Abstract
The mineral phosphate solubilizing (MPS) ability of a Serratia marcescens strain, namely CTM 50650, isolated from the phosphate mine of Gafsa, was characterized on a chemically defined medium (NBRIP broth). Various insoluble inorganic phosphates, including rock phosphate (RP), calcium phosphate (CaHPO4), tri-calcium phosphate (Ca3(PO4)2) and hydroxyapatite were tested as sole sources of phosphate for bacterial growth. Solubilization of these phosphates by S. marcescens CTM 50650 was very efficient. Indeed, under optimal conditions, the soluble phosphorus (P) concentration it produced reached 967, 500, 595 and 326 mg/l from CaHPO4, Ca3(PO4)2, hydroxyapatite and RP, respectively. Study of the mechanisms involved in the MPS activity of CTM 50650, showed that phosphate solubilization was concomitant with significant drop in pH. HPLC-analysis of culture supernatants revealed the secretion of gluconic acid (GA) resulting from direct oxidation pathway of glucose when the CTM 50650 cells were grown on NBRIP containing glucose as unique carbon source. This was correlated with the simultaneous detection by PCR for the first time in a S. marcescens strain producing GA, of a gene encoding glucose dehydrogenase responsible for GA production, as well as the genes pqqA, B, C and E involved in biosynthesis of its PQQ cofactor. This study is expected to lead to the development of an environmental-friendly process for fertilizer production considering the capacity of S. marcescens CTM 50650 to achieve yields of P extraction up to 75% from the Gafsa RP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is the second major macronutrient required for the growth and development of plants (Vance 2001). Although the soil P content (organic and inorganic forms) is generally about 0.04 to 0.12%, only 0.1% of the total P exists in a soluble form available for plant uptake (Zou et al. 1992). This P deficiency is one of the most important chemical factors limiting crop production in many soils worldwide (Arcand and Schneider 2006). Therefore, frequent application of important amounts of chemical fertilizers containing soluble forms of P is needed to achieve maximum plant productivity (Gyaneshwar et al. 2002). Yet, a great proportion of the applied soluble P is rapidly precipitated into forms of sparingly solubility, particularly Fe–P and Al–P complexes, which can be regarded as unavailable to plants (Johnson and Loepper 2006; Rengel and Marschner 2005). As an alternative strategy, phosphatidic-bearing minerals, particularly rock phosphate (RP) containing few P available to plants are also used (Rajan et al. 1996). RP can be applied directly to the soil and its agronomic efficiency varies depending on soil acidity and crop.
Traditional production of P fertilizers is based on chemical processing of insoluble mineral phosphate high-grade ore, which includes an energy intensive treatment with sulfuric acid at high temperature. This process has become an environmentally undesirable and costly affair (Vassilev et al. 2006). Furthermore, the current developments in sustainability require a strong reduction in agrochemical inputs and their replacement by more ecological, efficient and cheap natural products (Macias et al. 2003). For instance, insoluble inorganic phosphates including RP can be transformed into soluble forms available to plant roots by the action of some soil microorganisms termed as phosphate solubilizing microorganisms (PSM), which in turn metabolize root-borne C compounds, mainly sugars and organic acids for their growth (Arcand and Schneider 2006; Rodriguez and Fraga 1999; Whitelaw 2000). Mineral phosphate solubilization is an essential plant growth-promoting ability via which PSM have found extensive applications in agriculture as inoculants (Arcand and Schneider 2006; Lucy et al. 2004). The literature describes these PSM as belonging essentially to the bacterial genera Pseudomonas, Enterobacter, Serratia, Pantoea, Rhizobium… and to the fungal genera Aspergillus, Penicillium… (Antoun et al. 1998; Buch et al. 2008; Gulati et al. 2008; Son et al. 2006; Sulbarán et al. 2009; Whitelaw 2000). The mineral phosphate solubilizing (MPS) ability of these microorganisms is attributed to the secretion of a variety of low molecular weight organic acids mainly gluconic acid (GA), 2-ketogluconic acid, citric acid and oxalic acid, which via their hydroxyl and carboxyl groups chelate the cations bound to phosphate, or by the liberation of H+ protons, thereby converting it into soluble forms (Kpomblekou and Tabatabai 1994). The best characterized mechanism of MPS in many Gram-negative bacterial species, involves secretion of GA generated by a direct oxidation pathway of glucose via the membrane-bound quinoprotein glucose dehydrogenase (GDH) (Kim et al. 1997; Lin et al. 2006; Patel et al. 2008). This enzyme requires pyrroloquinoline quinone (PQQ) as a cofactor, whose biosynthesis involves a pqq operon consisting of six genes (pqqA, B, C, D, E, F) in Klebsiella pneumonia, Rahnella aquatilis and Enterobacter intermedium 60-2G (Kim et al. 1998, 2003; Meulenberg et al. 1992). Previous studies have proved the requirement of PQQ for the MPS ability. For instance, Kim et al. (1998) have shown that heterologous expression of the Rahnella aquatilis pqq genes in Escherichia coli (deficient for this cofactor), conferred to this bacterium the ability to solubilize hydroxyapatite due to the extracellular production of GA. Furthermore, Han et al. (2008) have demonstrated that the inactivation of pqq genes of Enterobacter intermedium 60-2G, abolished the production of 2-ketogluconic acid (generated by oxidation of GA by gluconic acid dehydrogenase) and eliminated its ability to solubilize hydroxyapatite.
