Abstract
Nisin is a small peptide produced by Lactococcus lactis ssp lactis that is currently industrially produced. This preservative is often used for growth prevention of pathogenic bacteria contaminating the food products. However, the use of nisin as a food preservative is limited by its low production during fermentation. This low production is mainly attributed to the multitude of parameters influencing the fermentation progress such as bacterial cells activity, growth medium composition (namely carbon and nitrogen sources), pH, ionic strength, temperature, and aeration. This review article focuses on the main parameters that affect nisin production by Lactococcus lactis bacteria. Moreover, nisin applications as a food preservative and the main strategies generally used are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid bacteria (LAB) are Gram-positive, non-spore forming, and catalase-lacking bacteria with cocci or rods morphology. LAB produce lactic acid as a main end product during carbohydrates fermentation. They grow only in complex environments, where fermentable carbohydrates and polyols are used as an energy source. Homofermentative LAB degrade hexoses to lactate, whereas heterofermentative ones degrade hexoses to lactate and other products such as CO2, acetate, formate, succinate or ethanol (Mattarelli et al. 2014).
LAB are widely used as starter-cultures in the food industry to produce fermented foods, including dairy products (yogurt, cheese), meat (sausage), grains (bread and drinks such as beer), fruits (malolactic fermentation in wine) and vegetables (sauerkraut, kimchi, silage). Most LAB are generally recognized as safe (GRAS) (George et al. 2018). Moreover, LAB are used to develop new sensory properties, improve the nutritional quality of foods, but also to preserve and ensure food safety. In fact, LAB have a strong antimicrobial activity against many related and unrelated microorganisms, including food spoiling microorganisms and pathogenic bacterial strains such those belonging to Listeria, Staphylococcus, Clostridium, and Bacillus spp. The antimicrobial effect of LAB is mainly due to the food pH lowering, competition for nutrients, and production of inhibitory metabolites (Wedajo 2015; Srivastava 2018; Bintsis 2018; Kaczmarek et al. 2019).
Bacteriocins are protein molecules with a broad activity spectrum but mainly against species phylogenetically close to the producing strain. Among the bacteriocin producing bacteria, strains belonging to the genera of Lactococcus, Lactobacillus, Pediococcus, Leuconostoc and Enterococcus are the most studied (Table 1). Lactococcus lactis subsp lactis produces nisin and lacticin 3147, two of the most extensively characterized lantibiotics (Table 1). Nisin is a one peptide antimicrobial composed of 34 amino acids. Lacticin 3147 is a two-peptide lantibiotic consisting of both LtnA1 and LtnA2, composed of 30 and 29 amino acids, respectively (Piper et al. 2009). Nisin has a wide spectrum of activity against gram-positive bacteria, such as Staphylococcus aureus, Listeria monocytogenes and Clostridium species (Vukomanović et al. 2017). Indeed, lacticin 3147 has shown an inhibitory activity against Listeria monocytogenes, Bacillus cereus, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecalis, penicillin-resistant Pneumococcus, Propionibacterium acnes and Streptococcus mutans (Ryan et al. 1996; Galvin et al. 1999). Unlike nisin which is poorly soluble, and thus less active, at pH 7, lacticin 3147 demonstrated greater potential as a therapeutic agent regarding its high activity at physiological pH 7 (Galvin et al. 1999; Cotter et al. 2005).
Bacteriocin producing bacteria are often used for all their above mentioned classic properties together, became the last few decades to be used for the production of bacteriocins. To achieve this goal, several basic and applied studies have helped to identify, categorize and better understand the biosynthesis mechanisms of these macromolecules. Bacteriocins are peptides produced by LAB to defend themself and are a part of innate immunity possessed by certain bacterial species. The main differences between bacteriocins and antibiotics are that antibiotics are not ribosomally synthesized, their mode of action is quite different, their antimicrobial spectrum is very diverse, and their applications are rather clinical than for food preservation (Cleveland et al. 2001; Cotter et al. 2013). However, bacteriocins are extracellular proteins synthesized by ribosomal pathways and having a bactericidal activity directed mainly against Gram-positive bacteria and the productive strain has specific protective mechanisms against their own bacteriocins (Cotter et al. 2013).
Nisin is the only bacteriocin which is used in currently permitted food products. This peptide was added to the list of food additives under the European number E234. In the present review, we give some properties and uses of nisin and we focus particularly on the main factors influencing the synthesis of nisin by lactic acid bacteria strains grown in controlled reactors.
Nisin production by Lactococcus lactis ssp. lactis
Nisin is an antimicrobial peptide of 34 amino acids produced by some strains of Lactococcus lactis ssp. lactis, which was discovered as a result of difficulties in delayed acidification experienced during cheese making by Rogers & Whittier (1928). A few years later the unidentified substance was found to be proteinaceous (Whitehead 1933), and in 1947 (Mattick and Hirsch 1947) it was called “NISIN” (group N Streptococcus Inhibitory Substance, -IN ending indicating an antibiotic). Nisin contains four unusual amino acids: dehydroalanine (DHA), dehydrobutyrine (DHB), lanthionine, and β-methyllanthionine that form thioether bridges in five positions (Fig. 1).
Lactococcus lactis is a Gram-positive, non-motile, and non-sporulating bacterium, measuring ordinarily between 0.5 and 1.5 µm. Cells of this bacterium are usually grouped in pairs or short chains. L. lactis metabolism is heterotrophic and facultative anaerobic (Song et al. 2017). Its optimum growth temperature is around 30 °C (Chen et al. 2015). L. lactis is classified into two major species: L. lactis ssp. lactis and L. lactis ssp. cremoris. Among the two subspecies, strains of L. lactis ssp. lactis are known for their better resistance to environmental changes such as pH and temperature. For example, L. lactis ssp. lactis can grow at 40 °C, pH 9.2 and even at a NaCl concentration up to 4%, whereas L. lactis ssp. cremoris cannot withstand any of these extreme conditions (Kim et al. 1999). In this same study, the authors showed that upon the L. lactis ssp. lactis medium acidification, the sub-lethal level is reached at pH 4.5, while the lethal level is reached at pH 2.5. Other specific properties of L. lactis ssp. lactis are summarized in Table 2. L. lactis ssp. lactis is very important commercially because of its wide use in the preparation of fermented dairy products. The main role of this bacterium during fermentation is acidification mainly through lactic acid production. It can also contribute to food texture modification by the production of exopolysaccharides and aroma improvement by the production of alcohols, ketones and aldehydes. L. lactis ssp. lactis can also be used for food preservation because of its ability to produce organic acids, hydrogen peroxide, diacetyl, as well as bacteriocins.
Pure nisin or nisin preparations can be obtained by culturing nisin-producing strains of L. lactis ssp. lactis followed by suitable extraction and purification methods. The study of 40 wild-type strains of L. lactis showed that 35 were capable of producing nisin (Hurst 1981). Several types of nisin have been identified. The main variants are called A, Z, and Q and possess different biological activities. Nisin A and Z are the most active forms that are often marketed. Nisin Z is produced by some subspecies such as L. lactis ssp. lactis biovar. diacetylactis and differs from nisin A by a single amino acid at position 27. Although the nisin A and Z have the same antimicrobial properties, nisin Z has better solubility at pH > 6 which is important for food applications (Angela Faustino Jozala 2015).
For a long time, it was believed that nisin is synthesized during the stationary phase when nutrients are exhausted (Hurst 1981). However, it has been reported that in batch fermentation, the nisin production follows a primary metabolite kinetic (Guerra et al. 2007): a production during the exponential growth phase and a full stop when the bacteria enter the stationary phase (De Vuyst and Vandamme 1992). It was observed that nisin was detected in the growth medium during the exponential phase and its production rate peaked at the end of this phase which confirms that the synthesis of this peptide follows a primary metabolite kinetic (Chinachoti et al. 1998; Zhu 2017).
