diazotrophs. nitrogen fixation with non-legumes. Springer, Dordrecht, pp 103–108. https://doi.org/10.1007/BF02226800
Eevers N, Gielen M, Sánchez-López A, Jaspers S, White JC, Vangronsveld J, Weyens N (2015) Optimization of isolation and cultivation of bacterial endophytes through addition of plant extract to nutrient media. Microb Biotechnol 8(4):707–715. https://doi.org/10.1111/1751-7915.12291
Escobar Diaz PA, Gil OJA, Barbosa CH, Desoignies N, Rigobelo EC (2021) Aspergillus spp. and Bacillus spp. as growth promoters in cotton plants under greenhouse conditions. Front Sustain Food Syst 5:709267. https://doi.org/10.3389/fsufs.2021.709267
Franco-Correa M, Quintana A, Duque C, Suarez C, Rodríguez MX, Barea JM (2010) Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol 45:209–217
Fu G, Wang R, Ding J, Qi H, Zhao Z, Chen C, Zhang H, Xue Z, Wang J, Wu M (2020) Micromonospora zhangzhouensis sp. nov., a novel Actinobacterium isolated from Mangrove soil, exerts a cytotoxic activity in vitro. Sci Rep 10(1):3889. https://doi.org/10.1038/s41598-020-60677-0
Gaur AC (1990) Phosphate solubilizing micro-organisms as biofertilizer. omega scientific publishers. Omega scientific publishers. https://books.google.co.in/books?id=1rnwAAAAMAAJ
Genilloud O (2012) Genus I Micromonospora Ørskov1923, 156 AL. In: Goodfellow M, Kämpfer P, Busse HJ, Trujillo ME, Suzuki K, Ludwig W, Whitman WB (eds) Bergey’s manual of systematic bacteriology. Springer, NewYork, pp 1039–1057
Glick BR (2015) Introduction to plant growth promoting bacteria. In: Glick BR (ed) Beneficial plant-bacterial interactions. Springer International Publishing, Cham, pp 1–28. https://doi.org/10.1007/978-3-319-13921-0_1
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26(1):192–195. https://doi.org/10.1104/pp.26.1.192
Gupta S, Pandey S (2019) ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front Micro Biol 10:1506. https://doi.org/10.3389/fmicb.2019.01506
Haggag WM, Timmusk S (2008) Colonization of peanut roots by biofilm-forming Paenibacillus polymyxa initiates biocontrol against crown rot disease. J Appl Microbiol 104(4):961–969. https://doi.org/10.1111/j.1365-2672.2007.03611.x
Hankin L, Anagnostakis SL (1975) The use of solid media for detection of enzyme production by fungi. Mycologia 67(3):597–607. https://doi.org/10.2307/3758395
Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79(3):293–320. https://doi.org/10.1128/MMBR.00050-14
Jacob S, Sajjalaguddam RR, Sudini H (2018) Streptomyces sp. RP1A-12 mediated control of peanut stem rot caused by Sclerotium rolfsii. J Integr Agric 17:1–9 (ISSN 2095-3119)
Jana SK, Islam MM, Hore S, Mandal S (2022) Rice seed endophytes transmit into the plant seedling, promote plant growth and inhibit fungal phytopathogens. Plant Growth Regul 99:373–388. https://doi.org/10.1007/s10725-022-00914-w
Jiang G, Wei Z, Xu J, Chen H, Zhang Y, She X, Macho AP, Ding W, Liao B (2017) Bacterial wilt in China: history, current status, and future perspectives. Front Plant Sci 11:8–1549. https://doi.org/10.3389/fpls.2017.01549
Khare E, Mishra J, Arora NK (2018) Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol 5:9–2732. https://doi.org/10.3389/fmicb.2018.02732
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120. https://doi.org/10.1007/BF01731581
Li HB, Singh RK, Singh P, Song QQ, Xing YX, Yang LT, Li YR (2017) Genetic diversity of nitrogen-fixing and plant growth promoting Pseudomonas Species isolated from sugarcane rhizosphere. Front Microbiol 14:8–1268. https://doi.org/10.3389/fmicb.2017.01268
Li L, Zhang Z, Pan S, Li L, Li X (2019) Characterization and metabolism effect of seed endophytic bacteria associated with peanut grown in South China. Front Microbiol 10:2659. https://doi.org/10.3389/fmicb.2019.02659
Liu D, Yang Q, Ge K, Hu X, Qi G, Du B, Liu K, Ding Y (2017) Promotion of iron nutrition and growth on peanut by Paenibacillus illinoisensis and Bacillus sp. strains in calcareous soil. Braz J Microbiol 48(4):656–670. https://doi.org/10.1016/j.bjm.2017.02.006
