Abstract
Cancer is characterized by mutagenic events that lead to disrupted cell signaling and cellular functions. It is one of the leading causes of death worldwide. Literature suggests that pathogens, mainly Helicobacter pylori and Epstein–Barr virus (EBV), have been associated with the etiology of human cancer. Notably, their co-infection may lead to gastric cancer. Pathogen-mediated DNA damage could be the first and crucial step in the carcinogenesis process that modulates numerous cellular signaling pathways. Altogether, it dysregulates the metabolic pathways linked with cell growth, apoptosis, and DNA repair. Modulation in these pathways leads to abnormal growth and proliferation. Several signaling pathways such RTK, RAS/MAPK, PI3K/Akt, NFκB, JAK/STAT, HIF1α, and Wnt/β-catenin are known to be altered in cancer. Therefore, this review focuses on the oncogenic roles of H. pylori, EBV, and its associated signaling cascades in various cancers. Scrutinizing these signaling pathways is crucial and may provide new insights and targets for preventing and treating H. pylori and EBV-associated cancers.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epstein–Barr virus (EBV) is a human pathogen involved in various tumorigenesis events such as nasopharyngeal carcinoma (NPC), Hodgkin's lymphoma (HL), Burkitt's lymphoma (BL), and gastric cancer (GC). The prevalence of EBV is about 90% among the human adult population. A study reported that 8.77% of GC patients were EBV-positive (Fig. 1A) (Tavakoli et al. 2020). Commonly, EBV infection does not cause any unfavorable effects on human health until a balance between the host and virus is sustained. EBV genome persists in episomal form; once human immune surveillance machinery is compromised, latent EBV can be reactivated to induce abnormal proliferation and transformation of host cells, eventually leading to malignancy (Kashyap et al. 2021). EBV is a DNA virus having two genome clusters that encode around 25 microRNAs that disturb the normal functioning of healthy cells (Zheng 2010). EBV encodes a viral oncogene LMP1 (latent membrane protein-1 or BZLF1 (BamHI Z fragment leftward open reading frame 1), essential for B cell transformation and disrupted cellular signal transduction (Siegler 2003). Although the EBV nuclear antigen 1 (EBNA1) is the earliest reported viral protein post-infection and is the only latent protein consistently expressed in virus-associated tumors, recent results indicate that EBNA1 is not a viral oncoprotein (Siegler 2003; Humme et al. 2003; Frappier 2012). BARF1 (BamHI-A reading frame1) is also an early gene but express as a latent gene in most NPCs (Hutajulu et al. 2010). Recent studies have suggested that BARF1 may have an essential role in NPC oncogenesis (Hutajulu et al. 2010; Young and Dawson 2014). Latent membrane protein LMP1 is produced by the BNLF1 gene, which contains C-terminal activating regions (CTAR1, CTAR2, and CTAR3) domains (Zheng 2010; Lo et al. 2020). These domains induce viral effect by targeting several signaling pathways, including nuclear transcription factor kappaB (NFkB), Delta-Notch, Renin-angiotensin system (RAS), mitogen-activated protein kinase (MAPK), signal transducer and activator of transcription 3 (STAT3) and phosphatidylinositol-3 kinase (PI3K) (Zheng 2010; Kung and Raab-Traub 2008; Jakhmola and Jha 2021). EBV-associated oncoproteins play an active role in the apoptotic inhibition of cancer cells by secreting survivin and rendering the pro-apoptotic protein Protease-activated receptor 4 (PAR4). EBV also inhibits apoptosis by increasing the expression of the anti-apoptotic gene B cell leukemia/lymphoma2 protein (BCL2) (Kung and Raab-Traub 2008; Sun et al. 2015). Studies revealed enhanced survivin levels in gastric carcinoma cell lines by LMP2A through an NFκB-mediated pathway (Kung and Raab-Traub 2008; Pei et al. 2016). LMP1 and 2 also stimulate the CD40 and B cell receptors (BCRs), respectively. However, EBNA1, 2, 3A, 3B, 3C, and one leader protein EBNA-LP are also encoded by the EBV and promote the oncogenic transformations through various host proteins like E2F6 and E2F1 (Pei et al. 2020a; Hooi et al. 2017). EBNA1, nucleolin, and nucleophosmin are crucial for EBV DNA replication and persistence. Moreover, EBNA2 and LP synergistically activate the transcription of viral and cellular genes, which can transform the EBV-exposed B cells into lymphoblastoid cell lines (LCLs). Moreover, EBNA3A, 3B, and 3C are similar in their genetic structure and promoter and regulate the transcription of host genes.
Incidence of individual infection of H. pylori and EBV in gastric cancer (GC). A Showing the total number of incidences of GC, and among that 90% of GC are H. pylori positive (Tavakoli et al. 2020). Meanwhile B graph depicts the total number of GC cases, and among that 8.77% of GC are EBV positive (Lu et al. 2022). Source: A Lu et al. (2022). Source: B Tavakoli et al. (2020)
Helicobacter pylori (H. pylori) is a Gram-negative, microaerophilic, and spiral bacterium. Human chronic H. pylori infection strikes about 4.4 billion people globally, and its prevalence varies geographically from 28 to 84% (Hooi et al. 2017). Moreover, group-1 carcinogen has potential to cause cancer and H. pylori is also declared as a group-1 carcinogen, which cause gastric cancer (GC) in only 2% of infected individuals (Conteduca et al. 2013). A systematic review and meta-analysis revealed that approximately 90% of GC patients are H. pylori positive (Fig. 1B) (Lu et al. 2022). H. pylori contains flagella and produces urease enzymes, which help avoid harsh environments and enhance survivability (Dunne 2014).
They established their infection by breaching the mucous lining of the stomach. H. pylori Cag pathogenicity island-encoded cytotoxin-associated antigen A (CagA) is a crucial factor for inflammatory stress within gastric mucous lining and oncogenesis. Nonetheless, H. pylori also encode another virulence factor, vacuolating cytotoxin A (VacA), which can predominantly induce cellular damage (Huang et al. 2016). H. pylori can also promote oncogenic transformation by inducing epigenetic alterations, viz. histone modification and DNA methylation.
Notably, the coinfection of EBV and H. pylori reduces the time span and promotes the aggressiveness of GC (Singh and Jha 2017). Study by Mulherkar et al. (2022) reported that the coinfection of retroviruses such as HIV-1 and HTLV-1 has implications for cancer malignancies. Another study by Shi et al., revealed that the coinfection of EBV and human papillomavirus (HPV) causes NPC in the endemic region. This study reported the coexistence of these two pathogens in the human host and its consequences towards malignancy. The cooperation between these two pathogens provides the cellular milieu for the growth and replication of each other. This study also shows that coinfection with H. pylori and EBV enhances tissue damage by the recruitment of immune cells (Al Moustafa et al. 2009). Nonetheless, more studies need to be conducted to understand the insight into molecular mechanisms controlling the interplay between these two pathogens. Herein, we provide the status of signaling pathways modulated by H. pylori and EBV and their further consequences in cancer development.
Role of Epstein–Barr virus and Helicobacter pylori in amendments of host cell signaling pathways
Janus kinase/signal transducer and activator of transcription pathway
EBV
The Janus kinase (JAK) and STAT play an important function in inflicting the host's immune response. This pathway is controlled by an array of regulatory proteins, such as the suppressor of cytokine signaling (SOCS), protein tyrosine phosphatases (PTPs), and protein inhibitors of activated STAT (PIAS) (Seif et al. 2017). JAK/STAT pathway gets hyperactivated in the presence of several pathogens. For instance, LMP1, LMP-2A, and LMP-2B interact with JAK3, which leads to the activation of the STAT3 pathway in diffused large B cell Lymphoma (Bousoik and Montazeri Aliabadi 2018). A study by Kilger et al. showed that endogenous LMP1 interacts with JAK3 in the EBV-immortalized cell line LCL 1852.4 and HEK293 cells upon ectopic expression. The interaction between the two proteins enhances the tyrosine phosphorylation of JAK3, which needs a proline-rich motif and the surrounding LMP1 33 bp repeat region sequence (Gires 1999). Subsequently, the signal gets transmitted to STAT and activates certain cytokine receptors. Thus, this pathway leads to the upregulation of HLX expression, which diminishes NKX6-3, SPIB, IL4R, and BCL2L11 to interfere with cell differentiation and subdue cell apoptosis (Luo et al. 2021). In cancerous cells, JAK/STAT helps to escape immune surveillance by increasing the expression of programmed cell death ligand-1 (PD-L1) (Green et al. 2010). LMP1 also utilized the NFκB-dependent interferons to regulate the STAT1 pathway (Najjar et al. 2005). Notably, interferons are a crucial component of the host defense system which protects against pathogenic viruses by activating the innate and adaptive immune systems. Interferons (IFNs) can activate JAK/STAT pathways by binding to cognate receptors (Michaud et al. 2010). In response to Interferon-gamma (IFNy), EBNA1 increases the phosphorylation of STAT1 and enhances the STAT1 expression in cancer cell lines (Najjar et al. 2005; Michaud et al. 2010). Interferons like IFN2 and IFN7 negatively regulate the EBV Qp promoter, whereas IFN1 and IFN2 can positively regulate it. Regulation of Qp is essential for EBV pathogenesis. Activation of STAT1 by LMP1 encompasses a mediator like IFNy, which is secreted in an NFκB-dependent manner (Luo et al. 2021; Moon et al. 2017; Wood et al. 2007). LMP1 also induces various chemokines like IP10, RANTES, and MIP1α (Vaysberg et al. 2009). The JAK/STAT pathway is always active during EBV latency along with STAT3, STAT4, and STAT5 (Wang 2016). CTAR3, the domain of LMP1, which resides between CTAR1 and CTAR2 domains, has been reported to show its binding activity with JAK3 and activate STAT1 (Dunne 2014; Vaysberg et al. 2009). This particular domain has been recently found to interact with UBC9, inducing SUMOlyation of IRF7 (Vaysberg et al. 2009; Wang 2016). Interestingly, higher IFN secretion and SOCS3 suppression were caused by EBV-infected monocytes, which further amplified IFN secretion. As mediated by JAK/STAT pathway, EBV blocks this pathway by IRF7, which was demonstrated by siRNA-suppressed SOCS3. Besides, EBNA1 knockdown reduced the expression of JAK2 and STAT1 signaling by a reduction in the activity of IFNγ-induced PD-L1 promoter (Moon et al. 2017; Ersing et al. 2013).
H. pylori
Helicobacter pylori induces the production and secretion of growth factors and pro-inflammatory cytokines, which activate the STAT3 and tend to cause GC (Menheniott et al. 2015). STAT3 controls various cellular processes, viz. basal homeostasis, angiogenesis proliferation, and apoptosis. It also gets activated by the phosphorylation of receptor tyrosine kinases, including JAK1, JAK2, and Src (Ersing et al. 2013; Menheniott et al. 2015). The H. pylori genome consisting of a 40-kb DNA fragment (known as cag PAI) encodes ~ 30 genes. One of these ~ 30 genes, the CagA gene, encodes the cagA protein, which is responsible for virulence. Intriguingly, infection of CagA-positive strains is related to a higher risk of gastric cancer. Activating the STAT3 pathway in cagA-dependent manner tends to trigger gastritis and ultimately cancer (Piao et al. 2016; Hatakeyama 2014). The Type 4 secretion system (T4SS) aids CagA to dock with the inner plasma membrane, thereby causing its delivery inside the epithelial cells. The phosphorylation of this protein occurs on C-terminal EPIYA (Glu-Pro-Ile-Tyr-Ala) motifs. Depending on adjacent amino acids, different EPIYA motifs are recognized and designated EPIYA-A to D (Piao et al. 2016). Several mouse model studies suggest that cagA triggers signal transduction via IL11 and IL6 by utilizing glycoprotein-130 (gp130), while IL11 activates gastric STAT3. Therefore, it can be corroborated that IL11-dependent activation may occur in the early stage of this pathway (Yin et al. 1950). Moreover, both recruitment and homo-dimerization of gp130 are induced by IL6. This potentially leads to a balance between the signaling of both JAK/STAT and SHP2/Ras/ERK pathways. Disrupting this signal transduction in the mutant mouse with gp130 knock-in mutation induces the formation of premalignant lesions, including atrophy, mucous metaplasia, and dysplasia, eventually leading to gastric cancer. Furthermore, ligand binding forms a signal transduction pathway wherein phosphorylation causes activation of gp130-associated JAK kinases and STAT3 (pTyr705). After its dissociation and dimerization, pSTAT3 moves to the nucleus, initiating gene transcription and targeting the expression of downstream effector genes that lead to cell survival, proliferation, angiogenesis, invasion, and migration (Yin et al. 1950).
Studies based on human gastric adenocarcinoma cells (AGS) revealed that phosphorylation of cagA preferentially activated SHP2 binding and activation of ERK. On the other hand, unphosphorylated CagA leads to the activation of STAT3 (Menheniott et al. 2015). In non-gastric tissues, H. pylori-induced Toll-like receptor manifest signaling through STAT3-dependent mechanisms (Tye et al. 2012). STAT3 causes upregulation of TLR2 gene expression, as observed in an IL11/STAT3-dependent mouse model of gastric cancer. Ablation of TLR2 does not affect inflammation but blocks tumor growth. In the immune pathologies of H. pylori, IL17 is involved in neoplastic growth that sets off chronic inflammation. Triggering the STAT3 pathway by H. pylori-induced IL23 secretion from dendritic cells results in increased prevalence and production of mucosal TH17 cells and pro-inflammatory IL17 cytokines (Menheniott et al. 2015). In addition, IL22 responses are mediated via its heterodimeric receptor complex (IL22R), composed of two subunits, IL10R2 and IL22R1. IL22 and its receptor form a complex that leads to the activation of STAT3 that further mediates an immunoregulatory effect on human gastric epithelial cells infected by H. pylori (Fig. 2) (Ersing et al. 2013; Tye et al. 2012; Chen et al. 2014).
Signaling pathways associated with H. pylori infection leading to oncogenic transformation of cells. H. pylori triggered the various host cell signaling pathways to transform the normal gastric epithelial cells into the gastric cancer (GC). Notably, H. pylori modulate the TNF Receptor-Associated Factor 2 (TRAF2), epidermal growth factor receptor (EGFR), mitogen-activated protein kinase kinase (MAPKK), Just Another Kinase (JAK), signal transducer and activator of transcription 3 (STAT3), β-catenin, nuclear factor kappa beta (NFkB) pathways to promotes the inflammation, cell proliferation, cell survival, invasion and alleviated the programmed cell death
Interferon regulatory factor signaling pathway
EBV
Mammalian interferon regulatory factors (IRFs) comprise nine members, namely IRF19. One of the factors, IRF7, is known for its post-translational phosphorylation and ubiquitination properties. Receptor-interacting protein kinase 1 (RIP1) and tumor necrosis factor receptor (TNFR)-associated factors (TRAF6) are important proteins that contribute to LMP1-mediated activation of IRF7 (Ning et al. 2011) SUMOylation of this pathway is carried forward by the physical interaction of ubiquitin-conjugating enzyme 9 (UBC9) and CTAR3 domain of LMP1 (Bentz et al. 2012). Through the C-terminal domain, LMP1 activates a multitude of transduction events (Wang 2016; Ning et al. 2011). Along with the C-terminal functional domain, the activator regions 1 and 2 of the C-terminal are required to activate IRF7 (Ning et al. 2011). LMP1 induces an upsurge in the transcriptional activity of IRF7 via phosphorylation (Bentz et al. 2012). The viral protein leads to deubiquitylation of interferon via A20 to repress its activity (Bentz et al. 2012). In EBV latency III, LMP1 introduces covalent modifications in IRF7 (Bentz et al. 2012). LMP1 uses small ubiquitin-related modifiers 1 (SUMO1) and FLAG-SUMO1 to SUMOylate IRF7 (Bentz et al. 2012). IRF7 in turn controls the expression of LMP2 (Brennan et al. 2001; Sgarbanti et al. 2007). There have been reports of gene overlapping between LMP1 targeted modification of the IRF7 pathway and NFκB pathway, indicating that the two pathways may cooperate during EBV infection. LMP1 activates IRF4 but does not interfere with the expression of IRF2 (Ning et al. 2011). Notably, siRNA-mediated suppression of c-Myc leads to reduction of ZEBRA and EBV early antigen, thereby inhibiting EBV reactivation (Gao et al. 2004). Antiapoptotic episomal maintenance of EBV is majorly due to EBNA1 which was inhibited by siRNA targeting endogenous EBNA1. This led to a reduction in tumorigenesis (Yin and Flemington 2006). RIG-1 targeting siRNA was used to elucidate the function of miR-BART6 that triggers IFN-β during EBV infection to evade immune surveillance (Lu et al. 2017). As miRs interfere with host gene expression, EBV miRs have been reported to indirectly target type 1 IFN pathways through TLR7 and TLR9 (Bouvet et al. 2021). Genome-wide CRISPR/Cas9 loss-of-function screening identified that LMP1 leads to cFLIP induction, which is critical against TNFα-mediated cell death (Ma et al. 2017).