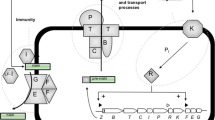
Although nisin synthesis occurs during the growth phase of cultured cells, the relationship between cell number and the amount of nisin produced is not linear in both batch and continuous modes (Abbasiliasi et al. 2017). This phenomenon can be explained by the complexity of the mechanism of nisin biosynthesis and its genetic regulation process. Genetically, a few remarks on the nisin biosynthesis stimulation deserve to be mentioned. Indeed, nisin is a molecule that self-regulates its own production (Hols et al. 2019). In cases of nisin A producing strains, two inducible promoters are located before genes nisA and nisF and a third before nisR gene (Kuipers et al. 1995). However, strains that produce nisin Z have two operons (nisZBTCIPRK and nisFEG) that are also nisin inducible (Qiao et al. 1996). In addition to these mechanisms based on genetic signal transduction, other studies have shown that the carbon source may also have a role in regulating nisin synthesis (Cheigh and Pyun 2005; Müller-Auffermann et al. 2015). During fermentation, the decreased production of nisin, even before the end of the exponential phase can also be attributed to its adsorption on the surface of producing cells: nisin is produced but remains adsorbed to cell surfaces (Parente and Ricciardi 1999). This adsorption becomes lower when pH decreases, which results in maintaining higher nisin concentrations in the reactor when the pH is not controlled (De Vuyst and Vandamme 1992). On the other hand, it was demonstrated that nisin production can be inhibited by high nisin concentrations when cells are cultured in a medium containing an excess of nutrients. This inhibition occurs even if the bacterial growth continues thanks to this excess of nutrients. However, it was also clearly observed that if the medium is deficient in nutrients, the production of nisin is limited by this nutrient depletion (Todorov and Dicks 2004).
A decrease in the amount of produced nisin can also be explained by the acidification due to lactate production. This acidification inhibits bacterial growth and consequently the synthesis of nisin. To avoid the inhibition related to acidification, several studies that have tried to remove lactate from the medium were published. Among the proposed strategies, we can mention fermentation in a mixed-culture system with another microorganism (Bouksaim et al. 2000), continuous separation of lactic acid-producing bacteria using a ceramic microfiltration membrane (Persson et al. 2001), using an anion exchange resin (Yu et al. 2002), changes in metabolic pathways of lactic acid synthesis (Wardani et al. 2006a; Hugenholtz 2008) or also the application of a magnetic field during fermentation for diverting the metabolism towards nisin synthesis (Alvarez et al. 2006).
Factors affecting nisin production by Lactococcus lactis ssp. lactis
Industrial nisin production
Nisin production by Lactococcus lactis demands optimized growth conditions. The complexity of the nisin purification step makes its industrial production a costly procedure. In addition, regarding the poor stability of nisin, the commercial nisin preparations contain only 2.5 wt% pure nisin, stabilized with denatured milk proteins and NaCl. The International Unit (IU) is defined as the amount of nisin dissolved in 1 mL of broth allowing the inhibition of one single Streptococcus agalactiae cell (Gharsallaoui et al. 2016). Cleveland et al. (2002) tested several commercial preparations standardized with salt and milk proteins. Results mainly demonstrated that the presence of insoluble substances influences the quantification and the activity of nisin. The authors considered that the low antimicrobial activity of commercial preparations may be due to nisin adsorption to milk proteins resulting in a decrease in its apparent activity.
Several industrial media were used to improve cell growth, neutralize the produced lactic acid, and increase nisin production. However, the multitude of parameters to control makes the nisin production conditions today far from being optimized. Papagianni et al. (2007) suggested that the optimum conditions that allow high production seem to be different from those that permit cell growth. Other authors such as Guerra et al. (2007) have even tried to use pseudo-mechanistic models to simulate the growth of L. lactis and its nisin production as a function of several experimental parameters. Development of such models can be seen as a necessary step towards controlling the nisin production at industrial scale.
The effect of carbon and nitrogen sources, minerals intake, pH, and various other parameters has been extensively studied. However, these studies were often criticized because they do not differentiate between factors that influence the nisin production and those that influence the growth of producing bacteria (Chandrapati and O’Sullivan 1998). The difficulty of this operation is also due to the lack of precision and the diversity of nisin quantification strategies used during all growth stages of L. lactis. In fact, the first nisin quantification method based on diffusion in a semi-solid matrix was developed by Mocquot and Lefebre (1956) and was subsequently improved by Tramer and Fowler (1964). However, Chandrapati and O’Sullivan (1998) have proposed a rapid method for nisin quantification during the growth of L. lactis based on the diameter of inhibition of a Micrococcus luteus strain. Other rapid methods based on nisin specific antibodies and specific microtitrer based bioassays have also been developed (Daoudi et al. 2001; Immonen and Karp 2007).
In the following sections, the effect of the main experimental parameters on nisin biosynthesis by L. lactis ssp. lactis will be summarized and Table 3 gives some examples of nisin produced amounts obtained during the last two decades.
Nisin producing strains
Several strains of L. lactis are able to produce nisin but not with the same yield (Alegria et al. 2010). The performance difference between these strains was attributed to gene expression intensity, the activity of enzymes that provide post-translational maturation, and resistance of the producing strain to nisin. Regarding this last factor, the introduction of a plasmid containing nisin resistance genes has improved nisin production and growth rate of L. lactis (López-González et al. 2018; Dzhavakhiya et al. 2018). In fact, the surface properties of the producing strains are different (Giaouris et al. 2009) and may influence the adsorption of nisin to the cell surface during production. The surface hydrophobicity should thus be a criterion for strains producing bacteriocin selection. Aiming to improve the nisin production, research is also currently active in the screening of new strains able to produce high amounts of this bacteriocin. These attempts include the encouraging results obtained by cultivation of the strain L. lactis UQ2 isolated from a Mexican cheese (García-Almendárez et al. 2008). Later, the same research team developed a medium based on whey powder for the culture of this strain. Using this optimized medium, they achieved a maximum nisin activity of 575 IU/mL (Gonzalez-Toledo et al. 2010). In addition to strain screening, nisin production by Lactococcus could be further optimized if the mechanisms and cellular pathways that guide the synthesis of this polypeptide were well understood (Wardani et al. 2006b).
Carbon source
The initial concentration of carbohydrates (carbon source) influences the amount of nisin produced by L. lactis at a given pH. The experiments performed by De Vuyst and Vandamme (1992) showed that the amount of produced nisin decreased from 19.1 to 10.9 mg per gram of produced biomass when sucrose concentration increased from 10 to 40 g L−1. In this study, it was also shown that the optimum concentration of sucrose that can produce the maximum amount of nisin is 30 g L−1. Beyond this sucrose level (30 g L−1), nisin production decreased, while bacterial growth was not significantly influenced (Lv et al. 2005). This imbalance between nisin production, biomass production, and substrate availability has been explained in terms of gene expression or posttranslational modifications regulation by carbon source (De Vuyst and Vandamme 1992).
Higher nisin production (4000 IU mL−1) was obtained using glucose as carbon source (Chinachoti et al. 1998). Compared to sucrose and fructose, glucose is the carbon source allowing the optimal production of nisin (Chandrapati and O’Sullivan 1998). It has also been shown, in this study, that glycerol exerts a suppressive action on the production of nisin. Xylose was also considered as a valuable carbon source for nisin production (3000 IU mL−1) by L. lactis simultaneously with lactic acid production. Nisin production was 4 times higher when the L. lactis ssp. lactis A164 strain was cultured in M17 medium supplemented with 3% lactose (Cheigh et al. 2002). Papagianni et al. (2007) showed that using the glucostat system to maintain a 10 g L−1 glucose concentration in the reactor causes a very significant nisin production increase (6100 IU mL−1) compared to that obtained in a batch growth medium containing an initial glucose concentration of 25 g L−1. The authors postulated that beyond a certain concentration, glucose transport inside the cell is saturated which leads to a decrease in the nisin synthesis.
Nitrogen source
After carbon, the most abundant element in the bacterial cell is nitrogen. A typical cell contains about 12% nitrogen (dry weight) which is the main component of nucleic acids, proteins, and other cell molecules such as antimicrobial peptides (OpenStax 2019). LAB are fastidious bacteria that require an exogenous source of amino acids or peptides which are provided by the hydrolysis of proteins in the growth medium. In addition, LAB are able to respond to changes in nitrogen availability by regulating their metabolism to ensure a nitrogen balance in the cell.
Usually, during LAB culture for the production of bacteriocins, semi-synthetic media and readily commercially available media such as MRS, TGE and APT are recommended (Abbasiliasi et al. 2017; Yang et al. 2018). Some authors consider that the role of proteins in nisin synthesis is limited and that the problem can be solved by the use of inorganic nitrogen (Guerra and Pastrana 2001). However, other studies have suggested that protein sources, particularly peptides, can act as inducers for nisin synthesis (Cheigh and Pyun 2005; Jenssen et al. 2006; Venegas-Ortega et al. 2019). This partly explains the results obtained by Kim et al. (1997) which show that the produced nisin concentration increases with the supply of organic nitrogen. In another study, De Vuyst and Vandamme (1993) have tested various organic nitrogen sources (cotton-seed meal, yeast extract, fish meal…). The obtained results showed that the concentration of produced nisin varies significantly depending on the nitrogen source. A proteolytic activity is first required for slow-metabolisable nitrogen sources, before making nitrogen available, in the fermentation medium. This nitrogen limitation state may result in the suppression of metabolic regulatory mechanisms and consequently to a low growth rate. Moreover, De Vuyst and Vandamme (1993) reported a positive correlation between nisin production levels and cell yield which is influenced by the organic nitrogen content.