Louden BC, Haarmann D, Lynne AM (2011) Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ 12:51–53. https://doi.org/10.1128/jmbe.v12i1.249
Ludueña LM, Anzuay MS, Angelini JG, McIntosh M, Becker A, Rupp O, Goesmann A, Blom J, Fabra A, Taurian T (2019) Genome sequence of the endophytic strain Enterobacter sp. J49, a potential biofertilizer for peanut and maize. Genomics 111(4):913–920. https://doi.org/10.1016/j.ygeno.2018.05.021
Madhaiyan M, Suresh Reddy BV, Anandham R, Senthilkumar M, Poonguzhali S, Sundaram SP, Sa T (2006) Plant growth-promoting Methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot pathogens. Curr Microbiol 53(4):270–276. https://doi.org/10.1007/s00284-005-0452-9
Maiti PK, Mandal S (2019) Majority of actinobacterial strains isolated from Kashmir Himalaya soil are rich source of antimicrobials and industrially important biomolecules. Adv Microbiol 9:220–238. https://doi.org/10.4236/aim.2019.93016
Maiti PK, Mandal S (2020) Lentzea indica sp. nov., a novel actinobacteria isolated from Indian Himalayan-soil. Antonie Van Leeuwenhoek 113(10):1411–1423. https://doi.org/10.1007/s10482-020-01449-8
Manter DK, Delgado JA, Holm DG, Stong RA (2010) Pyrosequencing reveals a highly diverse and cultivar-specific bacterial endophyte community in potato roots. Microb Ecol 60:157–166. https://doi.org/10.1007/s00248-010-9658-x
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol. https://doi.org/10.1016/S0022-2836(61)80047-8
Martínez-Hidalgo P, Galindo-Villardón P, Igual JM, Trujillo ME, Martínez-Molina E (2014) Micromonospora from nitrogen fixing nodules of alfalfa(Medicago sativa L.). a new promising plant probiotic bacteria. Sci Rep 4:6389. https://doi.org/10.1038/srep06389
Mir MI, Hameeda B, Quadriya H, Kumar BK, Ilyas N, Zuan ATK, Sayyed RZ (2021) Multifarious indigenous diazotrophic rhizobacteria of rice (Oryza sativa L.) rhizosphere and their effect on plant growth promotion. Front Nutr 3(8):781764. https://doi.org/10.1038/srep06389
Rojo FG, Reynoso MM, Ferez M, Chulze SN, Torres AM (2007) Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Protect 26:549–555
Rosenblueth M, Martínez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact 19(8):827–837. https://doi.org/10.1094/MPMI-19-0827
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Sharma S, Sharma A, Kaur M (2018) Extraction and evaluation of gibberellic acid from Pseudomonas sp.: plant growth promoting rhizobacteria. J Pharmacogn Phytochem 7:2790–2795
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340. https://doi.org/10.1099/00207713-16-3-313
Shridhar BS (2012) Nitrogen fixing microorganisms. Int J Microbiol Res 3:46–52. https://doi.org/10.5829/idosi.ijmr.2012.3.161103
Smith DL, Praslickova D, Ilangumaran G (2016) Inter-organismal signaling and management of the phytomicrobiome. Front Plant Sci 14:6–722. https://doi.org/10.3389/fpls.2015.00722
Smith DL, Gravel V, Yergeau E (2017) Editorial: signaling in the phytomicrobiome. Front Plant Sci 8:611. https://doi.org/10.3389/fpls.2017.00611
Stackebrandt SP (2000) The prokaryotes: an evolving electronic resource for the microbiological community. Springer-Verlag, New York NY
Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30. https://doi.org/10.1007/s00253-014-5720-9
Swamy MK, Akhtar MS, Sinniah UR (2016) Response of PGPR and AM fungi toward growth and secondary metabolite production in medicinal and aromatic plants. Plant, Soil Microb. https://doi.org/10.1007/978-3-319-29573-2_7
Tan KZ, Radziah O, Halimi MS, Khairuddin AR, Habib SH, Sham suddin ZH (2014) Isolation and characterization of rhizobia and plant growth-promoting rhizobacteria and their effects on growth of rice seedlings. Am J Agric Biol Sci 9:342–360
Tian X, Cao L, Tan H, Han W, Chen M, Liu Y, Zhou S (2007) Diversity of cultivated and uncultivated actinobacterial endophytes in the stems and roots of rice. Microb Ecol 53(4):700–707. https://doi.org/10.1007/s00248-006-9163-4