H. pylori
TLR7, 8, or 9 activation leads to an increase in type I interferon production in response to bacterial DNA or single-stranded RNA ingestion via phagosome. Inflammatory cytokines and type I interferons are transactivated by various factors such as NFκB, activator protein 1 (AP1), and IRF3/7. During H. pylori infection, activation of IRFs occurs through DC-SIGN receptors through an unidentified route (Sonkar et al. 2020). Retinoic acid-inducible gene I (RIG-I) and other RIG-I-like receptors (RLRs) are activated by 5' triphosphate (PPP) single-stranded RNA, which causes a conformational shift that activates tank-binding kinase 1 (TBK1). This ultimately results in the generation of type I interferons through the IRF3/7 signaling (Cheok et al. 2022).
H. pylori infection of gastric epithelial cells results in a wide range of intricate host protective immunological reactions. Type I interferon is produced by the nucleotide-binding oligomerization domain 1 (NOD1) signaling pathway, which drives chemokine and cytokine responses that control the severity of gastric H. pylori infection (Watanabe et al. 2011). Stomach adenocarcinoma (STAD) subgroup analyses based on race, gender, age, H. pylori infection status, histological subtypes, tumor grade, specific cancer stages, and nodal metastasis status revealed the increased expression of IRF3/7. Hence, the study revealed that only in 5–6% of all STAD patients, IRF3 and IRF7 were found to be altered. Additionally, IRF7 is strongly correlated with immune infiltration in STAD and is associated with the expression of the majority of immunological biomarkers as well as the number of immune cells (Guo et al. 2021). The activation of heterotrimeric transcription factor complexes known as IFN-stimulated gene factor 3 (ISGF3) and subsequent production of CXC motif chemokine ligand 10 (CXCL10) and type I IFN occurs as a result of IRF7-induced IFN beta production.
Nonetheless, Watanabe et al. (2010) revealed that NOD1-deficient mice lacking the IFN beta receptor showed a reduced CXCL10 response and elevated susceptibility to H. pylori infection. STAT1 and IFN regulatory factor 1 (IRF1) are the two elements of the IFN signaling pathway, which gets stimulated through the direct effects of H. pylori, leading to increased proinflammatory signaling in epithelial cells through the activation of the NOD1 pathway. It has been reported that H. pylori-mediated activation of the NOD1 pathway increases STAT1-Tyr701/Ser727 phosphorylation levels and IRF1 expression/synthesis in a cell, leading to increased production of the chemokines, which are controlled by NOD-1, IFNγ-, IL8- and IFNγ-induced protein 10 (IP10). As a result, H. pylori-induced inflammatory responses are influenced by the interaction between NOD1 and IFNγ signaling pathways, potentially revealing a new mechanism by which H. pylori strains promote severe illnesses (Allison et al. 2013) (Fig. 3). NOD1 enhances mucosal host resistance against H. pylori infection by activating type I IFN signaling pathways and producing AMPs. Intracellular NOD1 sensing of H. pylori-derived PGN in gastric ECs triggers production of type I IFN and CXCL10 via the RIP2-TRAF3-TBK1-IKKε-IRF7-ISGF3 pathway, promoting Th1 responses. Because IFNγ generated by Th1 cells increases NOD1 expression. During prolonged H. pylori infection, the type I IFN-CXCL10-IFNγ axis caused by NOD1 activation provides a positive feedback loop for the development of Th1 responses in the stomach mucosa (Minaga et al. 2018). The number of H. pylori organisms colonizing the stomach was lower in wild-type mice than in IFN gamma knockout (IFN−/−) mice (Sawai et al. 1999; Kong et al. 2020).
Outcomes of the altered signaling pathways in during H. pylori infection. The most important outcomes of H. pylori infection are gastritis or malignant transformation in abut 2–3% of infected populations. During the malignant transformation H. pylori altered the various host signaling pathways to promote the cell proliferation, inflammation, ROS generation, angiogenesis, and metastasis. Besides, H. pylori also disturbed the hemostasis between cell proliferation and apoptosis which leads to accumulation of cells
Nuclear factor kappa B pathway
EBV
NFκB tunes various host cellular processes like immune and inflammatory responses, transformation, proliferation, angiogenesis, and metastasis. Suppression of NFκB activation is known to reduce the oncogenic potential of transformed cells (Ma et al. 2017; Sonkar et al. 2020). The canonical and non-canonical NFκB pathways are recognized as being of utmost importance. Notably, LMP1 activates the canonical NFκB pathway (Vaysberg et al. 2009; Cheok et al. 2022). The N-terminal tail of LMP1 is composed of 24 amino acid residues, while the C terminal domain houses 200 residues along with six transmembrane domains. The TES1 domain starts from residues 187–231, and the TES2 domain spans residues 351–386 of LMP1. LMP1 uses the TES2 domain to mediate the activation of the canonical pathway and TES1 to activate the non-canonical pathway (Ersing et al. 2013). TES2 activates TRAF6 and leads to CTAR2-mediated pathway activation (Watanabe et al. 2011; Guo et al. 2021; Sethi et al. 2008). Proteins like BEX3, BEX5, and BS69 are essential to link LMP1 and TES2 to TRAF6 to activate the NFκB pathway (Hayden and Ghosh 2012; Schultheiss 2001) Apart from TRAF6, IRAK1 is also used to activate the NFκB pathway (Ersing et al. 2013; Gewurz et al. 2012; Luftig et al. 2003). The expression of EGFR activated by LMP1-CTAR1 provides a novel system to assess the contribution of NFκB pathway activation (Kung and Raab-Traub 2008). Increased glucose uptake is a prerequisite for cellular growth in B-cell lymphomas; this increased uptake is altered by LMP1-mediated NFκB, causing dysregulation of glucose transporter protein type 1 (GLUT1). Moreover, TES2 also upregulates several inhibitors of NFκB regulators, including ABIN1, CYLD, A20, RNF11, TAX1BP1, and IκBα (Ersing et al. 2013; Gewurz et al. 2011). Nonetheless, siRNA analysis of kinases identified that NFκB essential modifier (NEMO) is among the strongest inhibitors of LMP1 that further activate canonical NF-κB activation (Boehm et al. 2010).
H. pylori
Pathogens like H. pylori are known to modulate the level of NFκB through TLR activation and by other host oncoproteins. H. pylori alters the canonical and non-canonical pathways of NFκB activation depending on specific cell types (Lamb and Chen 2010). In epithelial cells, H. pylori activate the canonical NFκB cascade, whereas in B-lymphocytes it activates both pathways. Nonetheless, the binding of ligands with their receptor activates the kinase complex IκB kinase (IKK) (Lamb and Chen 2013). IKK in turn triggers the translocation of the canonical NFκB heterodimer of p50 and RelA/p65 and encourages the breakdown of inhibitor IκB phosphorylation. Canonical and non-canonical NFκB pathways differ in the signaling mechanism. However, the exact mechanisms of the NFκB non-canonical pathway are yet to be understood. Associated H. pylori LPS can activate this pathway in B-lymphocytes through the NFκB-inducing kinase (NIK) and IKK (Sun 2011). The receptor's downstream signaling activates the NIK and IKKα. Activated IKKα phosphorylates its downstream p100, which later induces the proteasomal degradation of p52 (Chen 2005). RelB and p52 work together to form a transcriptionally active complex that turns on the target genes involved in lymphoid organogenesis, B cell survival, maturation, and bone metabolism (Lamb and Chen 2013). In addition to LPS and peptidoglycan of H. pylori, other virulence genes also trigger the activation of NFκB. The multimerization of cagA also has a vital role in NFκB activation through the Met-PI3K-Akt pathway. CagA interacts with intramembrane hepatocyte growth factor receptor (HGFR)/MET in gastric epithelial cells to initiate PI3K-Akt signaling, which activates β-catenin and NFκB. Nonetheless, cagA activates TGF-β-activating kinase 1 (TAK1) by interacting with TRAF6 and TAK1. TAK1 later phosphorylates and activates the IKK complex leading to the activation of NFκB (Lamb and Chen 2013). Moreover, Hirata et al. (2006) showed that siRNA-mediated knockdown of NOD1 or RIP2 expression had no effect on H. pylori-induced NFkB/MAPK activation or CXCL8 synthesis in AGS cells. OMVs (Outer membrane vesicles) isolated from H. pylori activate NFkB in AGS cells, in a cagPAI-independent way. Interestingly, siRNA suppression of NOD1 expression prevented CXCL8 synthesis in AGS cells exposed to H. pylori-derived OMVs (Minaga et al. 2018).
c-Jun proto-oncogene signaling pathway
c-Jun pathway and EBV
TRAF2, along with other proteins, mediates the activation of JNK (Liu et al. 1996; Vanden Berghe et al. 1998). Proteins involved in LMP1-mediated activation of the JNK pathway include TAK1/TAB1, TRAF6, c-Jun N terminal kinase 1, 2, and TRAF6. The proteins mentioned above further recruit CTAR2 to activate the JNK pathway and also bridge the gap between TAB1 and LMP1 (Wu et al. 2006; Wan et al. 2004). The presence of 379PVQLSY384 motif on the C terminal of LMP1 establishes its interaction with TRAF6; this is why TRAF6 is an essential protein in activating this pathway (Wan et al. 2004). Receptor-interacting proteins (RIP) are also involved in the activation of the JNK pathway (Gewurz et al. 2011; Boehm et al. 2010). SEK1 kinase upstream of JNK is required for LMP1-mediated activation of JNK by TES2. JNK phosphorylation occurs with the help of BZLF1 and BRLF1, which further activates ATF2 (Mosialos 2001; Adamson et al. 2000). BGLF2, the tegument protein along with BNRF1, also plays a role in the phosphorylation of the JNK pathway, which in turn activates the BZLF1 (Liu and Cohen 2016). LMP1 expressing primary tumor cells derived from PTLD biopsies and EBV-transformed B cells survive and proliferate due to the IKK2–TPL2–JNK pathway (Voigt et al. 2020). The proteins that are important in LMP1-mediated activation of the JNK pathway are TRAF6, TAK1/TAB1, JNKK1, JNKK2, RIP, IKK2, NEMO, TES2/CTAR2, SEK1, IL1, IL5, TNIK, and TPL2 (Fig. 4).
Altered signaling pathways during EBV infection leading to oncogenic transformation of cells. Similarly, EBV also promotes the oncogenic transformation of gastric epithelial and B cell by altering the various host cell signaling pathways. The most important pathways utilized by the EBV to promotes the cancer are hypoxia-inducible factor 1-α, nuclear factor kappa beta (NFkB), Just Another Kinase (JAK), signal transducer and activator of transcription 3 (STAT3), interferon regulatory factor 7 (IRF-7), B cell receptor (BCR), Transforming growth factor beta (TGF-β), mitogen-activated protein kinase, phosphatidylinositol-3 kinase and PI3 kinase (PI3K), and protein 38
c-Jun pathway and H. pylori
H. pylori induces apoptosis signal-regulating kinase 1 (ASK1) in gastric epithelial cells in a reactive oxygen species (ROS) and cagPAI-dependent manner. JNK activation and H. pylori-mediated apoptosis are governed by ASK1 (Hayakawa et al. 2013). Early JNK activation and cytokine production driven by H. pylori are mediated through the transforming growth factor (TGF-β)-activated kinase 1 (TAK1). Whenever TAK1 or downstream p38 MAPK is inhibited, ASK1 is activated by the formation of ROS, and TAK1 is suppressed, which results in downstream NFκB activation in H. pylori-related responses (Hayakawa et al. 2013). H. pylori infection activates new signaling pathways like ROS/ASK1/JNK, which governs apoptotic cell death (Wen et al. 2018a). The fate of epithelial cells infected with H. pylori may rely on the balance of ASK1-induced apoptosis and TAK1-induced antiapoptotic or inflammatory responses, which may play a role in the etiology of gastritis and gastric cancer (Hayakawa et al. 2013). Thioredoxin (TRX), a protein that modulates reduction and oxidation, binds to ASK1 directly, and intracellular ROS can activate ASK1 by releasing it from TRX (Saitoh 1998). Through the phosphorylation of the MAP2Ks, MKK4, and MKK3, ASK1 activates the downstream MAPKs, JNK, and p38. Numerous human disorders involve the activation of ASK1 and subsequent MAPK. As a result, ASK1 is crucial for the emergence of colitis, colon cancer, liver damage, liver cancer, and gastric cancer (Hayakawa et al. 2011). TAK1 controls JNK activity both positively and negatively in a ROS-dependent manner. It also regulates ASK1 activation negatively via binding to TAB1. In contrast, ASK1 inhibits the effect of TAK1 on interleukin-1β (IL1β)-dependent NFκB activation (Hayakawa et al. 2013). ASK1 gets activated by H. pylori and regulates ROS-mediated and JNK-dependent apoptosis, and it has a reciprocal role in gastric cancer (Mochida et al. 2000).
Transforming growth factor-beta cascade
EBV
The TGF-β family consists of three members found in mammals, namely TGFβ1, TGFβ2, and TGFβ3. TGFβ induced p15 and p21, which stops the cells from entering the S-phase by preventing phosphorylation of tumor-suppressor pRB (Kashyap et al. 2022). Marked resistance to TGFβ1-mediated cell apoptosis is carried out by LMP1 due to an uprise in cyclin D2 levels and by LMP2A via AKT/PI3K pathway (Velapasamy et al. 2018). EBNA1, which is involved in the maintenance of viral genes, upregulates and phosphorylates STAT1 with the help of INFy and INFα in 8-azahypoxanthine-resistant epithelial cells (Ad-AH). TGF-β1 targets ID2 which is produced by EBNA1 and activates the Smad complex leading to an inhibition of SMAD2 by EBNA1 (Wood et al. 2007). LMP2A, on the other hand, eradicates the growth inhibitory effect of TGFβ by preventing Smad2 from getting phosphorylated with the help of miR-155-5p (Liu and Cohen 2016; Shi et al. 2020). Smad2 gets destabilized by EBNA1 and LMP2A, which keeps the inhibitory effect of TGFꞵ under check, thus favoring viral replication and boosting the infection capacity inside the host by increasing the expression of BZLF1 (Luo et al. 2021). LMP1 induces cancer-causing proteins like VEGF and MMP9 (Voigt et al. 2020; Hayakawa et al. 2013; Kondo et al. 2005). LMP1 induces its effect through the non-smad arm of TGFꞵ signaling. In epithelial cells, LMP1 activates TGFꞵ signaling in an NFκB-dependent manner (Morris et al. 2016). The EBV-induced proteins essential in the TGFꞵ pathway is smad1, 2, 4, AP-1, ID2, LMP2A, EBNA1, LMP1, and activin A. Moreover, study also revealed that knockdown of TGFβ by siRNA resulted in suppression of EBV transmission (Nanbo et al. 2018).
H. pylori
TGF-β signaling contributes to the pathogenesis of H. pylori infection (Li et al. 2015). H. pylori infection with a cagE-positive gene upregulates the TGFβ1-mediated epithelial–mesenchymal transition (EMT) pathway and consequently promotes EMT (Chang et al. 2015). Furthermore, through the TGFβ-mediated p38 MAPK signaling cascade, the H. pylori infection reduces the expression of cystic fibrosis transmembrane conductance regulator (CFTR) and solute carrier family 26 member 6 (SLC26A6) in duodenal epithelial cells. It provides insight into the pathophysiology of duodenal ulcers linked to H. pylori (Wen et al. 2018b). According to earlier research, TGFβ promotes H. pylori colonization and adhesion to host cells. It has also been linked to autoimmune disorders and gastritis, both caused by H. pylori. The severity of H. pylori-associated with non-metaplastic atrophic gastritis is correlated with the increased expression of TGFβ1.