Cheigh et al. (2002) confirmed this study by showing that the use of 3% yeast extract, as an organic nitrogen source, allows to produce higher nisin amounts. In general, slowly metabolizable organic nitrogen sources can cause a low specific growth rate, but promote the nisin biosynthesis (De Vuyst and Vandamme 1992).
In addition to this nutritional role, stimulating nisin and other bacteriocins production by organic nitrogen sources has been reported (Aasen et al. 2000; Vázquez et al. 2004). These studies have proposed several explanations such as enzyme induction by amino acids or the simultaneous need for many amino acids for the synthesis of the lanthionine ring. Other studies have provided more details, showing, for example, that cysteine and tryptophan stimulate nisin production, whereas proline inhibits it (Vázquez et al. 2004). Cabo et al. (2001) suggested that even if there is no induction of nisin synthesis by individual amino acids, tryptone and yeast extract may contain peptides that are essential to the synthesis of this bacteriocin or can act as inducers of its production. The effect of glycine on nisin production is debatable. Indeed, De Vuyst (1995) showed that the addition of glycine to the growth medium did not influence the production of nisin by L. lactis ssp. lactis NIZO 22,186 while later, Guerra and Pastrana (2001) showed that this amino acid exerts an inhibitory effect on cell growth and nisin production by L. lactis ssp. lactis CECT 539. According to the authors, this inhibitory action may be due to the synthesis inhibition of some membrane components such as peptidoglycan.
The use of some byproducts such as whey can significantly decrease the cost of nisin production and improve its production (Jozala 2011). Indeed, a nisin concentration of 11,120 mg L−1 was obtained by cultivation of L. lactis in bovine whey. This concentration is 22 times higher than that obtained in skim milk (De Arauz et al. 2008). The use of fermented barley extract enriched with glucose can also be considered as an alternative for the nisin production with lower costs (Furuta et al. 2008). The use of low/negative value soy whey (SW) was demonstrated as an alternative, inexpensive fermentation substrate to culture L. lactis for nisin production in MRS medium (Mitra et al. 2010).
pH
As mentioned above, nisin synthesis is associated with the growth phase. Thus, maintaining the optimal pH for L. lactis growth also improves nisin production. The optimal pH for nisin production is generally located around 5–6, slightly below the optimal pH for growth. Besides, Jozala (2011) explained that pH values could influence the extracellular liberation of nisin. The authors detected the highest nisin activity at pH < 5 for Lactococcus lactis ATCC 11,454 strain in milk whey. At pH values lower than 6.0, 80% of the nisin expressed by the cells were released in the culture medium. On the other hand, at pH values higher than 6.0, most of the nisin was retained in the cellular membrane or inside the cells.
However, the exact value of the optimum pH for nisin production may vary depending on the carbon source. For example, nisin Z production was highest at pH 6.0 in a medium containing xylose and 5.5 in a medium containing glucose (Parente and Ricciardi 1999).
Temperature
Usually, the growth optimal temperature allows the optimum production of nisin (Yang et al. 2018). This can easily be explained by the fact that production is associated with cell growth. However, Cheigh et al. (2002) showed that if the maximum growth temperature of the L. lactis ssp. lactis A164 strain was 37 °C, the optimum temperature for nisin and nisin-like bacteriocins production was 30 °C. In addition, heat stress can cause an increased nisin/biomass ratio (Lejeune and Crabbé 1998).
Ions
Some studies have shown that the presence of divalent cations like Ca2+ and Mg2+ may have a remarkable effect on the amount of produced nisin but the intensity of this effect is different from one strain to another. The Mg2+ ions preserve nisin from adsorption to L. lactis ssp. lactis ATCC 11,454 cells causing an increase in the apparent concentration of nisin (Meghrous et al. 1992). The addition of Ca2+ has helped to boost the nisin Z production, and the highest nisin production (3150 IU mL−1) was obtained with 0.1 M CaCl2 in a controlled pH reactor (Matsusaki et al. 1996). Like with Mg2+, this effect was explained by the displacement of nisin Z on the cell surface. Phosphate ions enhance the production of nisin by L. lactis ssp. lactis NIZO 22,186 to reach 3500 IU ml−1 (De Vuyst and Vandamme 1993).
Cell immobilization
The concordance of many studies, showing that nisin is synthesized simultaneously with cell growth, suggests that the improvement of nisin production requires a high concentration of cells in the medium (Parente and Ricciardi 1999). The immobilization of L. lactis cells may be a good alternative to increase cell density in fermentors and ensure the continued production of nisin (Desjardins et al. 2001) (Table 4). LAB immobilization in solid matrices for nisin production has been studied since the early 1990s (Zezza et al. 1993; Pasini et al. 1995; Wan et al. 1995). Overall, the results of these studies have shown that cell confinement in alginate beads enhances nisin production. By contrast, Sonomoto et al. (2000) reported that the use of alginate as a carrier did not improve the production of nisin when compared to the free cells. According to Sonomoto et al. (2000) the highest nisin production yielding good commercial satisfaction was mainly due to cell entrapment in chitosan beads (Table 3).
Since then, several studies aiming at finding new materials and new techniques to immobilize cells in a stable support having a better transfer of substrates and metabolites objectives were conducted. For instance, Sonomoto et al. (2000) tested several methods and matrices for immobilization of the L. lactis IO-1 strain. Results showed that the adsorption of cells in the pores of commercial chitosan beads can lead to a 1.7 times higher nisin production than by free cells. This study also showed the importance of the beads size. Indeed, small size beads have a higher specific surface permitting the adsorption of a larger amount of nisin mainly through hydrophobic interactions. On the other hand, cells incorporation in gels often leads to problems of nutrients transfer resulting in poor cell growth and hence low nisin production (Sonomoto et al. 2000). However, the release of nisin from alginate beads to the outside environment is less influenced by diffusion due to the small size of this peptide. Indeed, calcium alginate beads have a molecular cut-off point of about 20 kDa which is much higher than the molecular weight of nisin (Scannell et al. 2000).
The use of continuous bioreactors containing immobilized bacteria did not significantly improve nisin production compared to cultures using free cells (Sonomoto et al. 2000; Desjardins et al. 2001). These results have led researchers to suggest the existence of one or more limiting steps in the synthesis of nisin such as post-translational modifications, transport, or maturation (Desjardins et al. 2001). The use of culture in the repeated batch cycle (RCB) characterized by a first stage of bead colonization has increased significantly the amount of produced nisin (Bertrand et al. 2001). The immobilization of L. lactis ssp. lactis ATCC 11,454 by natural adsorption on cotton fibers based support has allowed the production of nisin in a continuous reactor for at least 6 months without interruption (Liu et al. 2005). However, since produced nisin amounts are generally expressed in arbitrary units (AU), the yields obtained cannot be compared from one study to another.
Other factors
Other parameters such as agitation and/or aeration are among the factors to be optimized during nisin production in batch mode. Aeration is particularly important because LAB oxygen tolerance is associated with different metabolic pathways, which leads to a decrease of nisin productivity. In the available literature, the conditions of nisin production varied from anaerobiosis to atmospheres containing 60% O2 (Fernández-Pérez et al. 2018). Several authors have determined the optimum rates of agitation and aeration (Desjardins et al. 2001; Mall et al. 2010; Jiang et al. 2015). However, it is not interesting to report these optimal values since they namely depend on the bioreactor size and design.
A comparison between the productions of different bacteriocins including nisin was published by Parente and Ricciardi (1999). This allowed to observe that continuous fermentations allow higher productivities compared to batch ones. This production can be improved by a factor of up to 4.5 times compared to batch culture by cell recycling (Taniguchi et al. 1994). Intermediate yields (~ 1.6–1.7 times higher than following batch mode) were also obtained by cultivating the strain L. lactis ssp. lactis ATCC11454 in a fed-batch mode by adding sucrose and organic nitrogen (yeast extract and soy peptone) (Lv et al. 2004). Continuous production of nisin is often confronted by the problem of bacterial cells loss (wash-out). This problem can be solved by immobilization or entrapment of L. lactis ssp. lactis cells in/on appropriate solid matrices as mentioned above.