Trujillo ME, Kroppenstedt RM, Schumann P, Carro L, Martinez Molina E (2006) Micromonospora coriariae sp. nov., isolated from root nodules of Coriaria myrtifolia. Int J Syst Evol Microbiol 56:2381–2385. https://doi.org/10.1099/ijs.0.64449-0
Trujillo ME, Bacigalupe R, Pujic P, Igarashi Y, Benito P, Riesco R, Médigue C, Normand P (2014a) Genome features of the endophytic actinobacterium Micromonospora lupini strain lupac 08: on the process of adaptation to an endophytic life style? PLoS ONE 9(9):e108522. https://doi.org/10.1371/journal.pone.0108522
Trujillo ME, Hong K, Genilloud O (2014b) Family micromonosporaceae. In: Ronsenberg E et al (eds) The prokaryotes the actinobacteria. Springer, pp 499–569
Trujillo ME, Riesco R, Benito P, Carro L (2015) Endophytic actinobacteria and the interaction of Micromonospora and nitrogen fixing plants. Front Microbiol 1:6–1341. https://doi.org/10.3389/fmicb.2015.01341
Turner S, Prayer KM, Miao VPW, Palmer JD (1999) Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol 46:327–338. https://doi.org/10.1111/j.1550-7408.1999.tb04612.x
Valdés M, Perez NO, de Los E, Santos P, Caballero-Mellado J, Peña Cabriales JJ, Normand P et al (2005) Non-Frankia actinomycetes isolated from surface sterilized roots of Casuarina equisetifolia fix nitrogen. Appl Environ Microbiol 71:460–466. https://doi.org/10.1128/AEM.71.1.460-466.2005
Verma SK, Sahu PK, Kumar K, Pal G, Gond SK, Kharwar RN, White JF (2021) Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J Appl Microbiol 131:2161–2177. https://doi.org/10.1111/jam.15111
Wan L, Wu Y, Huang J, Dai X, Lei Y, Yan L, Jiang H, Zhang J, Varshney RK, Liao B (2014) Identification of ERF genes in peanuts and functional analysis of AhERF008 and AhERF019 in abiotic stress response. Funct Integr Genom 14(3):467–477. https://doi.org/10.1007/s10142-014-0381-4
Wang X, Liang G (2014) Control efficacy of an endophytic Bacillus amyloliquefaciens strain BZ6-1 against peanut bacterial Wilt Ralstonia solanacearum. Biomed Res Int 2014:465435. https://doi.org/10.1155/2014/465435
Wang FP, Wang XF, Zhang J, Ma F, Hao YJ (2018) MdMYB58 modulates Fe homeostasis by directly binding to the MdMATE43 promoter in plants. Plant Cell Physiol 59(12):2476–2489. https://doi.org/10.1093/pcp/pcy168
Welbaum GE, Sturz AV, Dong Z, Nowak J (2004) Managing soil microorganisms to improve productivity of agro-eco systems. CRC Crit Rev Plant Sci 23:175–193. https://doi.org/10.1080/07352680490433295
Williams ST, Goodfellow M, Alderson G, Wellington EMH, Sneath PHA, Sackins MJ (1983) Numerical classification of Streptomyces and related genera. Microbiology 129:1743–1813. https://doi.org/10.1099/00221287-129-6-1743
Xing YX, Wei CY, Mo Y, Yang LT, Huang SL, Li YR (2016) Nitrogen-fixing and plant growth-promoting ability of two endophytic bacterial strains isolated from sugarcane stalks. Sugar Tech 18:373–379. https://doi.org/10.1007/s12355-015-0397-7
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Yu YY, Jiang CH, Wang C, Chen LJ, Li HY, Xu Q, Guo JH (2017) An improved strategy for stable biocontrol agents selecting to control rice sheath blight caused by Rhizoctonia solani. Microbiol Res 203:1–9. https://doi.org/10.1016/j.micres.2017.05.006
Acknowledgements
MMI is grateful to UGC, Govt. of India for providing the research fellowship.
Funding
This work is not supported by any financial support.
Author information
Authors and Affiliations
Contributions
MI performed all experiments, analyze the data, and wrote the initial manuscript; SM conceptualize and supervised the research, arranged resources, and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study does not need any ethical approval.
Consent for participate
Not applicable.
Consent for publication
Authors have consent to publish the manuscript in this journal.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Islam, M.M., Mandal, S. Unveiling growth-promoting attributes of peanut root endophyte Micromonospora sp.. Arch Microbiol 206, 182 (2024). https://doi.org/10.1007/s00203-024-03886-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-024-03886-9