Abstract
The discovery of new antimicrobials is the prime target in the fight against antimicrobial resistance. The continuous search for new lead compounds from bacteria of untapped and extreme ecosystems such as mangroves is currently being undertaken. This study describes the metabolite profiling of the Streptomyces euryhalinus culture extract. Previously, Streptomyces euryhalinus was isolated from the mangrove forest of Indian Sundarbans as a novel microorganism. The antimicrobial mechanism of action of Streptomyces euryhalinus culture extract against bacteria and fungi has been analyzed in this study. The gas chromatography–mass spectrometry profile of the ethyl acetate extract bacterial culture displayed the presence of several bioactive compounds with antibacterial, antifungal and antioxidant properties. The bacterial extract showed significant antimicrobial activity in terms of zone of inhibition, minimum inhibitory concentration, minimum bactericidal concentration, and minimum fungicidal concentration. Moreover, substantial capacity to alter or damage the inner membrane as well as the outer membrane of the gram-positive and gram-negative bacteria was exhibited by the bacterial extract. This membrane alteration or damaging potential of the extract is the mechanism of action. Biofilm formation inhibition property of the extract also signified its antimicrobial action, and possible use against resistant bacteria. The extract has shown notable activity against the virulence factors like prevention of hemolysis in bacteria and inhibition of secreted aspartyl proteinase in fungi. These functions of the bacterial extract have revealed the extent of its action in the prevention of infection by terminating the secretory virulence factors and by damaging the tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial infections are a grave concern to the world and their treatments are posing a serious threat to human life due to multidrug resistance. Methicillin-resistant Staphylococcus aureus (MRSA) has caused the spread of community-associated infections with increased multidrug resistance (Thomas 1994). In a review, Lord Jim O’Neill (2014) has warned that antibiotic resistance can cause the death of 10 million people by 2050. To reverse the situation or to prevent death from multidrug-resistant pathogens, new antibiotics are the need of the hour. Presently the discoveries of new antibiotics are limited due to the exit of the pharmaceutical companies from this area of research and lack of interest from the other research communities (Cooper and Shlaes 2011). Investigations on terrestrial microorganisms or microorganisms from traditional habitats for the discovery of new antibiotics have proved to be constrained options as it frequently leads to the rediscovery of old molecules and appeared to be less effective to treat multidrug-resistant infections (Xu 2012; Debbab et al. 2011). Currently, the search of new antibiotics has shifted from traditional habitats to new or extreme environment or ecology like marine, desert, or mangroves (Xu 2012, 2014). Mangroves are the locus of rich microbial diversity due to their distinctive characters like high rainfall, tidal flooding, light, salinity, and temperature (Feller et al. 2010; Andreote et al. 2012). Mangrove forests encompass numerous microorganisms and plants which could be the potential sources of new bioactive molecules owing to their distinct ecological and environmental attributes (Huang et al. 2012; Arumugam et al. 2010; Xu et al. 2014). Several novel bacteria and fungi have already been identified and reported from different mangrove forests (Biswas et al. 2017; Wei et al. 2011).
The Sundarbans is the largest tidal halophytic mangrove forest in the world and covers nearly 10,200 sq. km. in Bangladesh and India (Gopal and Chauhan 2006). The estuarine habitats of the Sundarbans harbor diverse biological entities with a constant supply of nutrients and biogeochemical cycling (Meire et al. 2005). Spatial differences and seasonal variations in biogeochemical processes help the region to maintain and produce the high biodiversity in this ecosystem. Though the Sundarbans is a detritus-based rich ecosystem, minimal data are available concerning its microbial diversity and abundance (Ghosh et al. 2010). Limited researches were carried out to date on bacterial identification and isolation of bioactive compounds from the Indian Sundarbans. However, few studies have highlighted the potential of the Sundarbans to yield novel bacteria and bioactive compounds (Arumugam et al. 2010, 2011; Biswas et al. 2017). The search for bacterial diversity and their novel bioactive metabolites from the Indian Sundarbans can address the global crisis of multidrug-resistant infections.