Additionally, the human gastric mucosal biopsy results showed that TGFβ1 mRNA expression is much higher in H. pylori-infected specimens compared to uninfected samples. The level of chronic inflammation and its impact are both positively correlated with the VacA genotype. Through the production of T-regs, TGFβ1 acts as a critical negative regulator of the immune response (Wan and Flavell 2008). Since T cell proliferation and immunological responses are inhibited by the H. pylori-related virulence factor vacA, this could increase the adherence of H. pylori to the gastric mucosa by upregulating TGFβ expression. Compared to H. pylori-negative populations, H. pylori-infected patients including those with gastritis and peptic ulcers have higher serum concentrations of IL17A, IL23, and TGFβ, indicating the degree of inflammation varies based on these cytokines concentrations (Arachchi et al. 2017). The immunohistochemical staining of TGFβ, TGFβ-RI, and Smad7 was higher in H. pylori-positive patients compared to H. pylori-negative patients, suggesting that the feedback loop involving TGFβ1 and Smad7 may be crucial for the development of H. pylori infection (Li et al. 2015).
TGFβ has also been shown to promote miR-155 expression via the Smad4 pathway. SMAD2 is the major signaling pathway downstream of TGFβ. Although the precise mechanism by which miR-155 regulates inflammation by influencing SMAD2 is unknown, considering the elevated level of TGFβ in H. pylori infection, we believe that miR-155 acts as negative feedback to lessen the pathogenesis of H. pylori infection (Wu et al. 2007).
Phosphatidylinositol 3-kinase pathway
EBV
PI3K pathway is one of the majors signaling pathways associated with various types of cancer. As an enzymatic antagonist of PTEN, PI3K is reported to be a carcinogen in humans and helps maintain a balance between the tumor-suppressor genes, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and PI3K (Kondo et al. 2005; Pei et al. 2020b). Class I PI3K consists of p110α, p110β, p110γ, and p110δ isoforms, products of PIK3CA, PIK3CB, and PIK3CD (Katso et al. 2001) which gets combined with p85 and p110 and expressed in several types of cancers (Zhao and Vogt 2008). Previous reports unveiled that Class II and III PI3Ks are non-carcinogenic, and it does not produce PIP3, an essential protein that controls cell growth and replication (Zhao and Vogt 2008). The p110 domain binds with p85 with a high affinity, and a serine/threonine protein kinase activity occurs, which phosphorylates p85 (Dhand et al. 1994). The product of serine/threonine kinase Akt is responsible for the inactivation of GSK3ꞵ (Cross et al. 1995) and also negatively impacts FOXO (Brunet et al. 1999). Inactivation of GSK3ꞵ leads to disrupted regulation of cAMP response element binding protein (CREB) and an upshot in cyclin D1 and Myc levels (Sears et al. 2000). Akt controls cell death by two proteins, BAD and Caspase-9, which are crucial to induce its effects on transcription factors through phosphorylation of BAD, caspase9, and FKHRL1 (Peso and González-Garcı́a M, Page C, 1997). Cancer causing viruses have transformed PI3K/Akt pathway to enhance their survival and infectivity (Engelman 2009). One such virus is EBV which uses LMP1 and LMP2A to activate PI3K pathway causing increased cell proliferation, genomic instability, apoptotic inhibition, and rearrangement of the cytoskeleton (Chen 2012). LMP1 induces secretion of IL10 in B cells via activation of PI3K (Lambert and Martinez 2007). LMP1 inhibits DNA repair and reduction in cyclin D2 and D3 levels via CTAR1-mediated activation of PI3K/Akt pathway (Chen et al. 2008). It also inactivates FOX3a and represses DDB1. LMP1 also indirectly affects microtubule activity by inducing the formation of stress fiber and activating cdc2 leading to phosphorylation of Op18, which regulates cell's entry into mitosis (Chen 2012; Lin et al. 2009). The viral oncoprotein also inhibits the proapoptotic activity of Par4 by de novo synthesis via multiple signaling pathways (Chen 2012; Lee et al. 2009). LMP1 increases the production of survivin through PI3K pathway to prevent apoptosis in cancer cell lines (Sun et al. 2015). Not only survivin but also Bcl2 expression increases when PI3K gets activated (Lee et al. 2009). LMP1 is associated with the p85 domain of PI3K (Dawson et al. 2003). PI3K in turn activates the Akt pathway, whose major role is to protect against apoptosis by inhibiting the proapoptotic ability of molecules (Dawson et al. 2003; Coffer et al. 1998). Once PKB/Akt is activated, two residues, namely Thr308 within the P loop and Ser473, get phosphorylated (Coffer et al. 1998). LMP1 leads to the downregulation of PTEN expression by increased expression of miR-21. This further leads to the activation of PI3K/Akt, causing the expression of cancer stem cell markers, thereby promoting the formation of tumor clusters and cancer development. LMP2A protects the EBV-mediated gastric cancer cells from the effects of chemotherapy by activating PI3K/Akt pathway, which provides resistance to apoptosis (Chen 2012; Shin et al. 2010). Furthermore, EBNA2 targets the p55α subunit of PI3K and CD40 and p85 subunit used by LMP1. Importantly, IL10 has been associated with the maintenance of EBV latency and tumors. siRNA-mediated IL10 knockdown prevented phosphorylation of PI3K/AKT further causing EBV lytic replication (Gao et al. 2019). Host miRNA-142 works in conjunction with a tumor-suppressor, PTEN, both of which regulate the PI3K/ Akt pathway. This pathway is suppressed due to miR-BART6-3p-mediated downregulation of PTEN (Zhou et al. 2016).
H. pylori
AP1 is a transcription factor existing in a dimeric complex of Jun and Fos proteins, which can be homo- and heterodimers. These protein subunits involve Jun (c-Jun, JunB, JunD), Fos (c-Fos, Fra1, Fra2, FosB), activating transcription factor (ATF), and musculoaponeurotic fibrosarcoma (MAF), which eventually regulate cell proliferation, cell cycle, and inflammation (Gazon et al. 2018). To form an active transcription complex, homo and heterodimeric complexes are formed with Fos, whereas c-Fos only heterodimerizes with members of the Jun family (Ding et al. 2008). Jun and Fos dimerize efficiently with several transcription factors, including members of the ATF/CREB or Maf/Nrl families of proteins (Mechta-Grigoriou et al. 2001). Several pathogens including H. pylori are known to hijack the AP1 pathway.
Among the strains of H. pylori, few are highly pathogenic due to their cagPAI. Type IV secretion system (T4SS) is expressed due to cag pathogenicity present in the genomes of highly virulent H. pylori strains. The T4SS form a pilus structure for delivering the virulent factor in CagA into the host cells achieved by several T4SS proteins that include CagI, CagL, CagY, and CagA. These proteins bind to host cell integrin member β1 delivering cagA across the host cell membrane (Tegtmeyer et al. 2011). The injection process also involves the interaction of CagA with phosphatidylserine. After getting delivered into the host cell, CagA mimics a host cell factor due to its phosphorylation by oncogenic tyrosine kinases, which is recognized by the NOD1 and activates NFκB, MAPK, and AP1 (Allison et al. 2009).
Studies have suggested that NOD1 is critical for the activation of AP1. In siNOD1 expressing gastric epithelial cells exposed to cagPAI+ H. pylori, a significant reduction was observed in p38 and ERK phosphorylation. On the other hand, there was no significant change in JNK phosphorylation. In contrast, only weak AP1 activation was observed in wild type or an isogenic strain without the VacA gene and isogenic strains of H. pylori with mutations in certain Cag genes (Naumann et al. 1999). Additionally, mutations in the CagA, Cag PAI, VacA, and non-phosphorylated CagA showed attenuated DNA binding activity of AP1. Also, the H. pylori flagella mutation does not affect AP1 activity. H. pylori induces the expression of c-Jun and c-Fos in gastric epithelial cells. The binding of these proteins to promoters of interleukin (IL6, IL8, matrix metalloproteinase (MMP1), cyclin D1, and cyclooxygenase2 (COX2) leads to the regulation of these genes. The binding of c-Jun, c-Fos, and ATF-2 to the COX-2 promoter occurs in a MAPK-dependent manner via a Toll-like receptor. This has been implicated in COX2-mediated cancer cell invasion and angiogenesis (Ding et al. 2008).
Besides, CagPAI activates AP1, which results in the cascade of cellular kinase responses such as c-Jun N-terminal kinase, MAP kinase 4, and p21-activated kinase, and small Rho-GTPases including Rac1 and Cdc42. The activation of AP1 is involved in activating pro-inflammatory cytokines/chemokines. The H. pylori-mediated AP1 activation in gastric epithelial cells (i.e., AGS and MKN45) vary increasingly with time- and dose-dependent expression of c-Jun, JunB, JunD, Fra1, and c-Fos proteins (Ding et al. 2008). AP1 acts as a downstream executioner protein for mitogen-activated protein kinases (MAPK), TGFβ, and Wnt (Jochum et al. 2001). Contrarily, AP1 also alters the extracellular signals for genes containing AP1 binding sites in their promoter or enhancer regions (Shaulian and Karin 2002). ERK, P38, and JNK each selectively regulate AP1 subcomponent activity and DNA binding activity (Ding et al. 2008). According to Nakayama et al. (2009), a short incubation of VacA causes β-catenin to activate and accumulate in the nucleus and is dependent on an inactive GSK3. Additionally, prolonged exposure to VacA causes Akt to be inactivated and GSK3 to be activated, which subsequently downregulates β-catenin activity. Another recent research shows that CagA adversely controls autophagy via the c-Met-PI3K/Akt-mTOR signaling pathway, which is linked to an upsurge in the release of proinflammatory cytokines. Therefore, we hypothesize that CagA's suppression of autophagy promotes gastric inflammation, which in turn starts the multistep process of gastric carcinogenesis (Li et al. 2017). Meanwhile, elevated miR-143-3p expression in H. pylori-positive gastric cancer tissues and cells. In the BGC-823 GC cell model, miR-143-3p mimics can reduce proliferation and induces apoptosis, whereas miR-143-3p inhibitors did the reverse. Subsequent research revealed that AKT2 modulated the effects of miR-143-3p. (Wang et al. 2017).
Hypoxia-inducible factor 1α pathway
EBV
The HIF1 transcription factor contains HIF1, HIF2α, and HIF3α subunits, which are needed to accelerate the progression of cancer (Kraus et al. 2017). HIF1α can activate a total of 60 genes including VEGF. HIF1α has a short half-life of 5–8 min (Berra et al. 2001; Zhu et al. 2016). HIF1α mediates the change of EBV from latent to lytic phase by activating the expression of the BZLF1 gene (Kraus et al. 2017). HIF gene products undergo degradation by prolyl hydroxylases (PHDs) (Demidenko and Blagosklonny 2011). It was seen that EBNA3A, 5, and LP are responsible for rendering PHDs inactive to stabilize HIF1α (Kraus et al. 2017; Darekar et al. 2012). EBNA3A targets PHD2, and EBNA5 attaches itself to PHD1 (Darekar et al. 2012). Due to the blockage of PHDs, hydroxylation and degradation of HIF1α cannot take place, and an accumulation of HIF1α occurs. LMP1 increases the level of H2O2 which results in the induction of HIF-1α (Kraus et al. 2017; Wakisaka et al. 2004). Once HIF1α stabilizes, it activates GLUT1, PDK1, and LDHA genes by forming a heterodimer with ARNT (Darekar et al. 2012). LMP1 is not only involved with HIF-1α activation but is also responsible for transcriptional activation of VEGF to some extent aided by COX2 (Luo et al. 2021; Darekar et al. 2012; Wakisaka et al. 2004). Signaling pathways like p42/p44 MAPK are also involved in enhancing LMP1-mediated HIF1α induction (Wakisaka et al. 2004). In NPC cells, LMP1 prohibits the degradation of HIF1α with the help of SIAH1 E3 ubiquitin ligase (Zhu et al. 2016; Kondo et al. 2006). The SIAH family of proteins has a high level of similarity. SIAH1 and SIAH2 only differ in their NH2 terminal and are 98% identical to one another (Hinshaw and Shevde 2019). LMP1 stabilizes SIAH proteins, leading to the degradation of PDH1 and PDH3 (Hinshaw and Shevde 2019). On the other hand, in B lymphoma cells, SIAH1 expression is inhibited by LMP1 which upregulates β-catenin (Hinshaw and Shevde 2019; Jang et al. 2005). Interestingly, Kraus et al. demonstrated that shRNA-mediated knockdown of HIF1α reduces EBV lytic replication. This is done as HIF1α binds directly to the promoter of the BZLF1 gene, Zp thereby associated with EBV related malignancies (Kraus et al. 2017). Furthermore, knockout of p53 using CRISPR-Cas9 resulted in failure of HIF-1α to activate Zp-promoted transcription of viral proteins (Kraus et al. 2020).
H. pylori
The activation of HIF1α aids the progression of malignant diseases, including GC. H. pylori starts the PI3K/mTOR signaling cascade, which triggers HIF-1α and releases the G0/G1 cell cycle arrest via CDK1. As a result, HIF1α has been recognized as a mediator between the cell cycle arrest signaling induced by H. pylori infection and survival (Canales et al. 2017). Usually, the canonical signaling pathway controls the expression of HIF1α through the hydroxylation of proline 402 and 564 by utilizing the proline hydroxylases and following the proteasomal degradation pathway. Moreover, hypoxia led to a decline in proline hydroxylase activity and elevated the HIF-1α protein (Canales et al. 2017). HIF1α can be triggered by hypoxia and hypoxia independent processes, such as the generation of ROS, activation of tyrosine kinase receptors, MEK/ERK, and PI3K/Akt/mTOR pathways (Laughner et al. 2001).
In cancer cells, the half-life of HIF1α is regulated by tumor suppressors p53 and VHL (Von Hippel–Lindau protein) (Laughner et al. 2001). Moreover, genetic alterations, alleviated intra-tumoral oxygen, loss-of-function mutations of VHL, p53, PTEN, and gain-of-function mutation in PI3K, SRC, and MAP signal cascade increases the HIF1α production (Laughner et al. 2001). HIF1α induces the VEGF expression in 3T3 and MCF7 cells via downregulating the expression of EGFR and PI3k/Akt pathway (Laughner et al. 2001; Wang et al. 2014). Precisely, gastric epithelial cells exposed to H. pylori stabilize the HIF1α through PI3K/Akt/mTOR activation, leading to GC (Canales et al. 2017). The elevation in AQP3 expression through activating the ROS pathway in the stomach may be a mechanistic component of H. pylori-infection-mediated carcinogenesis (Wen et al. 2018a). Through the ROS, HIF1α, and AQP3 axis, AQP3 can mediate ROS uptake and speed up intracellular ROS deposition, which aggravates AQP3 expression. Since human gastric cancer cells are encouraged to migrate and proliferate when aquaporin3 (AQP3) is overexpressed, AQP3 may play a significant role in the development of gastric carcinoma.
BCR signaling pathway
EBV
LMP2A mimics a B cell receptor in many ways starting from the pathways through which signal gets delivered to the tyrosine-based motifs in respective domains and triggering similar downstream signaling pathways just like the B cell receptor (Mancao and Hammerschmidt 2007). LMP2A and LMP1 are responsible for modulating the B cell receptor (BCR) pathway (Mrozek-Gorska et al. 2019). Igβ and Igα are the components that help to mediate signaling to BCR (Dykstra et al. 2001). There are several ways by which LMP2A inactivates this pathway, which leads to the inhibition of lytic signaling (Mancao and Hammerschmidt 2007; Brinkmann and Schulz 2006; Radolf and Samuels 2021). Several models have been hypothesized on how LMP2A downregulates BCR signaling. One of them considers the eradication of PTK and Syk by LMP2A from BCR and the inhibition of downstream effectors like PRKCD from getting phosphorylated by tyrosine (Brinkmann and Schulz 2006). Moreover, other researchers discuss how LMP2A restricts BCR from interacting with lipid rafts (Brinkmann and Schulz 2006). The immunoreceptor tyrosine-based activation motifs on B cells inhibit BCR signaling (Brinkmann and Schulz 2006). Erk1 is involved with the phosphorylation of S15 and S102 residues of LMP2A (Brinkmann and Schulz 2006). This pathway is involved with the demethylation of DNA and reactivation of EBV via activation of TPA, which eventually leads to binding between Tet1 and Zta promoter (Fig. 5) (Zhang et al. 2016). Furthermore, study revealed that Bruton’s tyrosine kinase (BTK) is a key component involved in BCR signaling. BTK knockdown with siRNA block BCR signaling thus involved in BCR mediated lytic induction (Kosowicz et al. 2017). Additionally, host miR-141 is exploited by EBV to mimic viral miR and block BCR signaling to promote lytic replication of the virus (Chen et al. 2021).