Agustin Wardani et al. (2006a, b) have shown that a symbiotic process system composed of L. lactis ssp. lactis ATCC11454 and the yeast Kluyveromyces marxianus MS1 is effective in improving the nisin production. In another study, Kim (1997) showed that the addition of an organic phase (phenyl-methyl silicone oil) to the growth medium can increase nisin production by 24%. This improvement of nisin synthesis has been explained by an improvement in growth due to the elimination from the aqueous phase of inhibitory molecules such as lactic acid.
On the other hand, the presence of a small amount of nisin (0.15 μg mL−1) in MRS medium seems necessary to stimulate the production of nisin by L. lactis ssp. lactis MTCC 440 (Mall et al. 2010). The autoregulating system of nisin allows transcription activation of the nisin structural gene by autophosphorylation of the histidine kinase enzyme (Chandrapati and O’Sullivan 1999; García-Parra et al. 2011). Besides, with the Lactococcus lactis UQ2 strain, García-Parra et al. (2011) added to the skim milk sub-inhibitory amounts of commercial nisin and a mixture of magnesium/manganese. The highest nisin production (75 ± 7 IU mL−1) was achieved after 10 h of incubation in skim milk supplemented with 1.87 µg L−1 of nisin and 0.5/0.1 g L−1 of Mg/Mn, while only 3.5 ± 0.5 IU mL−1 were produced by control cultures at 6 h.
Guo et al. (2010) optimized composition of L. lactis growth medium using an experimental design and a computational model. Results showed that the optimum composition is as follows (g L−1): 15.92 glucose; 30.57 peptone; 39.07 yeast extract; 5.25 NaCl; 10.00 KH2PO4; 0.20 MgSO4 7H2O. This composition has produced 21,423 IU mL−1 of nisin. This nisin concentration is about eight times higher than that obtained without computational optimization (Guo et al. 2010).
Nisin as a food preservative
Nisin is effective against several pathogenic Gram-positive bacteria, such as Staphylococcus aureus (Wang et al. 2020), Listeria monocytogenes (Zhao et al. 2020) and Clostridium tyrobutyricum (Ávila et al. 2020), but also against some Gram-negative pathogens such as Salmonella enterica, and Pseudomonas fluorescens when combined with chelators such as ethylenediaminetetraacetic acid (EDTA) (Liang et al. 2020) or heat treatment (Novickij et al. 2020). Indeed, the surface layer of Gram-negative bacteria, composed of lipopolysaccharides (LPS) acts as a barrier to the action of the nisin on the cytoplasmic wall. The action of chelating agents permits to confine the divalent magnesium and calcium ions of the LPS and destabilize the LPS layer. Thus, nisin can be transported through the LPS layer and create pores in the cytoplasmic membrane, causing a loss of the proton-motive force and a leakage of intracellular nutrients: its classical antimicrobial mechanism (Pattanayaiying et al. 2014).
This antimicrobial activity of nisin is largely dependent on its aqueous solubility and structural stability, which in turn depend on pH and temperature (Table 5). Nisin use for food preservation may offer several advantages: increasing the shelf life of the product, reducing the transmission risk of food-borne pathogens, reducing the use of chemical preservatives, salts, acids… and, permitting the use of soft treatments which better preserve vitamins and organoleptic properties. Moreover, it is important to highlight that nisin cannot be regarded as a “natural” preservative when used in concentrations higher than those naturally found in foods fermented with nisin-producing strains. Table 6 gives some recent examples of nisin amounts experimentally used for the shelf-life increase of some food products. When used for food preservation purpose, nisin can be directly added to food products (Younes et al. 2017), or incorporated in packaging films (Diblan and Kaya 2018), or also added as raw concentrates obtained from nisin-producer strain cultivated in milk or whey-derived substrates (Galvez et al. 2007).
The effect of direct addition of free nisin in dairy products is widely studied. Pinto et al., (2011) added nisin during Serro cheese manufacturing process against Staphylococcus aureus contamination. Pinto et al., (2011) observed that nisin did not affect the physicochemical and mechanical characteristics of obtained cheese. In the same way, Yoon et al. (2011) studied the inactivation of Listeria monocytogenes after adding nisin to the whole, low fat and skim milk. Their results showed that the anti-Listeria activity of nisin was dependent on fat contents in milk substrate. The anti-Listeria activity was moderate in whole milk, whereas remarkable in low fat and skim milk samples. The reaction between nisin and the listerial cell membrane was caused by hydrophobic interaction between amino acid residues of nisin and the fatty acids of the membrane phospholipids. So, the phospholipids present in milk fat could bind a large portion of the added nisin resulting in a reduced nisin amount available to interact with the cell membrane of Listeria spp. cells (Millette et al. 2004). This was not the case in skim milk where similar nisin concentrations were sufficient to cause disruption of the listerial cell membrane. This last case revealed that practical application of nisin can often be limited because of its variable solubility due to interactions with food components (such as protein and lipids) and its low activity at high pH, and consequently limited efficacy in certain food matrices (Malheiros et al. 2012). Moreover, the emergence of nisin tolerance in certain bacteria (Listeria monocytogenes) has been observed (Bergholz et al. 2013; Szendy et al. 2019). That is why several researchers combined nisin with other antimicrobial agents such as essential oil (Yoon et al. 2011), chitosan films (Cé et al. 2012) or with other antimicrobial treatments such as high-pressure processing (Marcos et al. 2013).
The second strategy for nisin use as a food preservative is its incorporation into polymeric films. The advantage of using bacteriocins in films, instead of their direct addition into food matrices, is associated with the increased stability of nisin and the control of its release (Barbosa et al. 2013). Nevertheless, as free nisin application, the effectiveness of antimicrobial packaging is dependent on the type of food packed, the film-forming polymer and the type and concentration of antimicrobial that will determine the release rate and therefore, the antimicrobial efficiency (Marcos et al. 2013). For example, Barbosa et al. (2013) formulated cellulose films containing nisin to improve the safety of minimally processed mangoes. Pathogen and spoilage microorganisms associated with fruit products were inhibited for 9 days without interfering in the organoleptic characteristics of mangoes appearance, texture, and nutritional value. This strategy was mainly beneficial to protect the product surface. Marcos et al. (2013) used nisin- polyvinyl alcohol film against L. monocytogenes for sliced fermented sausages.
As seen in Table 6, many studies have demonstrated the antimicrobial potential of polymeric gels, films, or plastic polymers containing nisin, but very few studies have reported the antimicrobial potential of immobilized living cells, potentially bacteriocin producers, on selected pathogenic bacteria (Millette et al. 2004; Gialamas et al. 2010; Brachkova et al. 2010; Concha-Meyer et al. 2011; Sánchez-González et al. 2013). To date, (Millette et al. 2004) are the only ones to have tested the antimicrobial potential of immobilized cells of a nisin-producing strain (Lactococcus lactis ATCC11454). They immobilized LAB cells in alginate-whey protein concentrate (WPC) beads with diameters between 1.6 and 2.2 mm. Their results showed that immobilization did not affect the inhibitory potential of L. lactis on Gram-positive bacteria Enterococcus sp., Lactobacillus sp., Pediococcus, Kocuria and Staphylococcus. The inhibition diameter measured ranged from 5 to 14 mm. To verify whether the inhibition of Gram-positive bacteria was attributable to nisin, they added a proteolytic enzyme to the culture medium. In the presence of proteases, no inhibition area was observed, which demonstrated that the production of a proteinaceous antimicrobial agent (such as nisin) was responsible for the inhibition.
Conclusion
Nisin is the only bacteriocin approved for food preservation although other bacteriocins produced by LAB are also effective against pathogenic bacteria. However, the direct use of nisin in antimicrobial packaging is limited due to its high price and low purity. This situation is probably due to low production, which is about an average of 100 mg L−1. The physicochemical properties of food matrix such as high-fat content, pH, and ionic force can reduce its activity of nisin. In addition to optimizing the composition of the growth medium, immobilization of L. lactis cells is today the most effective method that allows the reuse of cells, increasing the amount of produced nisin and the ease of its purification. The major developments occurring today in molecular biology could be used to improve both productions but also nisin stability without affecting its antimicrobial potency. However, the non-homogeneity of the units for measuring nisin production makes difficult the collection of global information concerning this bacteriocin production. On the other hand, nisin could be produced in situ in the preservation system. Recent development concerning food safety strategies revealed that immobilization of bacteriocin-producing LAB can preserve LAB ability to produce bacteriocins and to inhibit the growth of undesirable microorganisms.