Actinomycetes are well-known producers of different secondary metabolites like antibiotics. Xiamycin, Salinosporamide A, Chalcomycin B, Antimycin A18, etc., are examples of the molecules produced by mangrove-derived actinomycetes (Xu et al. 2014). Streptomyces euryhalinus sp. nov. was isolated from the sediment sample of the Lothian Island of the Indian Sundarbans. It is a Gram-positive, aerobic bacteria which can grow with 0–20% of sodium chloride and is resistant to penicillin (Biswas et al. 2017). The present research is focused on the metabolite profiling and assessment of antibacterial and antifungal properties of Streptomyces euryhalinus culture extract. This study also analyzed the activities of the culture derived metabolites on the permeability of the bacterial membrane along with the termination of secretion of virulence factors by bacteria and fungi.
Methods
Collection of bacteria
Streptomyces euryhalinus (Biswas et al. 2017) was isolated from the sediment soil of the Lothian Island (Lat. 20° 50′ N, Long. 88° 19′ E) of the Indian Sundarbans (Saha et al. 2005). It was grown on storage medium (units: g/L: starch (potato) 10.0, casein (protein rich) 3.0, peptone 1.0, malt 1.0, yeast extract 1.0, dipotassium phosphate 0.5, distilled water 500 ml and artificial sea water 500 ml, agar 2%, pH 7.2) described by Biswas et al. (2017). The strain was incubated at 28 °C for 4 days for optimum growth. It is available in the Leibniz Institute DSMZ–German Collection of Microorganisms and Cell Cultures (DSM 103378T) and Chinese Center for Industrial Culture Collection (CICC 11032T). For lab storage, cultures of the strain were preserved in glycerol stocks (10–15%) in – 80 °C and − 20 °C freezers as well as in lyophilized powder form.
Fermentation and extraction
Production of the antimicrobial component from the strain S. euryhalinus was carried out with the shake flask method as described by Saha et al. (2005) using a modified production medium (MPM: glucose 2.0, starch 2.0, yeast extract 0.5, soybean meal 2.0, CaCO3 0.32, NaCl 0.25, CuSO4 0.005, ZnSO4 0.005, MnCl2 0.005, distilled water 1000 ml, pH 7.2, units expressed in g/L) and incubated for 72–96 h at 30 °C. The filtered broth was extracted thrice using equal volume of ethyl acetate as solvent. Finally, the ethyl acetate extract was concentrated at 45 °C using rotary vacuum evaporator to get the crude extract.
GC–MS analysis of S. euryhalinus extract
Gas chromatography (GC) analysis of culture extract obtained from S. euryhalinus was performed using Perkin Elmer Clarus SQ8C gas chromatograph equipped with DB 5 MS capillary standard nonpolar column (30 m × 0.25 mm × 0.25 µm film thickness). Helium at a flow rate of 1 mL/min was selected as a carrier gas and the split mode ratio was kept as 1:12. The injector temperature was fixed at 250 °C and the obtained peaks were identified by NIST library data.
Antimicrobial activity
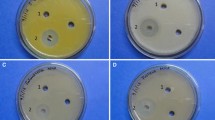
The antimicrobial activity of the ethyl acetate extract of S. euryhalinus was primarily evaluated by the agar well diffusion method (Hriduyatulla et al. 2018). About 20 µl of exponential phase cultures of Staphylococcus aureus MTCC 2940, E. coli MTCC-1195 and Candida albicans MTCC 227 were spread onto the nutrient agar and Sarbouraud dextrose agar plates. Different concentrations of crude extract were placed into the wells. The plates were then placed overnight in an incubator at 37 °C and the clearance zones were noted as an indicator of antimicrobial activity.
Determination of minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC)
The MIC values of the bacterial extract were evaluated against S. aureus MTCC 2940, E. coli MTCC-1195, and Candida albicans MTCC 227 following micro broth dilution method in 96-well microtiter plates (Sarker et al. 2007; Weigand et al. 2008). The bacterial and fungal strains were cultivated in Muller–Hinton Broth (MHB) and Sabouraud dextrose broth (SDB), respectively and incubated at 37 °C for 18 h. The turbidity for the test microbial cultures was adjusted to 106 CFU per ml. Total 50 µl of MHB or SDB, 50 µl of extracts, and 10 µl of microbial cultures were added to each well making the final concentration of 5 × 105 CFU per ml. Resazurin (10 µl stock solution prepared by dissolving 270-mg tablet in 40-ml sterile distilled water) was added to the each well of microtiter plates after 18 h of incubation at 37 °C. The change of colour was observed and the lowest concentration with no change in color was marked as the MIC value. Minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) were determined by spreading cultures from the wells onto the corresponding plates. MBC or MFC of the samples was determined by observing the plates with a minimum concentration of the extract having no colony growth (Devi et al. 2010; Sundararaman et al. 2013).