Outcomes of the altered signaling pathways in during EBV infection. EBV promotes the lytic replication, immune evasion, cell proliferation, epithelial-to-mesenchymal transformation, and metastasis to promote the oncogenic transformation. Moreover, during the oncogenic transformation EBV also utilized the various signaling molecules such as adaptor protein 1 (AP-1), programmed cell death ligand 1 (PD-L1), nuclear factor kappa beta (NFkB), c-Jun, signal transducer and activator of transcription 3 (STAT3) and beta catenin (β-catenin)
H. pylori
H. pylori can cause persistent infection due to its ability to hijack the host's immune response. BCR signaling is one of the crucial pathways identified in tumorigenesis. Diverse BCR activation mechanisms exist, ranging from a chronic antigenic drive by microbial or viral antigens, B cell auto-stimulation by self-antigens to activating mutations in intracellular components of this pathway. H. pylori infection is associated with the development of lymphomas of mucosa-associated lymphoid tissue (MALT) (Niemann and Wiestner 2013). Development of non-Hodgkin lymphomas occurs from nodal and extranodal lymphoid tissues. The marginal zone (MZ) of MALT is associated with a distinct subset of extranodal lymphomas. The number of microbes associated with such lymphomas is increasing with increased molecular investigations comprising members such as H. pylori, C. jejuni, B. burgdorferi, C. psittaci, and hepatitis C virus (HCV). These microbes have been associated with gastric lymphoma, immunoproliferative small intestinal disease, cutaneous lymphoma, ocular lymphoma, and spleen lymphoma, respectively (Suarez 2006). Studies have also demonstrated that in the case of gastric diffuse large B cell lymphoma (DLBCL), regression has been observed after eradication of H. pylori, but if the disease is unresponsive to H. pylori eradication, then DLBCL can progress rapidly. Hence, H. pylori eradication has become a primary therapeutic target for gastric DLBCL. Gene expression analysis shows a reduced expression of B cell signaling-related genes and increased tumor microenvironment-related genes indicating a positive association in response to H. pylori eradication (Torisu et al. 2021).
Mitogen-activated protein kinase pathway
EBV
The mitogen-activated protein kinase/extracellular signal regulated protein kinase (MAPK/ERK) pathway or Ras–Raf–MEK–ERK pathway involves a chain of proteins communicating with cell surface receptors to the nucleus. MAPK regulates various processes like proliferation, apoptosis, differentiation, and transformation in higher organisms (Zhang and Liu 2002). Mainly in mammals, three types of MAPK families have been discovered, which include classical (ERK), p38 kinase and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and ERK5 (Luo et al. 2021; Zhang and Liu 2002). The enzymes linked to the MAPK pathway are MAPKKK, MAPKK, and MAPK (Zhang and Liu 2002). LMP1 can activate the ERK pathway (Roberts and Cooper 1998). ERK pathway gets activated when Thr202 and Tyr204 undergo phosphorylation (Chen et al. 2002; Payne et al. 1991). Due to the presence of vimentin, LMP1 can interact with several cellular signaling pathways (Roberts and Cooper 1998). Such interactions of the oncogene with MAPK pathways result in tumorigenesis. The pathway undergoes a 3.8- to 5.0-fold rise in activity, which results in the proliferation of B cells (Roberts and Cooper 1998). LMP1 also reduces the expression of dual-phosphatase DUSP6 and DUSP8 (Lin et al. 2020). Interaction of MAPK with EBV oncogene not only results in proliferation and enhanced cell resistance to chemotherapy but also benefits the virus in replication and increases its infectivity by enabling the expression of BZLF1 and BGLF2 (Luo et al. 2021). BZLF1 is an indicator of EBV reactivation (Liu and Cohen 2016). The micro-RNA-BART22 of EBV also targets MAP3K5, which results in EMT and inhibition of cell apoptosis (Luo et al. 2021). For LMP2A, it is still unclear how it interacts with the MAPK pathway. However, it has been deduced that LMP2A indirectly uses the MAPK pathway to send proliferation signals to B cells (Liu and Cohen 2016; Anderson and Longnecker 2008). Besides, the p38 signaling pathway also plays a vital role in EBV-mediated cancer progression and metastasis. Importantly CTAR1 and CTAR2 mediated by TRAF2 are responsible for the activation of the p38 pathway (Eliopoulos et al. 1999). If the p38 pathway is inhibited, it affects the LMP-mediated expression of IL6 and TNF-mediated NFκB expression negatively (Eliopoulos et al. 1999; Eliopoulos et al. 1999). Inhibition of p38 also leads to apoptosis and further inhibits the reactivation of EBV in Raji cells (Matusali et al. 2009). The gene bzlf1 expression gets inhibited along with the inhibition of p38 phosphorylation (Matusali et al. 2009). Other proteins that are targeted by the p38 pathway include hsp27, Elk1 (Raingeaud et al. 1996), ATF2 (Eliopoulos et al. 1999; Raingeaud et al. 1995), CHOP/GADD153 (Eliopoulos et al. 1999; Wang and Ron 1996), and MAX30 (Eliopoulos et al. 1999; Zervos et al. 1995). In addition to CTAR1 and CTAR2, TAK1 is also required to activate the p38 pathway (Wan et al. 2004). Since p38 is activated in response to stimuli like stress or perhaps an initial infection, transient overexpression of LMP1 in response to p38 may prevent the cells from going through apoptosis. An indirect phosphorylation cascade involving the substrates MAPKAP-2 and MSK1 allows both p38α and p38β to activate CREB and ATF1 phosphorylation. The primary activating transcription factor that binds CRE in LMP1 is the ATF1-CREB heterodimer. Notably, siRNA-mediated knockdown of p38 MAPK prevents the LMP1-mediated thymidine phosphorylase (TP) induction and thus promotes the apoptosis and reduced the chances of cancer progression (Chen et al. 2002).
H. pylori
H. pylori are known to take over this pathway and result in cytokine production. In contrast, MAPK inhibitor treatment showed mitigation in the H. pylori-stimulated chemokines in the gastric cells (Seo et al. 2004). MAPK gets activated by the action of Ras and Raf proteins and directly upregulates ERK. At least two transcription factors, c-Myc, and Elk-1, are phosphorylated by specific ERK proteins that are activated by MEK and translocate to the nuclei (Chen 2006). H. pylori secretions have direct proliferation-stimulating effects through the MAPK pathway on gastric epithelial cells (Chen 2006). Additionally, H. pylori utilize T4SS to translocate peptidoglycan, which gets detected by the pathogen recognition protein NOD1, leading to NFκB activation. A significant decrease in p38 and ERK phosphorylation was seen in siNOD1-expressing cells driven by CagPAI+ H. pylori (Allison et al. 2009). The amount of IL8 produced by H. pylori considerably decrease through the suppression of p38 and ERK activity (Allison et al. 2009). By phosphorylating IKKβ and cytosolic phospholipase A2 (cPLA2), H. pylori LPS activates ERK, which increases NFκB nuclear translocation and induces the production of COX2 and iNOS (Slomiany 2012). The cells of the immune system like neutrophils, monocytes, and mast cells are triggered by the H. pylori neutrophil-activating protein (HP-NAP), which acts as a virulence factor. In human neutrophils, HP-NAP induces ERK and p38-MAPK activation, while the c-Jun N-terminal kinase does not (Nishioka et al. 2003). Additionally, H. pylori stimulated serum-responsive element (SRE)-dependent gene transcription and elevated c-Fos protein expression, demonstrating the signaling mechanism through which H. pylori activates ERK (Chen 2006).
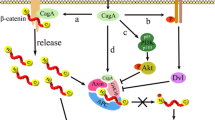
Wnt/β-catenin pathway
EBV
Differentiation and proliferation of mammalian cells involve the Wnt/β-catenin signaling pathway and have been linked to numerous cancers. Hence, this pathway is important for development, tissue homeostasis, and disease (Zwezdaryk et al. 2016). A multifunctional protein β-Catenin is critical for this signaling pathway, and the stabilization of this protein is disrupted due to mutations in numerous carcinomas. β-Catenin undergoes rapid degradation in type I B-lymphocytic lines but is stable in type III B cell lines. In contrast, its transcriptional activity is significantly higher in type III B cells. The association of β-catenin with deubiquitinating enzymes might be critical for its stabilization. Activation of this signaling pathway during EBV infection might contribute towards the characteristic lymphoproliferation of type III latency (Shackelford et al. 2003). Viral modulation of these cellular processes is increasingly interesting in understanding cancer development. Viruses interact with the Wnt pathway through numerous mechanisms, including epigenetic modifications, miR targeting, and altering signaling members, leading to nuclear translocation of β-catenin, thereby activating downstream signaling. Modulating this signaling pathway could be an approach for initiating and maintaining viral pathogenesis, resulting in virus-induced cancers due to dysregulation (Luo et al. 2021; Zuylen et al. 2016). Notably, oncogenic host miR-4721 modulates PI3K/Akt/c-Jun pathway further leading to Wnt/ β-catenin-mediated tumorigenesis as a result of induction by EBV-miR-BART22 (Tang et al. 2020).
H. pylori
The canonical Wnt pathway or Wnt/β-catenin pathway leads to the activation of T cell factor/lymphocyte enhancer factor (TCF/LEF) families of transcription factors via accumulation of β-catenin in the cytoplasm (Pai et al. 2017). Upstream stimulus triggers dissociation of β-catenin from degrading complex due to attachment of Wnt to its membrane receptor, namely Frizzled, and a co-receptor lipoprotein receptor-related protein 5/6 (LRP5/6) (Song et al. 2015). Subsequently, β-catenin escapes the degradation process due to phosphorylation by glycogen synthase kinase 3β (GSK3β) and the ubiquitin-proteasome system (UPS). This leads to the accumulation of β-catenin in the cytoplasm that is further translocated into the nucleus to combine with TCF/LEF. Contrarily, the anomaly in activating this pathway leads to cell proliferation and cell malignant transformation (Song et al. 2015). Certain pathogens like H. pylori also modulate the Wnt/β-catenin pathway through secreted glycoproteins Wnt1 and Wnt3a, leading to cancer-like pathologies. Besides, H. pylori causes activation of the Wnt/β-catenin pathway majorly via two gateways, c-Met and EGFR signals (Soutto et al. 2015).
The c-Met receptor gets activated in several cancers, such as gastric and colorectal cancer and further leads to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling. PI3K/Akt eventually triggers the accumulation of β-catenin in the cancer cells. Activating the PI3K/Akt pathway promotes cell proliferation, invasion, and escape from the apoptotic pathways. In contrast, c-Met inactivation accelerates GSK3β activity and degradation of β-catenin. Also, augmented c-met expression due to β-catenin suggests positive feedback between c-Met and β-catenin in cancerous cells (Song et al. 2015). Unlike c-Met, H. pylori activate EGFR signaling through VacA, CagE, CagL, secretory protein HP0175, and outer inflammatory protein A (OipA) but not CagA. Intriguingly, CagA inactivates the EGFR by binding to SH2 domain-containing protein tyrosine phosphatase (SHP-2). Further, the H. pylori factors induce EGFR phosphorylation, eventually leading to PI3K/Akt pathway activation.
EGFR-PI3K/Akt gives a suppression signal to GSK3β; thus, β-catenin gets accumulated (Soutto et al. 2015). Once activated, Akt further activates or inhibits downstream target proteins through phosphorylation, including GSK3β, NFκB, p21, etc. (Geng and Zhang 2017). H. pylori activates the PI3K pathway in a CagPAI-dependent manner. OMP outer inflammatory protein A (OipA) is also involved in the activation of AKT and phosphorylation of GSK3β. H. pylori also lead to the activation of PI3K and AKT in an Src and EGF receptor-dependent manner (Nagy et al. 2009). Apart from this, H. pylori activate this pathway through extracellular UreB, which binds to TLR 2 receptor in the gastric epithelial apical membrane (Toh and Wilson 2020). Even though H. pylori induce activation of the PI3K pathway, and the infection leads to opposite effects in gastric cells, like cell cycle arrest in the G1 phase and cell death by apoptosis (Canales et al. 2017). PI3K/AKT/GSK3β signal pathways might regulate the proliferation of gastric cancer cells (Geng and Zhang 2017). Constant activation of PI3K due to H. pylori infection might contribute to the development of gastric cancer (Peek and Crabtree 2006). N24P55γ, the regulatory subunit of PI3K, potentially inhibits the proliferation of gastric cancer cells and promotes apoptosis (Geng and Zhang 2017). VacA stimulates the activity of protein kinase B (PKB) by PI3K activation. This results in increased GSK3 phosphorylation that further releases β-catenin from the GSK3β/β-catenin complex translocating it to the nucleus. This whole process leads to the activation of the promoter of cyclin D1.
Furthermore, Runx3, a tumor-suppressor gene, usually undergoes downregulation in gastric cancer cells because of promoter hypermethylation. Runx3-mediated suppression of the Wnt/β-catenin pathway is reported when ternary complex forms in association with β-catenin/TCF4. The Runx3 loss increases the expression of Wnt/β-catenin genes and induces gastric carcinogenesis (Soutto et al. 2015). H. pylori virulence gene CagA through the WW domain gets associated with the PY motif of Runx3 and results in the ubiquitination and degradation of Runx3. Yet another factor trefoil factor 1 (TFF1) usually expresses in gastric mucosa but downregulates in gastric cancers due to mutations in the hypermethylation gene. Infection of H. pylori may lead to hypermethylation of TFF1. In the H. pylori-positive mucosa, TFF1 was found to be decreased and methylated compared to H. pylori-negative mucosa. TFF1 reduces the nuclear translocation of β-catenin by inhibiting the phosphorylation of both Akt and GSK3β through protein phosphatase 2A (PP2A) (Soutto et al. 2015). The ablation of TFF1 elevates H. pylori-induced β-catenin activation with oncogenic potential (MacDonald et al. 2009). H. pylori products involved in virulence include urease, OipA, the neutrophil-activating protein NapA, adhesins, heat-shock protein, and lipopolysaccharide.
The infection of H. pylori recruit’s macrophages via monocyte chemoattractant protein-1 (MCP-1) or Sonic Hedgehog (Shh) on gastric mucosa and secrete several proinflammatory cytokines (Oshima et al. 2011). In gastric cells, TNF-α potentially activates the Wnt/β-catenin signaling via Akt-GSK3β activation. In colon cancers, macrophage-derived IL1β inhibits GSK3β activity and degradation of β-catenin, enhancing TCF (T-cell factor) transcription activity (Oshima et al. 2011). The GSK3β suppression by IL1β depends on the activation of NFκB and Akt. Macrophages are also involved in cholangiocarcinoma, where they are involved in the activation of the Wnt/β-catenin pathway (Boulter et al. 2015). These observations identify macrophages as critical linkers between chronic inflammation and Wnt/β-catenin activation (Soutto et al. 2015).
Toll-like receptor signaling pathway
EBV
The antiviral responses of both infected cells and responding immune system cells are directly regulated by Toll-like receptors (TLRs). As a result, they are essential for responses against the related murine virus MHV68, oncogenic γ-herpesviruses (EBV), and Kaposi’s sarcoma-associated herpesvirus (KSHV), which directly infect the immune system cells (Sun et al. 2016). However, these viruses can also lead to infections that last for a lifetime. TLRs may also regulate inflammation during latent infection and aid in developing tumors brought on by viruses. These viruses may re-enter the replicative lytic cycle post-TLR activation (Gaglia 2021).