Data availability
Data and materials are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Aasen IM, Møretrø T, Katla T et al (2000) Influence of complex nutrients, temperature and pH on bacteriocin production by Lactobacillus sakei CCUG 42687. Appl Microbiol Biotechnol 53:159–166. https://doi.org/10.1007/s002530050003
Abbasiliasi S, Tan JS, Tengku Ibrahim TA et al (2017) Fermentation factors influencing the production of bacteriocins by lactic acid bacteria: a review. RSC Adv 7:29395–29420. https://doi.org/10.1039/C6RA24579J
Alegria A, Delgado S, Roces C et al (2010) Bacteriocins produced by wild Lactococcus lactis strains isolated from traditional, starter-free cheeses made of raw milk. Int J Food Microbiol 143:61–66. https://doi.org/10.1016/j.ijfoodmicro.2010.07.029
Al-Nabulsi AA, Osaili TM, Al-Holy MA et al (2009) Influence of desiccation on the sensitivity of Cronobacter spp. to lactoferrin or nisin in broth and powdered infant formula. Int J Food Microbiol 136:221–226. https://doi.org/10.1016/j.ijfoodmicro.2009.08.008
Alvarez DC, Pérez VH, Justo OR, Alegre RM (2006) Effect of the extremely low frequency magnetic field on nisin production by Lactococcus lactis subsp. lactis using cheese whey permeate. Process Biochem 41:1967–1973. https://doi.org/10.1016/j.procbio.2006.04.009
Angela FJ (2015) Nisin. In: Letícia CLN, Adalberto Pessoa Junior ED1 - Varaprasad Bobbarala (ed) Concepts, compounds and the alternatives of antibacterials. IntechOpen, Rijeka, p Ch. 5
Ávila M, Gómez-Torres N, Gaya P, Garde S (2020) Effect of a nisin-producing lactococcal starter on the late blowing defect of cheese caused by Clostridium tyrobutyricum. Int J Food Sci Tech. https://doi.org/10.1111/ijfs.14598
Barbosa AAT, Silva de Araújo HG, Matos PN et al (2013) Effects of nisin-incorporated films on the microbiological and physicochemical quality of minimally processed mangoes. Int J Food Microbiol 164:135–140. https://doi.org/10.1016/j.ijfoodmicro.2013.04.004
Basch CY, Jagus RJ, Flores SK (2013) Physical and antimicrobial properties of tapioca starch-HPMC edible films incorporated with nisin and/or potassium sorbate. Food Bioprocess Tech 6:2419–2428. https://doi.org/10.1007/s11947-012-0860-3
Bergholz TM, Tang S, Wiedmann M, Boor KJ (2013) Nisin resistance of Listeria monocytogenes is increased by exposure to salt stress and is mediated via LiaR. Appl Environ Microbiol 79:5682–5688. https://doi.org/10.1128/AEM.01797-13
Bertrand N, Fliss I, Lacroix C (2001) High nisin-Z production during repeated-cycle batch cultures in supplemented whey permeate using immobilized Lactococcus lactis UL719. Int Dairy J 11:953–960. https://doi.org/10.1016/S0958-6946(01)00129-7
Bi L, Yang L, Narsimhan G et al (2011) Designing carbohydrate nanoparticles for prolonged efficacy of antimicrobial peptide. J Control Release 150:150–156. https://doi.org/10.1016/j.jconrel.2010.11.024
Bintsis T (2018) Lactic acid bacteria as starter cultures: an update in their metabolism and genetics. AIMS Microbiol 4:665–684. https://doi.org/10.3934/microbiol.2018.4.665
Bouksaim M, Lacroix C, Audet P, Simard RE (2000) Effects of mixed starter composition on nisin Z production by Lactococcus lactis subsp. lactis biovar. diacetylactis UL 719 during production and ripening of Gouda cheese. Int J Food Microbiol 59:141–156. https://doi.org/10.1016/S0168-1605(00)00295-6
Brachkova MI, Duarte MA, Pinto JF (2010) Preservation of viability and antibacterial activity of Lactobacillus spp. in calcium alginate beads. Eur J Pharm Sci 41:589–596. https://doi.org/10.1016/j.ejps.2010.08.008
Cabo ML, Murado MA, González MP, Pastoriza L (2001) Effects of aeration and pH gradient on nisin production: a mathematical model. Enzyme Microb Tech 29:264–273. https://doi.org/10.1016/S0141-0229(01)00378-7
Cao-Hoang L, Chaine A, Grégoire L, Waché Y (2010) Potential of nisin-incorporated sodium caseinate films to control Listeria in artificially contaminated cheese. Food Microbiol 27:940–944. https://doi.org/10.1016/j.fm.2010.05.025
Cé N, Noreña CPZ, Brandelli A (2012) Antimicrobial activity of chitosan films containing nisin, peptide P34, and natamycin. CyTA J Food 10:21–26. https://doi.org/10.1080/19476337.2010.537371
Chandrapati S, O’Sullivan DJ (1998) Procedure for quantifiable assessment of nutritional parameters influencing nisin production by Lactococcus lactis subsp. lactis. J Biotechnol 63:229–233. https://doi.org/10.1016/s0168-1656(98)00090-x
Chandrapati S, O’Sullivan DJ (1999) Nisin independent induction of the nisA promoter in Lactococcus lactis during growth in lactose or galactose. FEMS Microbiol Lett 170:191–198. https://doi.org/10.1111/j.1574-6968.1999.tb13374.x
Cheigh C-I, Pyun Y-R (2005) Nisin biosynthesis and its properties. Biotech Lett 27:1641–1648. https://doi.org/10.1007/s10529-005-2721-x
Cheigh C-I, Choi H-J, Park H et al (2002) Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J Biotechnol 95:225–235. https://doi.org/10.1016/s0168-1656(02)00010-x
Chen J, Shen J, Ingvar Hellgren L et al (2015) Adaptation of Lactococcus lactis to high growth temperature leads to a dramatic increase in acidification rate. Sci Rep 5:14199–14199. https://doi.org/10.1038/srep14199
Chinachoti N, Matsusaki H, Sonomoto K, Ishizaki A (1998) Nisin Z production by Lactococcus lactis IO-1 using xylose as a carbon source. Biosci Biotechnol Biochem 62:1022–1024. https://doi.org/10.1271/bbb.62.1022
Choi MH, Park YH (2000) Selective control of lactobacilli in kimchi with nisin. Lett Appl Microbiol 30:173–177. https://doi.org/10.1046/j.1472-765x.2000.00719.x
Chollet E, Sebti I, Martial-Gros A, Degraeve P (2008) Nisin preliminary study as a potential preservative for sliced ripened cheese: NaCl, fat and enzymes influence on nisin concentration and its antimicrobial activity. Food Control 19:982–989. https://doi.org/10.1016/j.foodcont.2007.10.005
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20. https://doi.org/10.1016/s0168-1605(01)00560-8
Cleveland J, Chikindas M, Montville TJ (2002) Multimethod assessment of commercial nisin preparations. J Ind Microbiol Biot 29:228–232. https://doi.org/10.1038/sj.jim.7000315
Concha-Meyer A, Schöbitz R, Brito C, Fuentes R (2011) Lactic acid bacteria in an alginate film inhibit Listeria monocytogenes growth on smoked salmon. Food Control 22:485–489. https://doi.org/10.1016/j.foodcont.2010.09.032
Cotter PD, Hill C, Ross RP (2005) Bacterial lantibiotics: strategies to improve therapeutic potential. Curr Protein Pept Sci 6:61–75. https://doi.org/10.2174/1389203053027584
Cotter PD, Ross RP, Hill C (2013) Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105. https://doi.org/10.1038/nrmicro2937
da Malheiros P, S, Daroit DJ, Brandelli A, (2012) Inhibition of Listeria monocytogenes in minas frescal cheese by free and nanovesicle-encapsulated nisin. Braz J Microbiol 43:1414–1418. https://doi.org/10.1590/S1517-838220120004000024
Dal Bello B, Cocolin L, Zeppa G et al (2012) Technological characterization of bacteriocin producing Lactococcus lactis strains employed to control Listeria monocytogenes in Cottage cheese. Int J Food Microbiol 153:58–65. https://doi.org/10.1016/j.ijfoodmicro.2011.10.016
Daoudi L, Turcotte C, Lacroix C, Fliss I (2001) Production and characterization of anti-nisin Z monoclonal antibodies: suitability for distinguishing active from inactive forms through a competitive enzyme immunoassay. Appl Microbiol Biotechnol 56:114–119. https://doi.org/10.1007/s002530000560