Activity of S. euryhalinus extract on bacterial membrane permeability
Bacterial membrane integrity assay
The effect of the extract on bacterial cell membrane integrity was studied by recording the discharge of internal material which shows maximum absorbance at 260 nm (Li et al. 2014). Bacterial strains S. aureus MTCC 2940 and E. coli MTCC 1195 were used as test organisms. The bacterial cultures grown in nutrient broth were centrifuged at 8000 rpm for 10 min, cell pellets were washed and suspended in 0.5% NaCl. Specific concentrations (½ MIC, MIC, 2MIC) of S. euryhalinus extracts were added to the cell suspension and release of internal material was determined using a UV–visible spectrophotometer at 260 nm (Lambda 25 UV/visible spectrophotometer; Perkin Elmer, USA) for a period of 12 h. Cell suspensions of test organisms not treated with the bacterial extract were considered as controls.
Crystal violet assay for membrane permeability
Membrane permeability alternation capacity of the bacterial extract was evaluated using crystal violet assay (Li et al. 2014) with S. aureus MTCC 2940 and E. coli MTCC 1195. The bacterial strains were grown in nutrient broth and centrifuged at 9300g for 5 min to collect the cell masses. The bacterial cell pellets were washed and suspensions were prepared using 0.5% NaCl solution. Bacterial extract of ½ MIC, MIC, and 2 MIC doses were added in the cell suspension and incubated for 6 h at 37 °C. Centrifugation of the treated cell suspensions was carried out at 9300g for 5 min to collect the cell masses. The bacterial cells were suspended into 0.5% NaCl solution containing crystal violet (10 µg/ml), and finally incubated at 37 °C for 10 min. Finally, the optical density of the supernatant obtained after centrifugation of treated bacterial suspension at 13,400g for 15 min was measured using UV–Visible spectrophotometer at 590 nm. The crystal violet uptake of samples (as a percentage) was calculated as:
(Sample OD value)/(crystal violet solutions OD value) × 100.
Bacterial inner membrane permeability assay
Discharge of cytoplasmic β-galactosidase in the culture medium by E. coli MTCC-1195 and S. aureus MTCC 2940 which was complemented with substrate o-nitrophenyl –β-D-galactoside (ONPG) after treating with bacterial extract was determined as inner membrane permeabilization (Ibrahim et al. 2000). Bacterial culture has grown in Luria–Bertani (LB) broth supplemented with 2% lactose was collected at logarithmic phase and centrifuged for 10 min at 11,000 g, and suspended in 0.5% NaCl solution after washing. Cell suspension (200 µl), ONPG (10 µl), and bacterial extract of different concentrations (1/2 MIC, MIC, 2 MIC) were taken in a 96-well plate. Release of o-nitrophenol was recorded using a spectrophotometer (Lambda 25 UV/visible spectrophotometer; PerkinElmer, USA) at 415 nm. Bacterial culture suspension including extract was considered as control.
Evaluation for antibiofilm capacity
Inhibition of biofilm formation assay of the extract was carried out at different concentrations in 96-well microtiter plates. Cultures of S. aureus MTCC 2940 and E. Coli MTCC1195 were grown overnight in Luria broth and different concentrations (1/2MIC, MIC, 2MIC) of extract were added in 96-well microtiter plates which were incubated in a static position for 24 h at 37 °C. Thereafter the free-floating cells along with excess broth were discarded and dried for 30 min at 60 °C after washing with phosphate buffer solution (PBS) (pH 7.2). Crystal violet solution (1%) was used to stain the biofilm produced in the wells for 15 min. After washing with distilled water, crystal violet bound to the biofilm was resolubilized with 200 µl of ethanol and the absorbance was recorded at 595 nm with the help of a microplate reader (Spectra Max M5, Molecular Device, USA) (Wu et al. 2015, 2016).