TLR9 can recognize EBV and activate innate immune responses, which may help to regulate the spread of the virus and the development of latently infected B cells. EBV may manipulate the host immune system by interfering with TLR9 expression and function to promote the long-term survival of the virus. TLR9 activation by bacterial, viral, or parasitic DNA may affect the growth of EBV-infected B cells and the equilibrium between latent and lytic EBV. TLR9 signaling in EBV-infected B cells may be advantageous for the host and the highly adapted human gamma-herpesvirus (EBV) (Zauner 2012). The development of systemic lupus erythematosus (SLE, often known as lupus) is associated with TLR7's abnormal activation. TLR7 is also engaged in the host’s innate immunity against pathogens. The expression of EBV LMP1 and IRF7, which are involved in the stimulation process, gets upregulated by TLR7 activation. TLR7 activation does not cause IFNs to be produced by EBV-infected cells, but it does make them more susceptible to TLR3 or TLR9 activation, which can cause IFN production. LMP1 and IFNs are co-expressed in the same cells in some lupus patients. Therefore, LMP1-expressing cells may release IFNs in lupus patients due to the abnormal activation of TLR7. These findings suggest that EBV may contribute to some lupus patients by enhancing IFN production (Valente et al. 2012). Knockdown of TLR3 by siRNA leads to a reduction in EBER1-induced IFN indicating the role of EBER1 in viral pathogenesis and immune invasion (Iwakiri et al. 2009).
H. pylori
Multiple H. pylori antigens including LPS, flagellin A, etc., can activate TLR2, 4, 5, 8, and 9. MyD88 is identified to be a major protein in TLR signaling via induction of IRAK1 and TLR4 and subsequently NFκB and AP1 with numerous targets including IL6 (Pachathundikandi et al. 2013). Toll-like receptors (TLRs) are known to play important roles in gastric carcinogenesis. The signaling pathway starts with establishing antigen-specific immune responses against the pathogen-associated molecular patterns (PAMPs) of H. pylori. Likewise, chronic inflammation produces damage-associated molecular patterns (DAMPs) that may contribute to the development of gastric cancer (Uno 2014). Further, the extracellular domains of TLRs consist of leucine-rich motifs like PAMP proteins that lead to ligand binding. The cytoplasmic tail of TLR proteins has identified homology to the IL1 and IL18 receptors; thereby, it can trigger the intracellular signaling pathways via several adapter proteins (Su et al. 2003). These adaptor proteins include myeloid differentiation factor 88 (MyD88), toll-interleukin 1 receptor (TIR) domain-containing adapter protein (TIRAP), toll interacting protein (TOLLIP), (IRAK), (TRAF), TIR domain-containing adapter inducing interferon (IFN)-beta (TRIF), and TRIF-related adapter molecule (TRAM) (Uno 2014).
Besides, the TLR signaling works in both MyD88-dependent and MyD88-independent manner. The MyD88-dependent pathway gets activated by a different range of TLRs, namely TLR1, 2, 4, 5, 6, 7, and 9. After getting a signal from TLRs, MyD88 recruits various molecules, including IRAK1, IRAK4, and TRAF6, to the TLR-MyD88 complex. This further causes phosphorylation of IRAK1 and TRAF6, thereby phosphorylating multiple adaptor molecular complexes downstream to MAP kinases-AP1 complex and IKK complex NFκB (Uno 2014). On the other hand, the MyD88-independent pathway is associated with the activation of TL3 or TLR4 that induces IFNβ-mediated responses. The independent pathway involves propagating intracellular signals by TRIF and TRAM adaptor molecules, which consequently activate the IKK pathway, thereby producing IFNβ (Uno 2014).
Helicobacter pylori also activate TLR pathways and induce inflammation by producing proinflammatory cytokines, chemokines, and ROS, which form tumor microenvironment like gastric carcinogenesis (Uno 2014). LPS derived from H. pylori is considered a direct stimulator of TLR4, induces the NFκB pathway, and eventually promotes the production of proinflammatory cytokines as in the IL8 pathway. H. pylori-inflamed cells show an increase in TLR4 expression on the apical site of gastric epithelial cells in contrast to the basolateral site (Maeda et al. 2001). Moreover, the NAP has also been reported to work as a ligand of TLR2 via DAMP recognition (Ding et al. 2005). Besides, the H. pylori flagellin recognized by TLR5 triggers IL8 secretion via p38 MAP kinase, while TLR9 recognizes unmethylated CpG DNA and produces type- I IFN, IL6 and IL12 (Andersen-Nissen et al. 2005).
Role of MicroRNAs (miRs) in the regulation of H. pylori and EBV infection
EBV
MicroRNAs (miRs) exist in a wide spectrum of organisms including eukaryotic and viral genomes. miRs are of various lengths in between 20–25 nucleotides, which regulate the oncogenic processes (Kuroda et al. 2005; Noto et al. 2013). The viral miRNAs are known to interact with both host and viral mRNAs. These miRNAs bind to the 3’ untranslated region by getting incorporated into the RNA induced silencing complex (RISC) (Cullen 2009). This can plausibly either lead to suppression of translation or degradation of the mRNA. Viral miRs have been observed to evade the host immune system and lead to enhanced tumorigenicity as they aid in maintaining viral latency by targeting the host or the EBV genes (Kim et al. 2017). pri-miR-BHRF1, 2, and 3 encoded by EBV gene BHRF1 promote viral propagation as they favor lytic replication through the proliferation of virus-infected cells (Seto et al. 2010).
Suppression of host immune responses occurs as miR-BART6-3p targets the RIG1 gene (Lu et al. 2017). This miRs also regulates signaling in NFκB and PI3K/Akt pathways as it carries the potential to target PTEN and IL6R in Burkitt lymphoma cells (Ambrosio et al. 2014). Suppression of cellular immunity occurs when IFNγ and STAT1 are targeted by viral miRNAs, miR-BART20-5p and miR-BART8 (Huang and Lin 2014). Another miRNA, miR-BART16, leads to the inhibition of IFN signaling via targeting cAMP binding protein (CBP) in virus infected B cells and epithelial cells (Hooykaas et al. 2017).
EBV escapes the host immune system by its ability to exploit the host miRs. A latent EBV protein, EBNA2, leads to subsequent downregulation of MyD88 and IRAK1 as the viral protein causes upregulation of miR-21. Generally, miR-155 leads to the stabilization of persistent viral infection by attenuating NF-κB signaling. Activation of host NFκB signaling and miR-155 majorly occurs due to enhanced expression of viral LMP1 in virus-infected B-cells (Lu et al. 2008). This host miRNA also suppresses the JAK/STAT signaling by targeting the SOCS1 (Jiang et al. 2010). EBV-infected cells are observed to have high levels of SOCS1 due to upregulated miR-155 (Delgado-Ortega et al. 2013). miR-BART3 and miR-BART5, which are upregulated during EBV infections, were predicted to be potential targets of genes like p53, TGFβ, and the ones involved in Wnt signaling. This leads to the modulation of transformation and apoptosis in NPC carcinoma cells (Wan et al. 2015). EBV miRNAs promote EMT due to the activation of β-catenin by inhibiting E-cadherin and β-TrCP (Wang et al. 2019). Notably, Wang et al. (2022) reported that PD-L1 expression is also enhanced by the EBV-encoded miR-BART17-3p due to FOXP1 inhibition resulting in escalated tumor immune escape. Meanwhile, EBV-encoded EBERs are known to upregulate host miR-190 that prevent apoptosis, thereby preserving type 1 latency of the virus. (Lyu et al. 2014). Cellular miRNAs such as miR-93 and miRNA-19a are involved in the disruption of TGF-β signaling, thereby promoting EBV mediated oncogenic progression. (Lyu et al. 2014) (Fig. 6).
Role of miRNAs in EBV and H. pylori infection. Importantly, H. pylori and EBV also regulate the various host micro-RNAs (miRs). The modulation in the expression of miRs leads to altered homeostasis of various host signaling pathways and aggressive malignant transformation. H. pylori and EBV so far known to altered the B cell receptor (BCR), interleukin-1 receptor-associated kinase 1 (IRAK-1), transforming growth factor beta (TGF-β), phosphatidylinositol-3 kinase and PI3 kinase (PI3K), nuclear factor kappa beta (NFkB), Just Another Kinase (JAK), signal transducer and activator of transcription 3 (STAT3), interferon gamma (IFN-y) and beta catenin (β-catenin) signaling pathways to disturbed the homeostasis and enhance the malignant transformation
H. pylori
Altered expression of miRNAs is also reported in H. pylori-associated GC (Hayashi et al. 2013). H. pylori elevate the miR-21, miR-155 and miR-222 and downregulates the expression of mir-124a, miR-320, miR-101, miR-203 miR-210 (Song et al. 2015). H. pylori strain 7.13 downregulates the expression of miR320, which further regulates the expression of Mcl1-mediated apoptosis (Noto et al. 2013). Notably, compared to Cag− strains, the Cag+ strain causes markedly greater levels of Mcl1. Consequently, H. pylori-mediated apoptosis via miR-320 in a Cag-dependent manner induces activation of the Wnt/β-catenin signaling pathway (Noto et al. 2013). The Wnt signaling pathway is widely known for its role in embryogenesis and carcinogenesis. H. pylori, along with TNFα induces up-regulation of WNT10A and causes aggressive GC through Wnt/β-catenin/TCF signaling pathway (Kirikoshi et al. 2001). Notably, 20–30% of GC shows the nuclear accumulation of β-catenin (Song et al. 2015). Additionally, H. pylori infection may activate the Wnt co-receptor LRP6, which results in the accumulation of β-catenin in the nucleus (Song et al. 2015). The methylation patterns were discovered to correlate positively with H. pylori infection. Additionally, the hypermethylation of the promoters SFRP4 and SFRP5 has commonly led to the downregulation of Wnt antagonists in GC. H. pylori-mediated GC also expresses Wnt3 along with Wnt7a, Wnt7b, and Wnt receptor frizzled (Song et al. 2015). Through the activation of the WNT/β-catenin/TCF signaling pathway, H. pylori may play an important role in human gastric cancer (Kirikoshi et al. 2001). Exon 3 of the β-catenin gene, which codes for the serine-threonine phosphorylation sites for GSK3β, has certain mutations that prevent UPS from degrading the protein. The β-catenin degradation complex gets disassembled due to recurrent downregulation of APC expression in colon cancer. Unlike colon cancer, APC mutation and methylation are not involved in gastric cancer (Franco et al. 2005). In contrast to Cag− or uninfected individuals, H. pylori Cag+ specimens showed increased nuclear β-catenin, which is mainly localized in epithelial cells inside the proliferative zone in antral glands (MacDonald et al. 2009).
The role of MDM2 in the potential regulatory mechanisms of H. pylori LPS induced miR-375 and miR-106b expression has been explored and it can boost the expression of JAK1 and STAT3. Interestingly, in GC cells, JAK2 has been identified as a direct target gene of miR-375. LPS from H. pylori may activate the JAK/STAT signaling pathway in gastric epithelial cells by inhibiting miR-375 and miR-106b (Ye et al. 2015). This led to increased Bcl2 expression, which consequently encouraged the proliferation and carcinogenesis of gastric epithelial cells produced by H. pylori. The first miRNA studied in humans was miRNA lethal7 (let7). Let7b, which binds to the 3′-UTR regions of TLR4 mRNA and typically inhibits its activity at a post-transcriptional level, has been linked to the initiation of immunological responses in addition to its many roles in carcinogenesis. Infection with H. pylori reduces let7b levels, which increases TLR4 expression and activates NFkB, eventually resulting in inflammation (Săsăran et al. 2021). In case of H. pylori infection, miRNA-155 appears to be implicated in a negative feedback system by attenuating the NFkB response and reducing the production of pro-inflammatory cytokines such as IL8 (Tang et al. 2010; Ceppi et al. 2009). As a result of H. pylori infection, miRNA-146 was also shown to be a negative modulator of IL8, growth-related oncogene (GRO)-α, and macrophage inflammatory protein (MIP)-3α via the same NFkB pathway (Liu et al. 2010). miR-21 is a post-transcriptional suppressor of PDCD4, a protein that activates NFkB and suppresses IL10 production. As a result, miR-21 boosts IL10 levels and blocks NFkB, limiting inflammation (Sheedy et al. 2010). H. pylori infection promotes upregulation of miR-21 in gastric epithelial cells, and its persistence in the setting of GC implies that this pathogen disrupts the proliferation/apoptosis balance (Zhang et al. 2008). Several miRs have been reported to be up-regulated in gastric carcinomas; the question of their dysregulation in gastric epithelial cells during H. pylori infection is apparent. Zhang et al. (2008) showed a connection between H. pylori infection and miR-21. Petrocca et al. (2008a, 2008b) showed that miR-106b, miR-93, and miR-25 alter the physiological response of GC cells to TGFβ, influencing both the cell cycle and apoptosis. MiR-30a-3p and miR-30a-5p levels were considerably lower in H. pylori-infected MKN45 cells, miR-30a-3p could reduce COX-2 expression and β-catenin nuclear translocation. β-catenin impaired the function of TCF/LEF promoter targeting BCL9 to alter downstream target gene expression, therefore controlling the proliferation and migration of H. pylori-infected gastric GC (Liu et al. 2017). H. pylori infection also reduced miR-27b expression, and frizzled class receptor 7 (FZD7), a key receptor for the Wnt/β-catenin signaling pathway, was identified as a direct target gene of miR-27b. Overexpression of miR-27b inhibited GC cell proliferation and Wnt signal pathway activation caused by H. pylori, whereas FZD7 reversed these effects (Geng et al. 2016) (Fig. 6).
Coinfection of H. pylori and EBV in gastric cancer
Reports show that non-malignant gastroduodenal disorders (NMGDs) coinfection with EBV and H. pylori was present in 34% of cases (Dávila-Collado et al. 2020). Comparing studies where EBV infection was directly tested in stomach specimens to studies where EBV infection was established by serological methods, a greater coinfection rate (EBV + H. pylori) was identified. There were no studies comparing the coinfection rate in NMGDs with that in asymptomatic people despite the fact that the majority of these research was conducted in Latin America and India and most of them compared NMGDs with GC (Dávila-Collado et al. 2020). Coinfection of H. pylori and EBV also decreases the onset of GC (Rihane et al. 2020). Furthermore, a study by Kashyap et al. shows that prior exposure of H. pylori produced the cellular milieu for the growth and replication of EBV. Similarly, they were also determined that prior exposure of EBV also produced a cellular environment which promotes the growth and replication of H. pylori. Notably, same stud also demonstrated that coinfection of H. pylori and EBV promotes the aggressiveness of GC through the upregulation of oncoprotein Gankyrin and their downstream signaling molecules (Kashyap et al. 2021).
Conclusion
EBV and H. pylori exist in human systems where they are known to cause GC independently. Despite this, the coinfections are more common in biological systems where the pathogens interact with each other and enhance the aggressiveness of the disease. The coinfection of both microorganisms in GC specimens has been increasingly reported, suggesting that the interplay between both pathogens may be implicated in carcinogenesis. Multiple clinical reports explicitly describe the EBV and H. pylori coexistence in GC. Moreover, how these pathogens target host factors and downstream insight into molecular mechanisms is still unexplored. Henceforth, uncovering the mechanism of EBV and H. pylori-mediated aggressive carcinogenesis remains open for research. The open-ended knowledge regarding the H. pylori-mediated growth and replication of EBV remains to be answered. Another point of interest could be the antigens involved in understanding the progression of GC aggressiveness. Critical understanding of why gastric epithelial cells infected with H. pylori and EBV showing aggressive cancer properties remains unanswered. Thereby, our review has given a critical insight into the alteration of multiple signaling pathways in host cells during the infection of both the pathogens, which can find potential to interlink host–pathogen signaling pathways and their effects.