Davies EA, Bevis HE, Potter R et al (1998) Research note: the effect of pH on the stability of nisin solution during autoclaving. Lett Appl Microbiol 27:186–187. https://doi.org/10.1046/j.1472-765X.1998.t01-1-00401.x
Davies EA, Milne CF, Bevis HE et al (1999) Effective use of nisin to control lactic acid bacterial spoilage in vacuum-packed bologna-type sausage. J Food Prot 62:1004–1010. https://doi.org/10.4315/0362-028X-62.9.1004
De Vuyst L (1995) Nutritional factors affecting nisin production by Lactococcus lactis subsp. actis NIZO 22186 in a synthetic medium. J Appl Bacteriol 78:28–33. https://doi.org/10.1111/j.1365-2672.1995.tb01669.x
De Arauz LJ, Jozala AF, Pinheiro GS et al (2008) Nisin expression production from Lactococcus lactis in milk whey medium. J Chem Technol Biot 83:325–328. https://doi.org/10.1002/jctb.1813
Delves-Broughton J (1993) The use of EDTA to enhance the efficacy of nisin towards Gram-negative bacteria. Int Biodeter Biodegr 32:87–97. https://doi.org/10.1016/0964-8305(93)90042-Z
Desjardins P, Meghrous J, Lacroix C (2001) Effect of aeration and dilution rate on nisin Z production during continuous fermentation with free and immobilized Lactococcus lactis UL719 in supplemented whey permeate. Int Dairy J 11:943–951. https://doi.org/10.1016/S0958-6946(01)00128-5
Diblan S, Kaya S (2018) Antimicrobials used in active packaging films. Food Health. https://doi.org/10.3153/JFHS18007
Dos Santos Pires AC, De Fátima Ferreira Soares N, De Andrade NJ et al (2008) Development and evaluation of active packaging for sliced mozzarella preservation. Packag Technol Sci 21:375–383. https://doi.org/10.1002/pts.815
Dzhavakhiya VV, Glagoleva EV, Savelyeva VV et al (2018) New bacitracin-resistant nisin-producing strain of Lactococcus lactis and its physiological characterization. AIMS Microbiol 4:608–621. https://doi.org/10.3934/microbiol.2018.4.608
Ercolini D, Ferrocino I, La Storia A et al (2010) Development of spoilage microbiota in beef stored in nisin activated packaging. Food Microbiol 27:137–143. https://doi.org/10.1016/j.fm.2009.09.006
Fernández-Pérez R, Sáenz Y, Rojo-Bezares B et al (2018) Production and antimicrobial activity of nisin under enological conditions. Front Microbiol 9:1918–1918. https://doi.org/10.3389/fmicb.2018.01918
Ferreira MASS, Lund BM (1996) The effect of nisin on Listeria monocytogenes in culture medium and long-life cottage cheese. Lett Appl Microbiol 22:433–438. https://doi.org/10.1111/j.1472-765X.1996.tb01197.x
Furuta Y, Maruoka N, Nakamura A et al (2008) Utilization of fermented barley extract obtained from a by-product of barley shochu for nisin production. J Biosci Bioeng 106:393–397. https://doi.org/10.1263/jbb.106.393
Galvez A, Abriouel H, Lopez RL, Ben Omar N (2007) Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol 120:51–70. https://doi.org/10.1016/j.ijfoodmicro.2007.06.001
Galvin M, Hill C, Ross RP (1999) Lacticin 3147 displays activity in buffer against gram-positive bacterial pathogens which appear insensitive in standard plate assays. Lett Appl Microbiol 28:355–358. https://doi.org/10.1046/j.1365-2672.1999.00550.x
García-Almendárez BE, Cann IKO, Martin SE et al (2008) Effect of Lactococcus lactis UQ2 and its bacteriocin on Listeria monocytogenes biofilms. Food Control 19:670–680. https://doi.org/10.1016/j.foodcont.2007.07.015
García-Parra MD, García-Almendárez BE, Guevara-Olvera L et al (2011) Effect of sub-inhibitory amounts of nisin and mineral salts on nisin production by Lactococcus lactis UQ2 in Skim milk. Food Bioprocess Tech 4:646–654. https://doi.org/10.1007/s11947-009-0287-7
Garde S, Avila M, Medina M, Nuñez M (2004) Fast induction of nisin resistance in Streptococcus thermophilus INIA 463 during growth in milk. Int J Food Microbiol 96:165–172. https://doi.org/10.1016/j.ijfoodmicro.2004.03.023
George F, Daniel C, Thomas M et al (2018) Occurrence and dynamism of lactic acid bacteria in distinct ecological niches: a multifaceted functional health perspective. Front Microbiol 9:2899–2899. https://doi.org/10.3389/fmicb.2018.02899
Gharsallaoui A, Oulahal N, Joly C, Degraeve P (2016) Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr 56:1262–1274. https://doi.org/10.1080/10408398.2013.763765
Gialamas H, Zinoviadou KG, Biliaderis CG, Koutsoumanis KP (2010) Development of a novel bioactive packaging based on the incorporation of Lactobacillus sakei into sodium-caseinate films for controlling Listeria monocytogenes in foods. Food Res Int 43:2402–2408. https://doi.org/10.1016/j.foodres.2010.09.020
Giaouris E, Chapot-Chartier M-P, Briandet R (2009) Surface physicochemical analysis of natural Lactococcus lactis strains reveals the existence of hydrophobic and low charged strains with altered adhesive properties. Int J Food Microbiol 131:2–9. https://doi.org/10.1016/j.ijfoodmicro.2008.09.006
Gonzalez-Toledo SY, Dominguez-Dominguez J, Garcia-Almendarez BE et al (2010) Optimization of nisin production by Lactococcus lactis UQ2 using supplemented whey as alternative culture medium. J Food Sci 75:M347–353. https://doi.org/10.1111/j.1750-3841.2010.01670.x
Guerra RML, Pastrana L (2001) Nutritional factors affecting the production of two bacteriocins from lactic acid bacteria on whey. Int J Food Microbiol 70:267–281. https://doi.org/10.1016/s0168-1605(01)00551-7
Guerra NP, Agrasar AT, Macías CL et al (2007) Dynamic mathematical models to describe the growth and nisin production by Lactococcus lactis subsp. lactis CECT 539 in both batch and re-alkalized fed-batch cultures. J Food Eng 82:103–113. https://doi.org/10.1016/j.jfoodeng.2006.11.031
Guo W, Zhang Y, Lu J et al (2010) Optimization of fermentation medium for nisin production from Lactococcus lactis subsp. lactis using response surface methodology (RSM) combined with artificial neural network-genetic algorithm (ANN-GA). Afr J Biotechnol 9(38):6264–6272
Hols P, Ledesma-García L, Gabant P, Mignolet J (2019) Mobilization of microbiota commensals and their bacteriocins for therapeutics. Trends Microbiol 27:690–702. https://doi.org/10.1016/j.tim.2019.03.007
Hugenholtz J (2008) The lactic acid bacterium as a cell factory for food ingredient production. Int Dairy J 18:466–475. https://doi.org/10.1016/j.idairyj.2007.11.015
Hurst A (1981) Nisin. In: Perlman D, Laskin AI (eds) Adv Appl Microbiol. Academic Press, Cambridge, pp 85–123
Immonen N, Karp M (2007) Bioluminescence-based bioassays for rapid detection of nisin in food. Biosens Bioelectron 22:1982–1987. https://doi.org/10.1016/j.bios.2006.08.029
Jenssen H, Hamill P, Hancock REW (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511. https://doi.org/10.1128/CMR.00056-05
Jiang L, Liu Y, Yan G et al (2015) Aeration and fermentation strategies on nisin production. Biotechnol Lett 37:2039–2045. https://doi.org/10.1007/s10529-015-1886-1
Jozala (2011) Processing of byproducts to improve nisin production by Lactococcus lactis. Afr J Biotechnol. https://doi.org/10.5897/AJB11.979
Kaczmarek M, Avery SV, Singleton I (2019) Chapter two—microbes associated with fresh produce: Sources, types and methods to reduce spoilage and contamination. In: Gadd GM, Sariaslani S (eds) Adv Appl Microbiol. Academic Press, Cambridge, pp 29–82
Kim WS (1997) Nisin production by Lactococcus lactis using two-phase batch culture. Lett Appl Microbiol 25:169–171. https://doi.org/10.1046/j.1472-765x.1997.00195.x