Effects of S. euryhalinus extract on virulence factors produced by S. aureus MTCC 2940 and E. coli MTCC 1195
Hemolysis assay
Hemolytic toxicity of the bacterial extract was evaluated using murine erythrocytes. About 10 ml of murine blood was centrifuged for 5 min at 2500 rpm, and pellets were cleansed using PBS. Erythrocyte suspension (3%) was prepared using PBS. Different concentrations (1/2 MIC, MIC and 2MIC) of bacterial extract were added to the erythrocyte suspension (10 µl). The supernatant was collected after centrifugation of the mixture at 2500 rpm for 5 min and the optical density was recorded spectrophotometrically (Lambda 25 UV/visible spectrophotometer; PerkinElmer, USA) at 540 nm for the determination of hemoglobin released (Kaur et al. 2017). Erythrocyte suspension along with sterile distilled water was considered as blank.
Secreted aspartyl proteinase (Sap) inhibition assay using C. albicans
Candidal Sap inhibition activity of the bacterial extract was evaluated by following the method of Sundararaman et al. (2013). Mixture of C. albicans culture (100 µl), Sap induction medium (20 ml) (2.0 g yeast extract, 23.4 g yeast carbon base, and 4.0 g BSA in 1 L distilled H2O, pH 5.0) was treated with different concentrations (1/2 MIC, MIC, and 2MIC) of bacterial extract under the incubation at 37 °C for 48 h with shaking. Thereafter, culture supernatant (0.1 ml) was added to 0.9 ml medium which comprised of citrate buffer (0.1 M, pH 3.2) and BSA (0.2% w/v) which was incubated for 1 h at 37 °C for evaluation of proteinase inhibition. About 1 ml of 5% (w/v) ice-cold TCA was added into the mixture to cease the reaction. Centrifugation of the mixture was carried out for ten minutes at 1500g and optical density of the supernatant was recorded at 280 nm using a UV–visible spectrophotometer (Lambda 25 UV/visible spectrophotometer; PerkinElmer, USA). Sterile distilled water was considered as control.
Statistical analysis
Experiments were performed in triplicate, and results are presented as mean ± SD.
Results and discussion
GC–MS profile of extract
GC–MS study revealed the presence of several bioactive compounds in the ethyl acetate extract of S. euryhalinus. Different known compounds like triclosan, 2,4-di-tert-butylphenol, dibutyl phthalate, phenanthrene, 16-methyl-, methyl ester, 17-pentatriacontene, 7,9-di-tert-butyl -1-oxaspiro (4,5) deca-6,9-diene-2,8-dione, benzenepropanoic acid 3,5-bis (1,1–148 dimethylethyl)-4-hydroxy- methyl ester, heptadecanoic, etc., were present which are known for their antioxidant, antibacterial, antimicrobial, antibiofilm and antifungal activities (Table 1) (Kovács et al. 2008; Padmavathia et al. 2015; Varsha et al. 2015). Compounds with the percentage of an area more than 0.284 (%) in the GC–MS spectrogram of the bacterial extract were selected for determination. The presence of unknown or new compounds in the extract cannot be ruled out though it is not indicated in the result after GC–MS.
MIC, MBC and MFC of the extract
Zone of inhibition of extract against S. aureus MTCC 2940, E. coli MTCC 1195, and Candida albicans MTCC 227 in agar well diffusion method was found to be 25 mm, 20 mm and 14 mm, respectively. MIC of the extract against S. aureus MTCC 2940, E. coli MTCC-1195, and C. albicans MTCC 227 was recorded as 1 μg/ ml, 2 μg/ ml and 2 μg/ ml. MBC of the extract determined is 4 µg/ml and 8 µg/ml against S. aureus MTCC 2940 and E. coli MTCC 1195, respectively. MFC of the extract against C. albicans MTCC 227 was 6 µg/ml.