References
Adamson AL, Darr D, Holley-Guthrie E et al (2000) Epstein–Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol 74:1224–1233. https://doi.org/10.1128/JVI.74.3.1224-1233.2000
Al Moustafa A-E, Chen D, Ghabreau L, Akil N (2009) Association between human papillomavirus and Epstein–Barr virus infections in human oral carcinogenesis. Med Hypotheses 73:184–186. https://doi.org/10.1016/j.mehy.2009.02.025
Allison CC, Kufer TA, Kremmer E et al (2009) Helicobacter pylori Induces MAPK phosphorylation and AP-1 activation via a NOD1-dependent mechanism. J Immunol 183:8099–8109. https://doi.org/10.4049/jimmunol.0900664
Allison CC, Ferrand J, McLeod L et al (2013) Nucleotide oligomerization domain 1 enhances IFN-γ signaling in gastric epithelial cells during Helicobacter pylori infection and exacerbates disease severity. J Immunol 190:3706–3715. https://doi.org/10.4049/jimmunol.1200591
Ambrosio MR, Navari M, Di Lisio L et al (2014) The Epstein Barr-encoded BART-6-3p microRNA affects regulation of cell growth and immuno response in Burkitt lymphoma. Infect Agent Cancer 9:12. https://doi.org/10.1186/1750-9378-9-12
Andersen-Nissen E, Smith KD, Strobe KL et al (2005) Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci 102:9247–9252. https://doi.org/10.1073/pnas.0502040102
Anderson LJ, Longnecker R (2008) EBV LMP2A provides a surrogate pre-B cell receptor signal through constitutive activation of the ERK/MAPK pathway. J Gen Virol 89:1563–1568. https://doi.org/10.1099/vir.0.2008/001461-0
Arachchi PS, Fernando N, Weerasekera MM et al (2017) Proinflammatory cytokine IL-17 shows a significant association with Helicobacter pylori infection and disease severity. Gastroenterol Res Pract 2017:1–7. https://doi.org/10.1155/2017/6265150
Bentz GL, Shackelford J, Pagano JS (2012) Epstein–Barr virus latent membrane protein 1 regulates the function of interferon regulatory factor 7 by inducing its sumoylation. J Virol 86:12251–12261. https://doi.org/10.1128/JVI.01407-12
Berra E, Roux D, Richard DE, Pouysségur J (2001) Hypoxia-inducible factor-1α (HIF-1α) escapes O2-driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. EMBO Rep 2:615–620. https://doi.org/10.1093/embo-reports/kve130
Boehm D, Gewurz BE, Kieff E, Cahir-McFarland E (2010) Epstein–Barr latent membrane protein 1 transformation site 2 activates NF-κB in the absence of NF-κB essential modifier residues 133–224 or 373–419. Proc Natl Acad Sci 107:18103–18108. https://doi.org/10.1073/pnas.1011752107
Boulter L, Guest RV, Kendall TJ et al (2015) WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J Clin Invest 125:1269–1285. https://doi.org/10.1172/JCI76452
Bousoik E, Montazeri Aliabadi H (2018) “Do we know jack” about JAK? A closer look at JAK/STAT signaling pathway. Front Oncol 8:287. https://doi.org/10.3389/fonc.2018.00287
Bouvet M, Voigt S, Tagawa T et al (2021) Multiple viral microRNAs Regulate interferon release and signaling early during infection with Epstein–Barr virus. mBio 12:e03440-20. https://doi.org/10.1128/mBio.03440-20
Brennan P, Floettmann JE, Mehl A et al (2001) Mechanism of action of a novel latent membrane protein-1 dominant negative. J Biol Chem 276:1195–1203. https://doi.org/10.1074/jbc.M005461200
Brinkmann MM, Schulz TF (2006) Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J Gen Virol 87:1047–1074. https://doi.org/10.1099/vir.0.81598-0
Brunet A, Bonni A, Zigmond MJ et al (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868. https://doi.org/10.1016/S0092-8674(00)80595-4
Canales J, Valenzuela M, Bravo J et al (2017) Helicobacter pylori induced phosphatidylinositol-3-OH kinase/mTOR activation increases hypoxia inducible factor-1α to promote loss of cyclin D1 and G0/G1 cell cycle arrest in human gastric cells. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2017.00092
Ceppi M, Pereira PM, Dunand-Sauthier I et al (2009) MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci 106:2735–2740. https://doi.org/10.1073/pnas.0811073106
Chang H, Kim N, Park JH et al (2015) Helicobacter pylori might induce TGF-β1-mediated EMT by means of cagE. Helicobacter 20:438–448. https://doi.org/10.1111/hel.12220
Chen ZJ (2005) Ubiquitin signalling in the NF-κB pathway. Nat Cell Biol 7:758–765. https://doi.org/10.1038/ncb0805-758
Chen Y-C (2006) H pylori stimulates proliferation of gastric cancer cells through activating mitogen-activated protein kinase cascade. World J Gastroenterol 12:5972. https://doi.org/10.3748/wjg.v12.i37.5972
Chen J (2012) Roles of the PI3K/Akt pathway in Epstein–Barr virus-induced cancers and therapeutic implications. World J Virol 1:154. https://doi.org/10.5501/wjv.v1.i6.154
Chen S-Y, Lu J, Shih Y-C, Tsai C-H (2002) Epstein–Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J Virol 76:9556–9561. https://doi.org/10.1128/JVI.76.18.9556-9561.2002
Chen Y-R, Liu M-T, Chang Y-T et al (2008) Epstein–Barr virus latent membrane protein 1 represses DNA repair through the PI3K/Akt/FOXO3a pathway in human epithelial cells. J Virol 82:8124–8137. https://doi.org/10.1128/JVI.00430-08
Chen J-P, Wu M-S, Kuo S-H, Liao F (2014) IL-22 negatively regulates Helicobacter pylori-induced CCL20 expression in gastric epithelial cells. PLoS ONE 9:e97350. https://doi.org/10.1371/journal.pone.0097350
Chen Y, Fachko DN, Ivanov NS, Skalsky RL (2021) B cell receptor-responsive miR-141 enhances Epstein–Barr virus lytic cycle via FOXO3 inhibition. mSphere 6:e00093-21. https://doi.org/10.1128/mSphere.00093-21
Cheok YY, Tan GMY, Lee CYQ et al (2022) Innate immunity crosstalk with Helicobacter pylori: pattern recognition receptors and cellular responses. Int J Mol Sci 23:7561. https://doi.org/10.3390/ijms23147561
Coffer PJ, Jin J, Woodgett JR (1998) Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 335:1–13. https://doi.org/10.1042/bj3350001
Conteduca V, Sansonno D, Lauletta G et al (2013) H. pylori infection and gastric cancer: State of the art. Int J Oncol 42:5–18. https://doi.org/10.3892/ijo.2012.1701
Cross DAE, Alessi DR, Cohen P et al (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789. https://doi.org/10.1038/378785a0
Cullen BR (2009) Viral and cellular messenger RNA targets of viral microRNAs. Nature 457:421–425. https://doi.org/10.1038/nature07757
Darekar S, Georgiou K, Yurchenko M et al (2012) Epstein–Barr virus immortalization of human B-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the Warburg effect. PLoS ONE 7:e42072. https://doi.org/10.1371/journal.pone.0042072
Dávila-Collado R, Jarquín-Durán O, Dong LT, Espinoza JL (2020) Epstein–Barr virus and Helicobacter Pylori Co-infection in non-malignant gastroduodenal disorders. Pathogens 9:104. https://doi.org/10.3390/pathogens9020104
Dawson CW, Tramountanis G, Eliopoulos AG, Young LS (2003) Epstein–Barr Virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem 278:3694–3704. https://doi.org/10.1074/jbc.M209840200
del Peso L, González-Garcı́a M, Page C, et al (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687–689. https://doi.org/10.1126/science.278.5338.687
Delgado-Ortega M, Marc D, Dupont J et al (2013) SOCS proteins in infectious diseases of mammals. Vet Immunol Immunopathol 151:1–19. https://doi.org/10.1016/j.vetimm.2012.11.008
Demidenko ZN, Blagosklonny MV (2011) The purpose of the HIF-1/PHD feedback loop: To limit mTOR-induced HIF-1α. Cell Cycle 10:1557–1562. https://doi.org/10.4161/cc.10.10.15789
Dhand R, Hiles I, Panayotou G et al (1994) PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J 13:522–533. https://doi.org/10.1002/j.1460-2075.1994.tb06290.x
Ding S-Z, Torok AM, Smith MF, Goldberg JB (2005) Toll-like receptor 2-mediated gene expression in epithelial cells during Helicobacter pylori infection. Helicobacter 10:193–204. https://doi.org/10.1111/j.1523-5378.2005.00311.x
Ding S-Z, Olekhnovich IN, Cover TL et al (2008) Helicobacter pylori and mitogen-activated protein kinases mediate activator protein-1 (AP-1) subcomponent protein expression and DNA-binding activity in gastric epithelial cells. FEMS Immunol Med Microbiol 53:385–394. https://doi.org/10.1111/j.1574-695X.2008.00439.x
Dunne C (2014) Factors that mediate colonization of the human stomach by Helicobacter pylori. World J Gastroenterol 20:5610. https://doi.org/10.3748/wjg.v20.i19.5610
Dykstra ML, Longnecker R, Pierce SK (2001) Epstein–Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity 14:57–67. https://doi.org/10.1016/S1074-7613(01)00089-9
Eliopoulos AG, Gallagher NJ, Blake SMS et al (1999) Activation of the p38 mitogen-activated protein kinase pathway by Epstein–Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem 274:16085–16096. https://doi.org/10.1074/jbc.274.23.16085
Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9:550–562. https://doi.org/10.1038/nrc2664
Ersing I, Bernhardt K, Gewurz B (2013) NF-κB and IRF7 pathway activation by Epstein–Barr virus latent membrane protein 1. Viruses 5:1587–1606. https://doi.org/10.3390/v5061587
Franco AT, Israel DA, Washington MK et al (2005) Activation of β-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci 102:10646–10651. https://doi.org/10.1073/pnas.0504927102
Frappier L (2012) Contributions of Epstein–Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses 4:1537–1547. https://doi.org/10.3390/v4091537
Gaglia MM (2021) Anti-viral and pro-inflammatory functions of Toll-like receptors during gamma-herpesvirus infections. Virol J 18:218. https://doi.org/10.1186/s12985-021-01678-x
Gao X, Wang H, Sairenji T (2004) Inhibition of Epstein–Barr virus (EBV) reactivation by short interfering RNAs targeting p38 mitogen-activated protein kinase or c-myc in EBV-positive epithelial cells. J Virol 78:11798–11806. https://doi.org/10.1128/JVI.78.21.11798-11806.2004
Gao L, Han H, Wang H et al (2019) IL-10 knockdown with siRNA enhances the efficacy of Doxorubicin chemotherapy in EBV-positive tumors by inducing lytic cycle via PI3K/p38 MAPK/NF-kB pathway. Cancer Lett 462:12–22. https://doi.org/10.1016/j.canlet.2019.07.016
Gazon H, Barbeau B, Mesnard J-M, Peloponese J-M (2018) Hijacking of the AP-1 signaling pathway during development of ATL. Front Microbiol 8:2686. https://doi.org/10.3389/fmicb.2017.02686
Geng W, Zhang H-Y (2017) Research on the mechanism of HP mediated PI3K/AKT/GSK3β pathways in gastric cancer. Eur Rev Med Pharmacol Sci 21:33–37
Geng Y, Lu X, Wu X et al (2016) MicroRNA-27b suppresses Helicobacter pylori-induced gastric tumorigenesis through negatively regulating Frizzled7. Oncol Rep 35:2441–2450. https://doi.org/10.3892/or.2016.4572
Gewurz BE, Mar JC, Padi M et al (2011) Canonical NF-κB activation is essential for Epstein–Barr virus latent membrane protein 1 TES2/CTAR2 gene regulation. J Virol 85:6764–6773. https://doi.org/10.1128/JVI.00422-11
Gewurz BE, Towfic F, Mar JC et al (2012) Genome-wide siRNA screen for mediators of NF-κB activation. Proc Natl Acad Sci 109:2467–2472. https://doi.org/10.1073/pnas.1120542109
Gires O (1999) Latent membrane protein 1of Epstein–Barr virus interacts with JAK3 and activates STAT proteins. EMBO J 18:3064–3073. https://doi.org/10.1093/emboj/18.11.3064
Green MR, Monti S, Rodig SJ et al (2010) Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116:3268–3277. https://doi.org/10.1182/blood-2010-05-282780
Guo L, Fang T, Jiang Y, Liu D (2021) IRF7 is a Prognostic biomarker and associated with immune infiltration in stomach adenocarcinoma. Int J Gen Med 14:9887–9902. https://doi.org/10.2147/IJGM.S342607
Hatakeyama M (2014) Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe 15:306–316. https://doi.org/10.1016/j.chom.2014.02.008
Hayakawa Y, Hirata Y, Nakagawa H et al (2011) Apoptosis signal-regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci 108:780–785. https://doi.org/10.1073/pnas.1011418108
Hayakawa Y, Hirata Y, Kinoshita H et al (2013) Differential roles of ASK1 and TAK1 in Helicobacter pylori-induced cellular responses. Infect Immun 81:4551–4560. https://doi.org/10.1128/IAI.00914-13
Hayashi Y, Tsujii M, Wang J et al (2013) CagA mediates epigenetic regulation to attenuate let-7 expression in Helicobacter pylori -related carcinogenesis. Gut 62:1536–1546. https://doi.org/10.1136/gutjnl-2011-301625
Hayden MS, Ghosh S (2012) NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev 26:203–234. https://doi.org/10.1101/gad.183434.111
Hinshaw DC, Shevde LA (2019) The tumor microenvironment innately modulates cancer progression. Cancer Res 79:4557–4566. https://doi.org/10.1158/0008-5472.CAN-18-3962
Hirata Y, Ohmae T, Shibata W et al (2006) MyD88 and TNF receptor-associated factor 6 are critical signal transducers in Helicobacter pylori -infected human epithelial cells. J Immunol 176:3796–3803. https://doi.org/10.4049/jimmunol.176.6.3796
Hooi JKY, Lai WY, Ng WK et al (2017) Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153:420–429. https://doi.org/10.1053/j.gastro.2017.04.022
Hooykaas MJG, van Gent M, Soppe JA et al (2017) EBV MicroRNA BART16 suppresses type I IFN signaling. J Immunol 198:4062–4073. https://doi.org/10.4049/jimmunol.1501605
Huang W-T, Lin C-W (2014) EBV-encoded miR-BART20-5p and miR-BART8 inhibit the IFN-γ–STAT1 pathway associated with disease progression in nasal NK-Cell lymphoma. Am J Pathol 184:1185–1197. https://doi.org/10.1016/j.ajpath.2013.12.024
Huang Y, Wang Q, Cheng D et al (2016) Adhesion and invasion of gastric mucosa epithelial cells by Helicobacter pylori. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2016.00159
Humme S, Reisbach G, Feederle R et al (2003) The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc Natl Acad Sci 100:10989–10994. https://doi.org/10.1073/pnas.1832776100
Hutajulu SH, Hoebe EK, Verkuijlen SA et al (2010) Conserved mutation of Epstein–Barr virus-encoded BamHI-A Rightward Frame-1 (BARF1) gene in Indonesian nasopharyngeal carcinoma. Infect Agent Cancer 5:16. https://doi.org/10.1186/1750-9378-5-16
Iwakiri D, Zhou L, Samanta M et al (2009) Epstein–Barr virus (EBV)–encoded small RNA is released from EBV-infected cells and activates signaling from toll-like receptor 3. J Exp Med 206:2091–2099. https://doi.org/10.1084/jem.20081761
Jakhmola S, Jha HC (2021) Glial cell response to Epstein–Barr virus infection: a plausible contribution to virus-associated inflammatory reactions in the brain. Virology 559:182–195. https://doi.org/10.1016/j.virol.2021.04.005
Jang KL, Shackelford J, Seo SY, Pagano JS (2005) Up-regulation of β-catenin by a viral oncogene correlates with inhibition of the seven in absentia homolog 1 in B lymphoma cells. Proc Natl Acad Sci 102:18431–18436. https://doi.org/10.1073/pnas.0504054102
Jiang S, Zhang H-W, Lu M-H et al (2010) MicroRNA-155 functions as an OncomiR in breast cancer by targeting the Suppressor of Cytokine Signaling 1 gene. Cancer Res 70:3119–3127. https://doi.org/10.1158/0008-5472.CAN-09-4250
Jochum W, Passegué E, Wagner EF (2001) AP-1 in mouse development and tumorigenesis. Oncogene 20:2401–2412. https://doi.org/10.1038/sj.onc.1204389