Kim WS, Hall RJ, Dunn NW (1997) The effect of nisin concentration and nutrient depletion on nisin production of Lactococcuslactis. Appl Microbiol Biotechnol 48:449–453. https://doi.org/10.1007/s002530051078
Kim WS, Ren J, Dunn NW (1999) Differentiation of Lactococcus lactis subspecies lactis and subspecies cremoris strains by their adaptive response to stresses. FEMS Microbiol Lett 171:57–65. https://doi.org/10.1111/j.1574-6968.1999.tb13412.x
Kuipers OP, Rollema HS, Beerthuyzen MM et al (1995) Protein engineering and biosynthesis of nisin and regulation of transcription of the structural nisA gene. Int Dairy J 5:785–795. https://doi.org/10.1016/0958-6946(95)00032-1
Lauková A, Turek P (2011) Effect of enterocin 4231 in Slovak fermented salami Púchov after its experimental inoculation with Listeria innocua Li1. Acta Sci Pol Technol Aliment 10:423–431
Lejeune C, Crabbé DV (1998) Modelling the growth and bacteriocin production by Lactobacillus amylovorus DCE 471 in batch cultivation. J Appl Microbiol 84:159–168. https://doi.org/10.1046/j.1365-2672.1998.00266.x
Liang Z-R, Hsiao H-I, Jhang D-J (2020) Synergistic antibacterial effect of nisin, ethylenediaminetetraacetic acid, and sulfite on native microflora of fresh white shrimp during ice storage. J Food Safety. https://doi.org/10.1111/jfs.12794
Liu X, Chung Y-K, Yang S-T, Yousef AE (2005) Continuous nisin production in laboratory media and whey permeate by immobilized Lactococcus lactis. Process Biochem 40:13–24. https://doi.org/10.1016/j.procbio.2003.11.032
López-González MJ, Escobedo S, Rodríguez A et al (2018) Adaptive evolution of industrial Lactococcus lactis under cell envelope stress provides phenotypic diversity. Front Microbiol 9:2654. https://doi.org/10.3389/fmicb.2018.02654
Lv W, Cong W, Cai Z (2004) Nisin Production by Lactococcus Lactis Subsp. lactis under nutritional limitation in fed-batch culture. Biotechnol Lett 26:235–238. https://doi.org/10.1023/B:BILE.0000013721.78288.1d
Lv W, Cong W, Cai Z (2005) Effect of sucrose on nisin production in batch and fed-batch culture by Lactococcus lactis. J Chem Technol Bioechnol 80:511–514. https://doi.org/10.1002/jctb.1230
Mall P, Mohanty BK, Patankar DB et al (2010) Physiochemical parameters optimization for enhanced nisin production by Lactococcus lactis (MTCC 440). Braz Arch Biol Technol 53:203–209
Marcos B, Aymerich T, Garriga M, Arnau J (2013) Active packaging containing nisin and high pressure processing as post-processing listericidal treatments for convenience fermented sausages. Food Control 30:325–330. https://doi.org/10.1016/j.foodcont.2012.07.019
Matsusaki H, Endo N, Sonomoto K, Ishizaki A (1996) Lantibiotic nisin Z fermentative production by Lactococcus lactis IO-1: relationship between production of the lantibiotic and lactate and cell growth. Appl Microbiol Biot 45:36–40. https://doi.org/10.1007/s002530050645
Mattarelli P, Holzapfel W, Franz CMAP et al (2014) Recommended minimal standards for description of new taxa of the genera Bifidobacterium, Lactobacillus and related genera. Int J Syst Evol Micr 64:1434–1451. https://doi.org/10.1099/ijs.0.060046-0
Mattick ATR, Hirsch A (1947) Further observations on an inhibitory substance (nisin) from lactic streptococci. Lancet 2:5–8. https://doi.org/10.1016/s0140-6736(47)90004-4
Meghrous J, Huot E, Quittelier M, Petitdemange H (1992) Regulation of nisin biosynthesis by continuous cultures and by resting cell of Lactococcus lactis subsp. lactis. Res Microbiol 143:879–890. https://doi.org/10.1016/0923-2508(92)90075-Y
Millette M, Smoragiewicz W, Lacroix M (2004) Antimicrobial potential of immobilized Lactococcus lactis subsp. lactis ATCC 11454 against selected bacteria. J Food Prot 67:1184–1189. https://doi.org/10.4315/0362-028x-67.6.1184
Millette M, Le Tien C, Smoragiewicz W, Lacroix M (2007) Inhibition of Staphylococcus aureus on beef by nisin-containing modified alginate films and beads. Food Control 18:878–884. https://doi.org/10.1016/j.foodcont.2006.05.003
Mitra D, Pometto AL, Khanal SK et al (2010) Value-added production of nisin from soy whey. Appl Biochem Biotechnol 162:1819–1833. https://doi.org/10.1007/s12010-010-8951-y
Mocquot G, Lefebre E (1956) A simple procedure to detect nisin in cheese. J Appl Bacteriol 19:322–323. https://doi.org/10.1111/j.1365-2672.1956.tb00083.x
Müller-Auffermann K, Grijalva F, Jacob F, Hutzler M (2015) Nisin and its usage in breweries: a review and discussion: nisin and its usage in breweries. J Inst Brew 121:309–319. https://doi.org/10.1002/jib.233
Murillo-Martínez MM, Tello-Solís SR, García-Sánchez MA, Ponce-Alquicira E (2013) Antimicrobial activity and hydrophobicity of edible whey protein isolate films formulated with nisin and/or glucose oxidase. J Food Sci 78:M560–M566. https://doi.org/10.1111/1750-3841.12078
Neetoo H (2008) Use of nisin-coated plastic films to control Listeria monocytogenes on vacuum-packaged cold-smoked salmon. Int J Food Microbiol 122:8–15. https://doi.org/10.1016/j.ijfoodmicro.2007.11.043
Novickij V, Stanevičienė R, Staigvila G et al (2020) Effects of pulsed electric fields and mild thermal treatment on antimicrobial efficacy of nisin-loaded pectin nanoparticles for food preservation. LWT 120:108915. https://doi.org/10.1016/j.lwt.2019.108915
OpenStax (2019) Biology. OpenStax CNX. https://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@11.10
Papagianni M, Avramidis N, Filiousis G (2007) Investigating the relationship between the specific glucose uptake rate and nisin production in aerobic batch and fed-batch glucostat cultures of Lactococcus lactis. Enzyme Microb Tech 40:1557–1563. https://doi.org/10.1016/j.enzmictec.2006.10.035
Parada JL, Caron CR, Medeiros ABP, Soccol CR (2007) Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Braz Arch Biol Technol 50:512–542. https://doi.org/10.1590/S1516-89132007000300018
Parente E, Ricciardi A (1999) Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl Microbiol Biotechnol 52:628–638. https://doi.org/10.1007/s002530051570
Pasini G, Crapisi A, Lante A et al (1995) Evaluation of bacteriocin activity produced in milk by Lactococcus lactis, Subspecies lactis, immobilized in barium alginate beads. Ann NY Acad Sci 750:465–468. https://doi.org/10.1111/j.1749-6632.1995.tb19997.x
Pattanayaiying R, H-Kittikun A, Cutter CN (2014) Effect of lauric arginate, nisin Z, and a combination against several food-related bacteria. Int J Food Microbiol 188:135–146. https://doi.org/10.1016/j.ijfoodmicro.2014.07.013
Persson A, Jonsson AS, Zacchi G (2001) Separation of lactic acid-producing bacteria from fermentation broth using a ceramic microfiltration membrane with constant permeate flow. Biotechnol Bioeng 72:269–277. https://doi.org/10.1002/1097-0290(20010205)72:3<269:aid-bit3>3.0.co;2-h
Pinto MS, de Carvalho AF, Pires ACS et al (2011) The effects of nisin on Staphylococcus aureus count and the physicochemical properties of Traditional Minas Serro cheese. Int Dairy J 21:90–96. https://doi.org/10.1016/j.idairyj.2010.08.001
Piper C, Draper LA, Cotter PD et al (2009) A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J Antimicrob Chemother 64:546–551. https://doi.org/10.1093/jac/dkp221
Qiao M, Ye S, Koponen O et al (1996) Regulation of the nisin operons in Lactococcus lactis N8. J Appl Bacteriol 80:626–634. https://doi.org/10.1111/j.1365-2672.1996.tb03267.x
Rogers LA, Whittier EO (1928) Limiting factors in the lactic fermentation. J Bacteriol 16:211–229