Effects of S. euryhalinus extract on bacterial cell membrane integrity
Disfigurement of the bacterial membrane was evaluated by the release of UV absorbing intracellular components. The S. euryhalinus extract showed the highest membrane damage activity at 2 MIC concentration against the test bacteria. The OD value increased from 0.081 to 0.412 at 2MIC concentration and at MIC concentration it was increased up to 0.379 in case of E. coli. In case of S. aureus, OD value reached 0.496 from 0.194 at 2MIC concentration whereas at MIC concentration OD value reached 0.412. (Fig. 1a, b). Results indicated that the presence of S. euryhalinus extract decreases the rise of absorbance and it remains nearly constant over a prolonged time in case of Gram-negative bacteria. Contrarily, the absorbance changed abruptly and increased slowly after a long period in case of Gram-positive bacteria. This occurred as a result of dissimilarities in the membrane structure of gram-positive and gram-negative bacteria. Gram-positive bacteria have peptidoglycan layer with several pores which facilitate the entry of antimicrobial molecules into cell and thereby cause cellular damage (Tao et al. 2011). Results also corroborated damage of the cell membrane by the S. euryhalinus extract after interaction with cytoplasmic membrane (Denyer 1990). Damage of cell membrane causes release of K+, PO4–, DNA, RNA, etc. (Li et al. 2014).
a Cell membrane permeability effect of S. euryhalinus extract on E. coli MTCC 1195. Error bars are displaying the ± SD of three replicates. b Cell membrane permeability effect of S. euryhalinus extract on S. aureus MTCC 2940. Error bars are displaying the ± SD of three replicates *p value < 0.05, **p value < 0.005, ***p value < 0.001
Crystal violet assay of S. euryhalinus extract on bacterial membrane permeability
Crystal violet assay is helpful to evaluate the alteration in the bacterial membrane by the antimicrobial agents. In case of an altered or damaged membrane, crystal violet penetrates the membrane easily whereas it fails to penetrate if the membrane is intact. The S. euryhalinus extract at 2 MIC concentration exhibited an increment of crystal violet uptake from 18.59 (control) to 74.49%, in case of E. coli, and in case of S. aureus, it is increased from 19.09 (control) to 79.63% (Fig. 2). Results exhibited by S. euryhalinus extract are comparable with studies conducted by Li et al. (2014), Sana et al. (2018). Extract showed higher membrane alteration property at MIC dose in comparison with carvacrol and ciprofloxacin (Khan et al. 2017; Sana et al. 2018). This result displayed the significant alteration in bacterial membrane permeability and membrane damage property of S. euryhalinus extract.
Activity of S. euryhalinus extract on the permeability of the bacterial inner membrane
The capacity of the antimicrobials for permeability or penetration of bacterial inner membrane is manifested by the release of cytoplasmic beta-galactosidase in both gram-positive and gram-negative bacteria. The obtained results revealed discharge of cytoplasmic beta-galactosidase is dose and time-dependent (Fig. 3a, b). Greater absorbance was found at 2MIC concentration than MIC concentration which signifies that S. euryhalinus extract showed a greater inner membrane permeability effect at dose of 2MIC. The obtained results showed a sharp increase of absorbance up to 80 min for gram-positive and gram-negative bacteria, followed by steady-state. This result is significant and comparable with the research conducted by Je and Kim (2006) and Ibrahim et al. (2000). These empirical values indicate occurrences of changes or damage of the inner membrane of bacteria by the extract. Therefore, S. euryhalinus extract has the potential of causing both inner as well as outer membrane alterations of the targeted bacterial strains.
a Evaluation of permeability of inner cell membrane recorded through release of cytoplasmic β- galactosidase from E. coli MTCC 1195 upon treatment with S. euryhalinus extract. Error bars are displaying the ± SD of three replicates. b Evaluation of permeability of inner cell membrane recorded through release of cytoplasmic β- galactosidase from S. aureus MTCC 2940 upon treatment with S. euryhalinus extract. Error bars are displaying the ± SD of three replicates *p value < 0 .05, **p value < 0.005, ***p value < 0.001
Inhibition of biofilm formation by S. euryhalinus extract
Biofilm formation by the microbes or microbial communities is directly involved with antibiotic resistance (Michalska and Wolf 2015; Simones et al. 2009). S. euryhalinus extract demonstrated notable biofilm inhibition activity in a dose-dependent manner. In case of E. coli, inhibition of biofilm formation at MIC and 2 MIC dose was observed as 53.64% (± 0.60) and 67.90% (± 0.027), respectively (Fig. 4a). 2 MIC doses of the extract displayed 78. 49% (± 0.037) inhibition of biofilm formation against S. aureus whereas 62.99% (± 0.049) inhibition was observed at MIC concentration (Fig. 4b). The antibiofilm potential of S. euryhalinus extract can be compared with earlier researches (Kaur et al. 2017; Yadav et al. 2015). Extract with significant antibiofilm activity could be a potential agent to fight against the biofilm forming bacterial pathogens with antibiotic resistance.