Kashyap D, Baral B, Jakhmola S et al (2021) Helicobacter pylori and Epstein–Barr virus coinfection stimulates aggressiveness in gastric cancer through the regulation of gankyrin. mSphere 6:e00751-21. https://doi.org/10.1128/mSphere.00751-21
Kashyap D, Varshney N, Parmar HS, Jha HC (2022) Gankyrin: at the crossroads of cancer diagnosis, disease prognosis, and development of efficient cancer therapeutics. Adv Cancer Biol Metastasis 4:100023. https://doi.org/10.1016/j.adcanc.2021.100023
Katso R, Okkenhaug K, Ahmadi K et al (2001) Cellular function of phosphoinositide 3-kinases: implications for development, immunity, homeostasis, and cancer. Annu Rev Cell Dev Biol 17:615–675. https://doi.org/10.1146/annurev.cellbio.17.1.615
Kim H, Iizasa H, Kanehiro Y et al (2017) Herpesviral microRNAs in cellular metabolism and immune responses. Front Microbiol 8:1318. https://doi.org/10.3389/fmicb.2017.01318
Kirikoshi H, Sekihara H, Katoh M (2001) Up-regulation of WNT10A by tumor necrosis factor α and Helicobacter pylori in gastric cancer. Int J Oncol. https://doi.org/10.3892/ijo.19.3.533
Kondo S, Wakisaka N, Schell MJ et al (2005) Epstein–Barr virus latent membrane protein 1 induces the matrix metalloproteinase-1 promoter via an Ets binding site formed by a single nucleotide polymorphism: enhanced susceptibility to nasopharyngeal carcinoma. Int J Cancer 115:368–376. https://doi.org/10.1002/ijc.20849
Kondo S, Seo SY, Yoshizaki T et al (2006) EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1α through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res 66:9870–9877. https://doi.org/10.1158/0008-5472.CAN-06-1679
Kong H, You N, Chen H et al (2020) Helicobacter pylori-induced adrenomedullin modulates IFN-γ-producing T-cell responses and contributes to gastritis. Cell Death Dis 11:189. https://doi.org/10.1038/s41419-020-2391-6
Kosowicz JG, Lee J, Peiffer B et al (2017) Drug modulators of B Cell signaling pathways and Epstein–Barr virus lytic activation. J Virol 91:e00747-e817. https://doi.org/10.1128/JVI.00747-17
Kraus RJ, Yu X, Cordes BA et al (2017) Hypoxia-inducible factor-1α plays roles in Epstein–Barr virus’s natural life cycle and tumorigenesis by inducing lytic infection through direct binding to the immediate-early BZLF1 gene promoter. PLOS Pathog 13:e1006404. https://doi.org/10.1371/journal.ppat.1006404
Kraus RJ, Cordes BA, Sathiamoorthi S et al (2020) Reactivation of Epstein–Barr virus by HIF-1α requires p53. J Virol 94:e00722-e820. https://doi.org/10.1128/JVI.00722-20
Kung C-P, Raab-Traub N (2008) Epstein–Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3. J Virol 82:5486–5493. https://doi.org/10.1128/JVI.00125-08
Kuroda T, Kitadai Y, Tanaka S et al (2005) Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res 11:7629–7636. https://doi.org/10.1158/1078-0432.CCR-05-0798
Lamb A, Chen L-F (2010) The many roads traveled by Helicobacter pylori to NF-κB activation. Gut Microbes 1:109–113. https://doi.org/10.4161/gmic.1.2.11587
Lamb A, Chen L-F (2013) Role of the Helicobacter pylori -induced inflammatory response in the development of gastric cancer. J Cell Biochem 114:491–497. https://doi.org/10.1002/jcb.24389
Lambert SL, Martinez OM (2007) Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J Immunol 179:8225–8234. https://doi.org/10.4049/jimmunol.179.12.8225
Laughner E, Taghavi P, Chiles K et al (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21:3995–4004. https://doi.org/10.1128/MCB.21.12.3995-4004.2001
Lee J-W, Liu P-F, Hsu L-P et al (2009) EBV LMP-1 negatively regulates expression and pro-apoptotic activity of Par-4 in nasopharyngeal carcinoma cells. Cancer Lett 279:193–201. https://doi.org/10.1016/j.canlet.2009.01.037
Li N, Xie C, Lu N-H (2015) Transforming growth factor-β: an important mediator in Helicobacter pylori-associated pathogenesis. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2015.00077
Li N, Tang B, Jia Y et al (2017) Helicobacter pylori CagA protein negatively regulates autophagy and promotes inflammatory response via c-Met-PI3K/Akt-mTOR signaling pathway. Front Cell Infect Microbiol 7:417. https://doi.org/10.3389/fcimb.2017.00417
Lin X, Liu S, Luo X et al (2009) EBV-encoded LMP1 regulates Op18/stathmin signaling pathway by cdc2 mediation in nasopharyngeal carcinoma cells. Int J Cancer 124:1020–1027. https://doi.org/10.1002/ijc.23767
Lin K-M, Lin S-J, Lin J-H et al (2020) Dysregulation of dual-specificity phosphatases by Epstein–Barr virus LMP1 and Its impact on lymphoblastoid cell line survival. J Virol 94:e01837-e1919. https://doi.org/10.1128/JVI.01837-19
Liu X, Cohen JI (2016) Epstein–Barr virus (EBV) tegument protein BGLF2 promotes EBV reactivation through activation of the p38 mitogen-activated protein kinase. J Virol 90:1129–1138. https://doi.org/10.1128/JVI.01410-15
Liu Z, Hsu H, Goeddel DV, Karin M (1996) Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565–576. https://doi.org/10.1016/S0092-8674(00)81375-6
Liu Z, Xiao B, Tang B et al (2010) Up-regulated microRNA-146a negatively modulate Helicobacter pylori-induced inflammatory response in human gastric epithelial cells. Microbes Infect 12:854–863. https://doi.org/10.1016/j.micinf.2010.06.002
Liu X, Ji Q, Zhang C et al (2017) miR-30a acts as a tumor suppressor by double-targeting COX-2 and BCL9 in H. pylori gastric cancer models. Sci Rep 7:7113. https://doi.org/10.1038/s41598-017-07193-w
Lo AK-F, Dawson CW, Lung HL et al (2020) The therapeutic potential of targeting BARF1 in EBV-associated malignancies. Cancers 12:1940. https://doi.org/10.3390/cancers12071940
Lu F, Weidmer A, Liu C-G et al (2008) Epstein–Barr virus-induced miR-155 attenuates NF-κB signaling and stabilizes latent virus persistence. J Virol 82:10436–10443. https://doi.org/10.1128/JVI.00752-08
Lu Y, Qin Z, Wang J et al (2017) Epstein–Barr virus miR-BART6-3p inhibits the RIG-I pathway. J Innate Immun 9:574–586. https://doi.org/10.1159/000479749
Lu Y, Xiao F, Wang Y et al (2022) Prevalence of Helicobacter pylori in non-cardia gastric cancer in China: a systematic review and meta-analysis. Front Oncol 12:850389. https://doi.org/10.3389/fonc.2022.850389
Luftig M, Prinarakis E, Yasui T et al (2003) Epstein–Barr virus latent membrane protein 1 activation of NF-κB through IRAK1 and TRAF6. Proc Natl Acad Sci 100:15595–15600. https://doi.org/10.1073/pnas.2136756100
Luo Y, Liu Y, Wang C, Gan R (2021) Signaling pathways of EBV-induced oncogenesis. Cancer Cell Int 21:93. https://doi.org/10.1186/s12935-021-01793-3
Lyu X, Fang W, Cai L et al (2014) TGFβR2 is a major target of miR-93 in nasopharyngeal carcinoma aggressiveness. Mol Cancer 13:51. https://doi.org/10.1186/1476-4598-13-51
Ma Y, Walsh MJ, Bernhardt K et al (2017) CRISPR/Cas9 screens reveal Epstein–Barr virus-transformed B cell host dependency factors. Cell Host Microbe 21:580-591.e7. https://doi.org/10.1016/j.chom.2017.04.005
MacDonald BT, Tamai K, He X (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26. https://doi.org/10.1016/j.devcel.2009.06.016
Maeda S, Akanuma M, Mitsuno Y et al (2001) Distinct mechanism of Helicobacter pylori-mediated NF-κB activation between gastric cancer cells and monocytic cells. J Biol Chem 276:44856–44864. https://doi.org/10.1074/jbc.M105381200
Mancao C, Hammerschmidt W (2007) Epstein–Barr virus latent membrane protein 2A is a B-cell receptor mimic and essential for B-cell survival. Blood 110:3715–3721. https://doi.org/10.1182/blood-2007-05-090142
Matusali G, Arena G, De Leo A et al (2009) Inhibition of p38 MAP kinase pathway induces apoptosis and prevents Epstein Barr virus reactivation in Raji cells exposed to lytic cycle inducing compounds. Mol Cancer 8:18. https://doi.org/10.1186/1476-4598-8-18
Mechta-Grigoriou F, Gerald D, Yaniv M (2001) The mammalian Jun proteins: redundancy and specificity. Oncogene 20:2378–2389. https://doi.org/10.1038/sj.onc.1204381
Menheniott TR, Judd LM, Giraud AS (2015) STAT3: a critical component in the response to Helicobacter pylori infection: STAT3 in Helicobacter pylori infection. Cell Microbiol 17:1570–1582. https://doi.org/10.1111/cmi.12518
Michaud F, Coulombe F, Gaudreault E et al (2010) Epstein–Barr virus interferes with the amplification of IFNα secretion by activating suppressor of cytokine signaling 3 in primary human monocytes. PLoS ONE 5:e11908. https://doi.org/10.1371/journal.pone.0011908
Minaga K, Watanabe T, Kamata K et al (2018) Nucleotide-binding oligomerization domain 1 and Helicobacter pylori infection: a review. World J Gastroenterol 24:1725–1733. https://doi.org/10.3748/wjg.v24.i16.1725
Mochida Y, Takeda K, Saitoh M et al (2000) ASK1 inhibits interleukin-1-induced NF-κB activity through disruption of TRAF6-TAK1 interaction. J Biol Chem 275:32747–32752. https://doi.org/10.1074/jbc.M003042200
Moon JW, Kong S-K, Kim BS et al (2017) IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep 7:17810. https://doi.org/10.1038/s41598-017-18132-0
Morris MA, Dawson CW, Laverick L et al (2016) The Epstein–Barr virus encoded LMP1 oncoprotein modulates cell adhesion via regulation of activin A/TGFβ and β1 integrin signalling. Sci Rep 6:19533. https://doi.org/10.1038/srep19533
Mosialos G (2001) Cytokine signaling and Epstein–Barr virus-mediated cell transformation. Cytokine Growth Factor Rev 12:259–270. https://doi.org/10.1016/S1359-6101(00)00035-6
Mrozek-Gorska P, Buschle A, Pich D et al (2019) Epstein–Barr virus reprograms human B lymphocytes immediately in the prelatent phase of infection. Proc Natl Acad Sci 116:16046–16055. https://doi.org/10.1073/pnas.1901314116
Mulherkar TH, Gómez DJ, Sandel G, Jain P (2022) Co-infection and cancer: host–pathogen interaction between dendritic cells and HIV-1, HTLV-1, and other oncogenic viruses. Viruses 14:2037. https://doi.org/10.3390/v14092037
Nagy TA, Frey MR, Yan F et al (2009) Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J Infect Dis 199:641–651. https://doi.org/10.1086/596660
Najjar I, Baran-Marszak F, Le Clorennec C et al (2005) Latent membrane protein 1 regulates STAT1 through NF-κB-dependent interferon secretion in Epstein–Barr virus-immortalized B cells. J Virol 79:4936–4943. https://doi.org/10.1128/JVI.79.8.4936-4943.2005
Nakayama M, Hisatsune J, Yamasaki E et al (2009) Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. J Biol Chem 284:1612–1619. https://doi.org/10.1074/jbc.M806981200
Nanbo A, Ohashi M, Yoshiyama H, Ohba Y (2018) The role of transforming growth factor β in cell-to-cell contact-mediated Epstein–Barr virus transmission. Front Microbiol 9:984. https://doi.org/10.3389/fmicb.2018.00984
Naumann M, Wessler S, Bartsch C et al (1999) Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J Biol Chem 274:31655–31662. https://doi.org/10.1074/jbc.274.44.31655
Niemann CU, Wiestner A (2013) B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol 23:410–421. https://doi.org/10.1016/j.semcancer.2013.09.001
Ning S, Pagano JS, Barber GN (2011) IRF7: activation, regulation, modification and function. Genes Immun 12:399–414. https://doi.org/10.1038/gene.2011.21
Nishioka H, Baesso I, Semenzato G et al (2003) The neutrophil-activating protein of Helicobacter pylori (HP-NAP) activates the MAPK pathway in human neutrophils. Eur J Immunol 33:840–849. https://doi.org/10.1002/eji.200323726
Noto JM, Piazuelo MB, Chaturvedi R et al (2013) Strain-specific suppression of microRNA-320 by carcinogenic Helicobacter pylori promotes expression of the antiapoptotic protein Mcl-1. Am J Physiol Gastrointest Liver Physiol 305:G786–G796. https://doi.org/10.1152/ajpgi.00279.2013
Oshima H, Hioki K, Popivanova BK et al (2011) Prostaglandin E2 signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology 140:596-607.e7. https://doi.org/10.1053/j.gastro.2010.11.007
Pachathundikandi SK, Tegtmeyer N, Backert S (2013) Signal transduction of Helicobacter pylori during interaction with host cell protein receptors of epithelial and immune cells. Gut Microbes 4:454–474. https://doi.org/10.4161/gmic.27001
Pai SG, Carneiro BA, Mota JM et al (2017) Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol 10:101. https://doi.org/10.1186/s13045-017-0471-6
Payne DM, Rossomando AJ, Martino P et al (1991) Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J 10:885–892. https://doi.org/10.1002/j.1460-2075.1991.tb08021.x
Peek RM, Crabtree JE (2006) Helicobacter infection and gastric neoplasia. J Pathol 208:233–248. https://doi.org/10.1002/path.1868
Pei Y, Banerjee S, Sun Z et al (2016) EBV nuclear antigen 3C mediates regulation of E2F6 to inhibit E2F1 transcription and promote cell proliferation. PLOS Pathog 12:e1005844. https://doi.org/10.1371/journal.ppat.1005844
Pei Y, Wong JH, Jha HC et al (2020a) Epstein–Barr virus facilitates expression of KLF14 by regulating the cooperative binding of the E2F-Rb-HDAC complex in latent infection. J Virol 94:e01209-e1220. https://doi.org/10.1128/JVI.01209-20
Pei Y, Hwang N, Lang F et al (2020b) Quassinoid analogs with enhanced efficacy for treatment of hematologic malignancies target the PI3Kγ isoform. Commun Biol 3:267. https://doi.org/10.1038/s42003-020-0996-z
Petrocca F, Visone R, Onelli MR et al (2008a) E2F1-Regulated MicroRNAs impair TGFβ-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell 13:272–286. https://doi.org/10.1016/j.ccr.2008.02.013
Petrocca F, Vecchione A, Croce CM (2008b) Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor β signaling. Cancer Res 68:8191–8194. https://doi.org/10.1158/0008-5472.CAN-08-1768
Piao J-Y, Lee HG, Kim S-J et al (2016) Helicobacter pylori activates IL-6-STAT3 Signaling in human gastric cancer cells: potential roles for reactive oxygen species. Helicobacter 21:405–416. https://doi.org/10.1111/hel.12298
Radolf JD, Samuels DS (eds) (2021) Lyme disease and relapsing fever spirochetes: genomics, molecular biology, host interactions and disease pathogenesis. Caister Academic Press, Poole
Raingeaud J, Gupta S, Rogers JS et al (1995) Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 270:7420–7426. https://doi.org/10.1074/jbc.270.13.7420
Raingeaud J, Whitmarsh AJ, Barrett T et al (1996) MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16:1247–1255. https://doi.org/10.1128/MCB.16.3.1247
Rihane FE, Hassou N, Nadifi S, Ennaji MM (2020) Status of Helicobacter pylori coinfection with Epstein–Barr virus in gastric cancer. In: Ennaji MM (ed) Emerging and reemerging viral pathogens. Elsevier, Amsterdam, pp 571–585
Roberts ML, Cooper NR (1998) Activation of a Ras–MAPK-dependent pathway by Epstein–Barr virus latent membrane protein 1 Is essential for cellular transformation. Virology 240:93–99. https://doi.org/10.1006/viro.1997.8901
Saitoh M (1998) Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17:2596–2606. https://doi.org/10.1093/emboj/17.9.2596
Săsăran MO, Meliț LE, Dobru ED (2021) MicroRNA modulation of host immune response and inflammation triggered by Helicobacter pylori. Int J Mol Sci 22:1406. https://doi.org/10.3390/ijms22031406