Rollema HS, Kuipers OP, Both P et al (1995) Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl Environ Microbiol 61:2873–2878. https://doi.org/10.1128/AEM.61.8.2873-2878.1995
Ryan MP, Rea MC, Hill C, Ross RP (1996) An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl Environ Microbiol 62:612–619. https://doi.org/10.1128/AEM.62.2.612-619.1996
Sánchez-González L, Quintero Saavedra JI, Chiralt A (2013) Physical properties and antilisterial activity of bioactive edible films containing Lactobacillus plantarum. Food Hydrocoll 33:92–98. https://doi.org/10.1016/j.foodhyd.2013.02.011
Sarika AR, Lipton AP, Aishwarya MS (2012) Comparative assessment of bacteriocin production in free and immobilized Lactobacillus plantarum MTCC B1746 and Lactococcus lactis MTCC B440. J Appl Sci Res 8(4):2197–2202
Scannell AGM, Hill C, Ross RP et al (2000) Continuous production of lacticin 3147 and nisin using cells immobilized in calcium alginate. J Appl Microbiol 89:573–579. https://doi.org/10.1046/j.1365-2672.2000.01149.x
Song AA-L, In LLA, Lim SHE, Rahim RA (2017) A review on Lactococcus lactis: from food to factory. Microb Cell Fact 16:55. https://doi.org/10.1186/s12934-017-0669-x
Sonomoto K, Chinachoti N, Endo N, Ishizaki A (2000) Biosynthetic production of nisin Z by immobilized Lactococcus lactis IO-1. J Mol Catal B Enzym 10:325–334. https://doi.org/10.1016/S1381-1177(00)00133-8
Srivastava RK (2018) Enhanced shelf life with improved food quality from fermentation processes. J Food Process Preserv 2(3):8–14
Szendy M, Kalkhof S, Bittrich S et al (2019) Structural change in GadD2 of Listeria monocytogenes field isolates supports nisin resistance. Int J Food Microbiol 305:108240. https://doi.org/10.1016/j.ijfoodmicro.2019.108240
Taniguchi M, Hoshino K, Urasaki H, Fujii M (1994) Continuous production of an antibiotic polypeptide (nisin) by Lactococcus lactis using a bioreactor coupled to a microfiltration module. J Ferment Bioeng 77:704–708. https://doi.org/10.1016/0922-338X(94)90159-7
Todorov SD, Dicks LMT (2004) Effect of medium components on bacteriocin production by Lactobacillus Pentosus ST151BR, a strain isolated from beer produced by the fermentation of maize, barley and soy flour. World J Microb Biot 20:643–650. https://doi.org/10.1023/B:WIBI.0000043196.09610.de
Tramer J, Fowler GG (1964) Estimation of nisin in foods. J Sci Food Agric 15:522–528. https://doi.org/10.1002/jsfa.2740150802
Vázquez JA, Cabo ML, González MP, Murado MA (2004) The role of amino acids in nisin and pediocin production by two lactic acid bacteria: a factorial study. Enzyme Microb Technol 34:319–325. https://doi.org/10.1016/j.enzmictec.2003.11.005
Venegas-Ortega MG, Flores-Gallegos AC, Martínez-Hernández JL et al (2019) production of bioactive peptides from lactic acid bacteria: a sustainable approach for healthier foods. Compr Rev Food Sci Food Saf 18:1039–1051. https://doi.org/10.1111/1541-4337.12455
Vukomanović M, Žunič V, Kunej Š et al (2017) Nano-engineering the antimicrobial spectrum of lantibiotics: activity of nisin against gram negative bacteria. Sci Rep 7:4324. https://doi.org/10.1038/s41598-017-04670-0
Vuyst De, Vandamme EJ (1992) Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J Gen Microbiol 138:571–578. https://doi.org/10.1099/00221287-138-3-571
Vuyst De, Vandamme EJ (1993) Influence of the phosphorus and nitrogen source on nisin production in Lactococcus lactis subsp. lactis batch fermentations using a complex medium. Appl Microbiol Biot 40:17–22. https://doi.org/10.1007/BF00170422
Wan J, Hickey MW, Coventry MJ (1995) Continuous production of bacteriocins, brevicin, nisin and pediocin, using calcium alginate-immobilized bacteria. J Appl Bacteriol 79:671–676. https://doi.org/10.1111/j.1365-2672.1995.tb00953.x
Wandling LR, Sheldon BW, Foegeding PM (1999) Nisin in milk sensitizes Bacillus spores to heat and prevents recovery of survivors. J Food Prot 62:492–498. https://doi.org/10.4315/0362-028x-62.5.492
Wang H, Niu Y, Pan J et al (2020) Antibacterial effects of Lactobacillus acidophilus surface-layer protein in combination with nisin against Staphylococcus aureus. LWT 124:109208. https://doi.org/10.1016/j.lwt.2020.109208
Wardani AK, Egawa S, Nagahisa K et al (2006a) Robustness of cascade pH and dissolved oxygen control in symbiotic nisin production process system of Lactococcus lactis and Kluyveromyces marxianus. J Biosci Bioeng 101:274–276. https://doi.org/10.1263/jbb.101.274
Wardani AK, Egawa S, Nagahisa K et al (2006b) Computational prediction of impact of rerouting the carbon flux in metabolic pathway on cell growth and nisin production by Lactococcus lactis. Biochem Eng J 28:220–230. https://doi.org/10.1016/j.bej.2005.10.003
Wedajo B (2015) Lactic Acid Bacteria: benefits, selection criteria and probiotic potential in fermented food. J Prob Health. https://doi.org/10.4172/2329-8901.1000129
Whitehead HR (1933) A substance inhibiting bacterial growth, produced by certain strains of lactic streptococci. Biochem J 27:1793–1800. https://doi.org/10.1042/bj0271793
Yang E, Fan L, Yan J et al (2018) Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Express 8:10. https://doi.org/10.1186/s13568-018-0536-0
Yoon JI, Bajpai VK, Kang SC (2011) Synergistic effect of nisin and cone essential oil of Metasequoia glyptostroboides Miki ex Hu against Listeria monocytogenes in milk samples. Food Chem Toxicol 49:109–114. https://doi.org/10.1016/j.fct.2010.10.004
Younes M, Aguilar F, Crebelli R et al (2017) Safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J 15:e05063. https://doi.org/10.2903/j.efsa.2017.5063
Yu P-L, Dunn NW, Kim WS (2002) Lactate removal by anionic-exchange resin improves nisin production by Lactococcuslactis. Biotechnol Lett 24:59–64. https://doi.org/10.1023/A:1013865502420
Zezza N, Pasini G, Lombardi A et al (1993) Production of a bacteriocin active on lactate-fermenting clostridia by Lactococcus lactis subsp. lactis immobilized in coated alginate beads. J Dairy Res 60:581–591. https://doi.org/10.1017/S002202990002793X
Zhao X, Chen L, Zhao L et al (2020) Antimicrobial kinetics of nisin and grape seed extract against inoculated Listeria monocytogenes on cooked shrimps: survival and residual effects. Food Control 115:107278. https://doi.org/10.1016/j.foodcont.2020.107278
Zhu X (2017) Optimization of nutritional factors for nisin yield improvement by Lactococcus lactis E15 using corn steep liquor powder as nitrogen source. AIBM 2. https://doi.org/https://doi.org/10.19080/AIBM.2017.02.555585
Zohri M, Shafiee Alavidjeh M, Mirdamadi SS et al (2013) Nisin-loaded chitosan/alginate nanoparticles: a hopeful hybrid biopreservative. J Food Safety 33:40–49. https://doi.org/10.1111/jfs.12021
Zottola EA, Yezzi TL, Ajao DB, Roberts RF (1994) Utilization of cheddar cheese containing nisin as an antimicrobial agent in other foods. Int J Food Microbiol 24:227–238. https://doi.org/10.1016/0168-1605(94)90121-X
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors participated equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Additional information
Communicated by Erko stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khelissa, S., Chihib, NE. & Gharsallaoui, A. Conditions of nisin production by Lactococcus lactis subsp. lactis and its main uses as a food preservative. Arch Microbiol 203, 465–480 (2021). https://doi.org/10.1007/s00203-020-02054-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02054-z