a The inhibition of E. coli MTCC 1195 biofilm formation by S. euryhalinus extract recorded at 595 nm. Error bars are displaying the ± SD of three replicates. b The inhibition of S. aureus MTCC 2940 biofilm formation by S. euryhalinus extract recorded at 595 nm. Error bars are displaying the ± SD of three replicates, *p < 0.005, **p < 0.001
Hemolysis activity of S. euryhalinus extract
Reduction of hemolysis was displayed by extract at 2 MIC dose from 61.29% (± 0.0009) to 18.28% (± 0.0002) in case of S. aureus (Fig. 5). In case of E. coli, hemolysis is reduced from 68.88% (± 0.002) to 19.6% (± 0.001) at 2 MIC concentration (Fig. 5). Hemolysin toxin is a virulence factor produced by the pathogenic bacteria which helps to expedite the infection (Kupferwasser et al. 2003). This result may be attributed to the capacity of S. euryhalinus extract for inhibition of bacterial virulence factor.
Action of extract on secreted aspartyl proteinase (Sap) inhibition
The S. euryhalinus extract showed a gradual drop of Sap inhibition activity in C. albicans. The 2 MIC dose of the extract was found to exhibit the highest inhibition activity among other concentrations. The effective concentration of the extract was comparatively lower than that of standard drugs (Fig. 6). The observed results were found to be comparable with previous studies (Schaller et al. 2003; Sundararaman et al. 2013). Proteinase activity of C. albicans corresponded to the virulence factor (MacDonald and Odds 1983), the tissue invasion, and damage capacity (Schaller et al. 1999; Borg and Rüchel 1988). Therefore S. euryhalinus extract at 2 MIC dose could suppress virulence factor and tissue damage capacity of C. albicans.
Conclusion
New or novel antibiotics are indispensable to fight against antibiotic resistance. Actinomycetes are a promising source of bioactive metabolites. Mangrove being a unique ecology, may serve as the potential supplier of new or novel bacteria and their secondary metabolites. GC–MS profile of ethyl acetate extract obtained from S. euryhalinus culture has shown the presence of several important metabolites which are biologically active. The S. euryhalinus extract exhibited significant MIC, MBC and MFC values against both bacteria and fungi. This study showed that ethyl acetate extract can alter or damage the inner membrane as well as the outer membrane of gram-negative and gram-positive bacteria. 2 MIC concentration of the extract has shown the highest activity in bacterial membrane damage. The membrane damaging properties of S. euryhalinus extract were found more potent against gram-positive bacteria than gram-negative bacteria due to dissimilarities in their cell membrane structure. Antibiofilm formation capacity of the extract validated antimicrobial activity in relation to antibiotic resistance. Hemolysis prevention and Sap inhibition activities of the bacterial extract have strengthened the evidence of prevention or termination of bacterial and fungal virulence factors. This bioassay guided comprehensive study indicated that the ethyl acetate extract obtained from S. euryhalinus has potent antimicrobial activity expressed through cell membrane destruction mechanism.