Sawai N, Kita M, Kodama T et al (1999) Role of gamma interferon in Helicobacter pylori -induced gastric inflammatory responses in a mouse model. Infect Immun 67:279–285. https://doi.org/10.1128/IAI.67.1.279-285.1999
Schultheiss U (2001) TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J 20:5678–5691. https://doi.org/10.1093/emboj/20.20.5678
Sears R, Nuckolls F, Haura E et al (2000) Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14:2501–2514. https://doi.org/10.1101/gad.836800
Seif F, Khoshmirsafa M, Aazami H et al (2017) The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 15:23. https://doi.org/10.1186/s12964-017-0177-y
Seo JH, Lim JW, Kim H, Kim KH (2004) Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-κB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest 84:49–62. https://doi.org/10.1038/labinvest.3700010
Sethi G, Sung B, Aggarwal BB (2008) Nuclear factor-κB activation: from bench to bedside. Exp Biol Med 233:21–31. https://doi.org/10.3181/0707-MR-196
Seto E, Moosmann A, Grömminger S et al (2010) Micro RNAs of Epstein–Barr virus promote cell cycle progression and prevent apoptosis of primary human B cells. PLoS Pathog 6:e1001063. https://doi.org/10.1371/journal.ppat.1001063
Sgarbanti M, Marsili G, Remoli AL et al (2007) IRF-7: new role in the regulation of genes involved in adaptive immunity. Ann N Y Acad Sci 1095:325–333. https://doi.org/10.1196/annals.1397.036
Shackelford J, Maier C, Pagano JS (2003) Epstein–Barr virus activates β-catenin in type III latently infected B lymphocyte lines: association with deubiquitinating enzymes. Proc Natl Acad Sci 100:15572–15576. https://doi.org/10.1073/pnas.2636947100
Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–E136. https://doi.org/10.1038/ncb0502-e131
Sheedy FJ, Palsson-McDermott E, Hennessy EJ et al (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11:141–147. https://doi.org/10.1038/ni.1828
Shi Q, Zhang Y, Liu W et al (2020) Latent membrane protein 2A inhibits expression level of Smad2 through regulating miR-155-5p in EBV-associated gastric cancer cell lines. J Med Virol 92:96–106. https://doi.org/10.1002/jmv.25579
Shin J-Y, Kim J-O, Lee SK et al (2010) LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein–Barr virus-positive gastric cancer cells. BMC Cancer 10:425. https://doi.org/10.1186/1471-2407-10-425
Siegler G (2003) Epstein–Barr virus encoded latent membrane protein 1 (LMP1) and TNF receptor associated factors (TRAF): colocalisation of LMP1 and TRAF1 in primary EBV infection and in EBV associated Hodgkin lymphoma. Mol Pathol 56:156–161. https://doi.org/10.1136/mp.56.3.156
Singh S, Jha HC (2017) Status of Epstein–Barr virus coinfection with Helicobacter pylori in GASTRIC CANcer. J Oncol 2017:1–17. https://doi.org/10.1155/2017/3456264
Slomiany BL (2012) Helicobacter pylori induction in gastric mucosal prostaglandin and nitric oxide generation is dependent on MAPK/ERK-mediated activation of IKK-β and cPLA2: modulatory effect of ghrelin. Open J Cell Biol 02:21–31. https://doi.org/10.4236/ojcb.2012.22003
Song X, Xin N, Wang W, Zhao C (2015) Wnt/β-catenin, an oncogenic pathway targeted by H. pylori in gastric carcinogenesis. Oncotarget 6:35579–35588. https://doi.org/10.18632/oncotarget.5758
Sonkar C, Kashyap D, Varshney N et al (2020) Impact of gastrointestinal symptoms in COVID-19: a molecular approach. SN Compr Clin Med 2:2658–2669. https://doi.org/10.1007/s42399-020-00619-z
Soutto M, Peng D, Katsha A et al (2015) Activation of β-catenin signalling by TFF1 loss promotes cell proliferation and gastric tumorigenesis. Gut 64:1028–1039. https://doi.org/10.1136/gutjnl-2014-307191
Su B, Ceponis PJM, Lebel S et al (2003) Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun 71:3496–3502. https://doi.org/10.1128/IAI.71.6.3496-3502.2003
Suarez F (2006) Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood 107:3034–3044. https://doi.org/10.1182/blood-2005-09-3679
Sun S-C (2011) Non-canonical NF-κB signaling pathway. Cell Res 21:71–85. https://doi.org/10.1038/cr.2010.177
Sun L, Zhao Y, Shi H et al (2015) LMP-1 induces survivin expression to inhibit cell apoptosis through the NF-κB and PI3K/Akt signaling pathways in nasal NK/T-cell lymphoma. Oncol Rep 33:2253–2260. https://doi.org/10.3892/or.2015.3847
Sun Z, Jha HC, Pei Y, Robertson ES (2016) Major histocompatibility complex class II HLA-DRα is downregulated by Kaposi’s sarcoma-associated herpesvirus-encoded lytic transactivator RTA and MARCH8. J Virol 90:8047–8058. https://doi.org/10.1128/JVI.01079-16
Tang B, Xiao B, Liu Z et al (2010) Identification of MyD88 as a novel target of miR-155, involved in negative regulation of Helicobacter pylori-induced inflammation. FEBS Lett 584:1481–1486. https://doi.org/10.1016/j.febslet.2010.02.063
Tang Z, Chen W, Xu Y et al (2020) miR-4721, induced by EBV-miR-BART22, targets GSK3β to enhance the tumorigenic capacity of NPC through the WNT/β-catenin pathway. Mol Ther Nucleic Acids 22:557–571. https://doi.org/10.1016/j.omtn.2020.09.021
Tavakoli A, Monavari SH, Solaymani Mohammadi F et al (2020) Association between Epstein–Barr virus infection and gastric cancer: a systematic review and meta-analysis. BMC Cancer 20:493. https://doi.org/10.1186/s12885-020-07013-x
Tegtmeyer N, Wessler S, Backert S (2011) Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J 278:1190–1202. https://doi.org/10.1111/j.1742-4658.2011.08035.x
Toh JWT, Wilson RB (2020) Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. Int J Mol Sci 21:6451. https://doi.org/10.3390/ijms21176451
Torisu T, Kawano S, Miyawaki K et al (2021) B cell receptor signaling related to resistance to Helicobacter pylori eradication therapy in gastric diffuse large B cell lymphoma. Hematol Oncol 39:145–147. https://doi.org/10.1002/hon.2816
Tye H, Kennedy CL, Najdovska M et al (2012) STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell 22:466–478. https://doi.org/10.1016/j.ccr.2012.08.010
Uno K (2014) Novel role of toll-like receptors in Helicobacter pylori—induced gastric malignancy. World J Gastroenterol 20:5244. https://doi.org/10.3748/wjg.v20.i18.5244
Valente RM, Ehlers E, Xu D et al (2012) Toll-like receptor 7 stimulates the expression of Epstein–Barr virus latent membrane protein 1. PLoS ONE 7:e43317. https://doi.org/10.1371/journal.pone.0043317
van Zuylen WJ, Rawlinson WD, Ford CE (2016) The Wnt pathway: a key network in cell signalling dysregulated by viruses: Wnt signalling and viral infection. Rev Med Virol 26:340–355. https://doi.org/10.1002/rmv.1892
Vanden Berghe W, Plaisance S, Boone E et al (1998) p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-κB p65 transactivation mediated by tumor necrosis factor. J Biol Chem 273:3285–3290. https://doi.org/10.1074/jbc.273.6.3285
Vaysberg M, Lambert SL, Krams SM, Martinez OM (2009) Activation of the JAK/STAT pathway in Epstein Barr virus+-associated posttransplant lymphoproliferative disease: role of interferon-γ. Am J Transplant 9:2292–2302. https://doi.org/10.1111/j.1600-6143.2009.02781.x
Velapasamy S, Dawson C, Young L et al (2018) The dynamic roles of TGF-β signalling in EBV-associated cancers. Cancers 10:247. https://doi.org/10.3390/cancers10080247
Voigt S, Sterz KR, Giehler F et al (2020) A central role of IKK2 and TPL2 in JNK activation and viral B-cell transformation. Nat Commun 11:685. https://doi.org/10.1038/s41467-020-14502-x
Wakisaka N, Kondo S, Yoshizaki T et al (2004) Epstein–Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1α. Mol Cell Biol 24:5223–5234. https://doi.org/10.1128/MCB.24.12.5223-5234.2004
Wan YY, Flavell RA (2008) TGF-β and regulatory T cell in immunity and autoimmunity. J Clin Immunol 28:647–659. https://doi.org/10.1007/s10875-008-9251-y
Wan J, Sun L, Mendoza JW et al (2004) Elucidation of the c-Jun N-terminal kinase pathway mediated by Epstein–Barr virus-encoded latent membrane protein 1. Mol Cell Biol 24:192–199. https://doi.org/10.1128/MCB.24.1.192-199.2004
Wan X-X, Yi H, Qu J-Q et al (2015) Integrated analysis of the differential cellular and EBV miRNA expression profiles in microdissected nasopharyngeal carcinoma and non-cancerous nasopharyngeal tissues. Oncol Rep 34:2585–2601. https://doi.org/10.3892/or.2015.4237
Wang L (2016) Inactivation of type I IFN Jak-STAT pathway in EBV latency. Cancer Biol Treat 3:1–4. https://doi.org/10.24966/CBT-7546/100009
Wang X, Ron D (1996) Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272:1347–1349. https://doi.org/10.1126/science.272.5266.1347
Wang J, Ni Z, Duan Z et al (2014) Altered expression of hypoxia-inducible factor-1α (HIF-1α) and its regulatory genes in gastric cancer tissues. PLoS ONE 9:e99835. https://doi.org/10.1371/journal.pone.0099835
Wang F, Liu J, Zou Y et al (2017) MicroRNA-143-3p, up-regulated in H. pylori-positive gastric cancer, suppresses tumor growth, migration and invasion by directly targeting AKT2. Oncotarget 8:28711–28724. https://doi.org/10.18632/oncotarget.15646
Wang M, Gu B, Chen X et al (2019) The function and therapeutic potential of Epstein–Barr virus-encoded MicroRNAs in cancer. Mol Ther Nucleic Acids 17:657–668. https://doi.org/10.1016/j.omtn.2019.07.002
Wang J, Ge J, Wang Y et al (2022) EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat Commun 13:866. https://doi.org/10.1038/s41467-022-28479-2
Watanabe T, Asano N, Fichtner-Feigl S et al (2010) NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest 120:1645–1662. https://doi.org/10.1172/JCI39481
Watanabe T, Asano N, Kitani A et al (2011) Activation of type I IFN signaling by NOD1 mediates mucosal host defense against Helicobacter pylori infection. Gut Microbes 2:61–65. https://doi.org/10.4161/gmic.2.1.15162
Wen J, Wang Y, Gao C et al (2018a) Helicobacter pylori infection promotes Aquaporin 3 expression via the ROS–HIF-1α–AQP3–ROS loop in stomach mucosa: a potential novel mechanism for cancer pathogenesis. Oncogene 37:3549–3561. https://doi.org/10.1038/s41388-018-0208-1
Wen G, Deng S, Song W et al (2018b) Helicobacter pylori infection downregulates duodenal CFTR and SLC26A6 expressions through TGFβ signaling pathway. BMC Microbiol 18:87. https://doi.org/10.1186/s12866-018-1230-8
Wood VHJ, O’Neil JD, Wei W et al (2007) Epstein–Barr virus-encoded EBNA1 regulates cellular gene transcription and modulates the STAT1 and TGFβ signaling pathways. Oncogene 26:4135–4147. https://doi.org/10.1038/sj.onc.1210496
Wu L, Nakano H, Wu Z (2006) The C-terminal activating region 2 of the Epstein–Barr virus-encoded latent membrane protein 1 activates NF-κB through TRAF6 and TAK1. J Biol Chem 281:2162–2169. https://doi.org/10.1074/jbc.M505903200
Wu M, Lin J, Hsu P et al (2007) Preferential induction of transforming growth factor–β production in gastric epithelial cells and monocytes by Helicobacter pylori soluble proteins. J Infect Dis 196:1386–1393. https://doi.org/10.1086/522520
Ye F, Tang C, Shi W et al (2015) A MDM2-dependent positive-feedback loop is involved in inhibition of miR-375 and miR-106b induced by Helicobacter pylori lipopolysaccharide: H. pylori LPS inhibits miR-375 and miR106b. Int J Cancer 136:2120–2131. https://doi.org/10.1002/ijc.29268
Yin Q, Flemington EK (2006) siRNAs against the Epstein Barr virus latency replication factor, EBNA1, inhibit its function and growth of EBV-dependent tumor cells. Virology 346:385–393. https://doi.org/10.1016/j.virol.2005.11.021
Yin T, Taga T, Tsang ML et al (1950) (1993) Involvement of IL-6 signal transducer gp130 in IL-11-mediated signal transduction. J Immunol Baltim Md 151:2555–2561
Young LS, Dawson CW (2014) Epstein–Barr virus and nasopharyngeal carcinoma. Chin J Cancer. https://doi.org/10.5732/cjc.014.10197
Zauner L (2012) Understanding TLR9 action in Epstein–Barr virus infection. Front Biosci 17:1219. https://doi.org/10.2741/3982
Zervos AS, Faccio L, Gatto JP et al (1995) Mxi2, a mitogen-activated protein kinase that recognizes and phosphorylates Max protein. Proc Natl Acad Sci 92:10531–10534. https://doi.org/10.1073/pnas.92.23.10531
Zhang W, Liu HT (2002) MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12:9–18. https://doi.org/10.1038/sj.cr.7290105
Zhang Z, Li Z, Gao C et al (2008) miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest 88:1358–1366. https://doi.org/10.1038/labinvest.2008.94
Zhang W, Han D, Wan P et al (2016) ERK/c-Jun recruits Tet1 to induce Zta expression and Epstein–Barr virus reactivation through DNA demethylation. Sci Rep 6:34543. https://doi.org/10.1038/srep34543
Zhao L, Vogt PK (2008) Class I PI3K in oncogenic cellular transformation. Oncogene 27:5486–5496. https://doi.org/10.1038/onc.2008.244
Zheng Z-M (2010) Viral oncogenes, noncoding RNAs, and RNA splicing in human tumor viruses. Int J Biol Sci. https://doi.org/10.7150/ijbs.6.730
Zhou L, Bu Y, Liang Y et al (2016) Epstein–Barr virus (EBV)-BamHI-a rightward transcript (BART)-6 and cellular MicroRNA-142 synergistically compromise immune defense of host cells in EBV-positive burkitt lymphoma. Med Sci Monit 22:4114–4120. https://doi.org/10.12659/MSM.897306
Zhu C, Zhu Q, Wang C et al (2016) Hostile takeover: Manipulation of HIF-1 signaling in pathogen-associated cancers (Review). Int J Oncol 49:1269–1276. https://doi.org/10.3892/ijo.2016.3633
Zwezdaryk KJ, Combs JA, Morris CA, Sullivan DE (2016) Regulation of Wnt/β-catenin signaling by herpesviruses. World J Virol 5:144–154. https://doi.org/10.5501/wjv.v5.i4.144
Acknowledgements
We gratefully acknowledge the DST-FIST Project No. SR/FST/LS-I/2020/621 and Indian Institute of Technology Indore for providing facilities and support. This project was supported by the Department of Science and Technology DST-EMR project no. DST-EMR: EMR/2017/001637. We are thankful to CSIR, UGC, and DBT for fellowship to Dharmendra Kashyap, Pranit Hemant Bagde, and Vaishali Saini respectively in the form of a research stipend. We appreciate Ms. Annu Rani, Dr. Tarun Prakash Verma, Samiksha Rele, Siddharth Singh, Sonali Adhikari, and our other laboratory colleagues for insightful discussions and advice.
Funding
This project was supported by the Department of Science and Technology grant no. DST-EMR: EMR/2017/001637 and Center for Rural Development and Technology, IIT Indore grant no. IITI/CRDT/2022-23/05.
Author information
Authors and Affiliations
Contributions
HCJ and DK contributed to the design, data acquisition, analysis, conceptualization, interpretation, and drafted. VS, PB and SR contributed to the analysis, interpretation, and drafting. DC, AKJ and RKP critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest associated with this article.
Ethical approval
Not applicable.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashyap, D., Rele, S., Bagde, P.H. et al. Comprehensive insight into altered host cell-signaling cascades upon Helicobacter pylori and Epstein–Barr virus infections in cancer. Arch Microbiol 205, 262 (2023). https://doi.org/10.1007/s00203-023-03598-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03598-6