References
Andreote FD, Jiménez DJ, Chaves D, Dias Franco AC, Luvizotto DM, Dini-Andreote F, Fasanella CC, Lopez MV, Baena S, Taketani RG, de Melo IS (2012) The microbiome of Brazilian mangrove sediments as revealed by metagenomics. PloSOne 7:e38600
Arumugam M, Mitra A, Jaisankar P, Dasgupta S, Sen T, Gachhui R, Mukhopadhyay UK, Mukherjee J (2010) Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl Microbiol Biotechnol 86:109–117
Arumugam M, Mitra A, Pramanik M, Saha M, GachhuiMukjerjeea RJ (2011) Streptomyces sundarbansensis sp. Nov., a novel actinomycete that produces 2-allyloxyphenol. Int J Syst Evol Microbiol 61:2664–2669
Biswas K, Chowdhury JD, Mahansaria R, Mukherjee J (2017) Streptomyces euryhalinus sp. nov., a new actinomycete isolated from a mangrove forest. J Antibiot 70:747–753
Borg M, Rüchel R (1988) Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun 56:626–631
Cooper MA, Shlaes D (2011) Fix the antibiotics pipeline. Nature 472:32
Debbab A, Aly AH, Proksch P (2011) Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers 49:1–12
Denyer SP (1990) Mechanisms of action of biocides. Int Biodeterior 26:89–100
Devi KP, Nisha SA, Sakthivel R, Pandian SK (2010) Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol 130:107–115
Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC (2010) Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2:395–417
Ghosh A, Dey N, Bera A et al (2010) Culture independent molecular analysis of bacterial communities in the mangrove sediment of Sundarban. India Saline Syst 6:1
Gopal B, Chauhan M (2006) Biodiversity and its conservation in the Sundarban mangrove ecosystem. Aquat Sci 68:338–354
Hidayathulla S, Shahat AA, Ahamad SR, Al Moqbil AAN, Alsaid MS, Divakar DD (2018) GC/MS analysis and characterization of 2-Hexadecen-1-oland beta sitosterol from Schimpera arabica extract for its bioactive potential as antioxidant and antimicrobial. J Appl Microbiol 124:1082–1091
Huang Z, Yang J, Cai X et al (2012) A new furanocoumarin from the mangrove endophytic fungus Penicillium sp. (ZH16). Nat Prod Res 26:1291–1295
Ibrahim HR, Sugimoto Y, Aoki T (2000) Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria througha membrane damage mechanism. Biochim Biophys Acta 1523:196–205
Je JY, Kim Sk (2006) Antimicrobial action of novel chitin derivative. Biochem Biophys Acta 1760:104–109
Kaur G, Balamurugan P, Vasudevan S, Jadav S, Princy SA (2017) Antimicrobial and antibiofilm potential of acyclic amines and diamines against multi-drug resistant Staphylococcus aureus. Front Microbiol 8:1767
Khan I, Bahuguna A, Kumar P, Bajpai VK, Kang SC (2017) Antimicrobial potential of carvacrol against uropathogenic Escherichia coli via membrane disruption, depolarization, and reactive oxygen species generation. Front Microbiol 8:2421
Kovács A, Vasas A, Hohmann J (2008) Natural phenanthrenes and their biological activity. Phytochemistry 69:1084–1110
Kupferwasser LI, Yeaman MR, Nast CC, Kupferwasser D, Xiong YQ, Palma M, Cheung AL, Bayer AS (2003) Salisylic acid attenuates virulence in endovascular infection by targeting global regulatory pathways in Staphylococcus aureus. J Clin Invest 112:222–223
Li N, Sheng-Nan T, Cui J, Guo N, Wang W, Yuan-gang Z, Jin S, Xian-xiu X, Liu Q, Yu-jie F (2014) PA-1, a novel synthesized pyrrolizidine alkaloid, inhibits the growth of Escherichia coli and Staphylococcus aureus by damaging the cell membrane. J Antibiot 67:689–696
MacDonald F, Odds FC (1983) Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol 129:431–438
Meire P, Ysebaert T, Van Damme S et al (2005) The Scheldt estuary: a description of a changing ecosystem. Hydrobiologia 540:1–11
Michalska M, Wolf P (2015) Pseudomonas Exotoxin A: optimized by evolution for effective killing. Front Microbiol 6:963
O’Neill J (2014) Review on antimicrobial resistance antimicrobial resistance: tackling a crisis for the health and wealth of nations. Review on Antimicrobial Resistance, London
Padmavathia AR, Bakkiyaraja D, Thajuddinb N, Pandian SK (2015) Effect of 2, 4-di-tert-butylphenol on growth and biofilm formation by an opportunistic fungus Candida albicans. Biofouling 31:565–574
Saha M, Ghosh D Jr, Ghosh D, Garai D, Jaisankar P, Sarkar KK, Dutta PK, Das S, Jha T, Mukherjee J (2005) Studies on the production and purification of an antimicrobial compound and taxonomy of the producer isolated from the marine environment of the Sundarbans. Appl Microbiol Biotechnol 66:497–505
Sana S, Datta S, Biswas D, Sengupta D (2018) Assessment of synergistic antibacterial activity of combined biosurfactants revealed by bacterial cell envelop damage. Biochim Biophys Acta Biomembr 1860:579–585
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitreplate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324
Schaller M, Korting HC, Schafer W, Bastert J, Chen W, Hube B (1999) Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral Candidosis. Mol Microbiol 34:169–180
Schaller M, Krnjaic N, Niewerth M, Hamm G, Hube B, Korting HC (2003) Effect of antimycotic agents on the activity of aspartyl proteinases secreted by Candida albicans. J Med Microbiol 52:247–249
Simoes M, Bennett RN, Rosa EAS (2009) Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep 26:746–757
Sundararaman M, Kumar RR, Venkatesan P, Ilangovan A (2013) 1-Alkyl-(N, N-dimethylamino) pyridinium bromides: inhibitory effect on virulence factors of Candida albicans and on the growth of bacterial pathogens. J Med Microbial 62:241–248
Tao Y, Qian L-H, Xie J (2011) Effect of chitosan on membrane permeability and cell morphology of Pseudomonas aeruginosa and Staphyloccocus aureus. Carbohydr Polym 86:969–974
Tomasz A (1994) Multiple-antibiotic resistant pathogenic bacteria. N Engl J Med 330:1247–1251
Varsha KK, Devendra L, Shilpa G, Priya S, Pandey A, Nampoothiri KM (2015) 2,4-Di-tert-butyl phenol as the antifungal, antioxidant bioactive purified from a newly isolated Lactococcus sp. Int J Food Microbiol 211:44–50
Wei YH, Liou GY, Liu HY, Lee FL (2011) Sympodiomycopsis kandeliae sp. nov., a basidiomycetous anamorphic fungus from mangroves, and reclassification of Sympodiomycopsis lanaiensis as Jaminaea lanaiensis comb. nov. Int J Syst Evol Micrbiol 61:469–473
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175
Wu S, Liu G, Zhang D, Li C, Sun C (2015) Purification and biochemical characterization of analkaline protease from marinebacteria Pseudoalteromonas sp. J Basic Microbiol 55:1427–1434
Wu S, Liu G, Jin W, Xiu P, Sun C (2016) Antibiofilm and anti-infection of a marine bacterial exopolysaccharide against Pseudomonas aeruginosa. Front Microbiol 7:102
Xu MJ, Liu XJ, Zhao YL, Liu D, Xu ZH, Lang XM, Ao P, Lin WH, Yang SL, Zhang ZG, Xu J (2012) Identification and characterization of an anti-fibrotic benzopyran compound isolated from mangrove-derived Streptomyces xiamenensis. Mar Drugs 10:639–646
Xu DB, Ye WW, Han Y, Deng Z-X, Hong K (2014) Natural products from mangrove actinomycetes. Mar Drugs 12:2590–2613
Yadav MK, Chae SW, Im GJ, Chung JW, Song JJ (2015) Eugenol: a phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 10(3):e0119564
Acknowledgements
We are thankful to Council of Scientific and Industrial Research for funding this research (Grant No. 09/096(0717)/2012-EMR-I).
Author information
Authors and Affiliations
Contributions
KB conceptualized and performed all experiments, and framed the article, DB supported to perform the cell membrane related experiments, interpretation of data and reviewed the article, MS helped to analyses the data and reviewed the article, JM conceptualized the study, supervised the study, and reviewed the article, SK supported in antibiofilm assay and hemolysis assay, and reviewed the article.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest.
Accession numbers
Chinese Centre for Industrial Culture Collection 11032T, Leibniz Institute DSMZ–German Collection of Microorganisms and Cell Cultures DSM 103378T.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biswas, K., Bhattarcharya, D., Saha, M. et al. Evaluation of antimicrobial activity of the extract of Streptomyces euryhalinus isolated from the Indian Sundarbans. Arch Microbiol 204, 34 (2022). https://doi.org/10.1007/s00203-021-02698-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02698-5