The aim of this work was to characterize the MPS activity of novel bacterial isolates for the first time from the Tunisian phosphate mine of Gafsa, toward various insoluble inorganic phosphates, including the Gafsa RP. We also report on the mechanisms involved in phosphate solubilization by the Serratia marcescens CTM 50650 strain (the best phosphate solubilizing bacterium) and their correlation with the presence of genes commonly involved in the MPS phenotype of Gram-negative bacteria.
Materials and methods
Screening of bacterial isolates with MPS activity
Soil samples were collected in April 2006 from three distant sites within the phosphate mine of Gafsa, an original biotope rich in insoluble rock phosphate (RP). From each site, approximately 500 g of soil sample were taken in duplicate from the upper 30 cm of the soil profile aseptically. Samples were homogenized in sterile saline solution (NaCl 9 g/l). Then, serially diluted soil samples were spread onto Lauria-Bertani (LB) agar to allow isolation of bacterial strains. Colonies exhibiting different morphological aspect were picked up individually and further purified by replating on agar plate. In total, 279 isolates were screened for their MPS ability on National Botanical Research Institute’s phosphate growth medium (NBRIP) agar (Nautiyal 1999). The NBRIP medium (pH 7) contained the following ingredients per litre: glucose, 10 g; (NH4)2SO4, 0.1 g; KCl, 0.2 g; MgSO4·7H2O, 0.25 g and hydroxyapatite, 5 g. After 3 days of incubation at 30°C, the plates were examined for the presence of phosphate solubilizing bacteria identified as colonies developing clear solubilization halos (Gyaneshwar et al. 1999). Of the 279 screened isolates, 52 strains showed reproducibly MPS activity on NBRIP agar. 5 among these 52 strains, designed US540, US468, US537, US549 and US557 exhibiting the largest clarification zones (halo size greater than 10 mm diameter), were further screened on NBRIP broth to confirm their MPS phenotype. Hence, for each isolate, 500-ml Erlenmeyer flasks containing 100 ml of NBRIP medium were inoculated at 0.1 OD600 final with 16 h old culture grown on LB and incubated on a rotary shaker (250 rpm) at 30°C for 72 h. In addition to hydroxyapatite, these five isolates were also checked for their ability to solubilize the following inorganic phosphates: the Gafsa RP, calcium phosphate (CaHPO4) and tri-calcium phosphate (Ca3(PO4)2). Aliquots of 1.5 ml were withdrawn from all the cultures after 0, 2, 6, 24, 30, 48, 54 and 72 h of cultivation. Cultures supernatants were collected by centrifugation at 14,000 rpm for 30 min and used for estimating the concentration of the released soluble P or P2O5 (for the RP), which was generally utilized as criteria for choosing the most efficient PSM. One bacterium named strain US540 having the highest MPS activity was preserved as pure culture in the Collection of Tunisian Microorganisms “CTM” of the Centre of Biotechnology of Sfax (deposit no. CTM 50650), and selected for further investigations.
DNA manipulation
General molecular biology techniques were performed as described by Sambrook et al. (1989). Polymerase chain reaction (PCR) amplifications were performed using Pfu DNA polymerase from BIOTOOLS (Madrid-Spain). PCR products were purified from agarose gels by using the GFX™ PCR DNA and Gel Band Purification Kit (Amersham Bioscience) and sequenced. The nucleotide sequences were determined by an automated 3100 Genetic Analyser (Applied Biosystems) and homology search was performed using the Basic Local Alignment Search Tool BlastN program.
Taxonomic identification of the selected CTM 50650 isolate
Determination of biochemical characteristics by API 20E
Biochemical characteristics of the CTM 50650 strain (best MPS ability) were analyzed by the API 20E bacterial biochemical identification system (BioMérieux) widely used in routine in microbiology laboratories for identification of members of the family Enterobacteriaceae.
Sequencing of 16S rDNA and 16S-23S intergenic region
The gene encoding 16S rRNA and the 16S-23S intergenic region were amplified from CTM 50650 by PCR using primers corresponding to highly conserved regions within the rRNA operon of Escherichia coli (Ol1: 5′AGAGTTTGATCCTGGCTCAG3′ and Ol2: 5′AAGGAGGTGATCCAAGCC3′ for the 16S rRNA gene; Ol3: 5′GGCTTGGATCACCTCCTCCTT3′ and Ol4: 5′ACTTAGATGTTTCAGTTC3′ for the 16S-23S intergenic region). Cycling conditions were performed as reported by Farhat et al. (2008).
Characterization of the MPS activity of Serratia marcescens CTM 50650
In order to characterize the MPS activity of the S. marcescens CTM 50650 strain, we performed cultures on NBRIP broth containing 5 g/l of one of the four previously mentioned inorganic phosphates sources. Inoculated cultures were incubated for 72 h at 30°C with shaking (250 rpm). Cultures samples were taken at 0, 2, 6, 24, 30, 48, 54 and 72 h of cultivation. Cell growth pattern and medium acidification were monitored during growth and the supernatants fractions were used for determination of the concentration of liberated soluble P or P2O5. For the analysis of organic acids secretion in the supernatant, we carried out cultures on NBRIP liquid amended with 10 g/l glucose (GDH substrate) or fructose (non GHD substrate).
Effect of cultural conditions on RP and hydroxyapatite solubilization by Serratia marcescens CTM 50650
In order to observe the effect of cultural conditions for inorganic phosphates solubilization, the S. marcescens CTM 50650 strain was cultured on NBRIP broth containing RP or hydroxyapatite at 5 g/l as inorganic phosphate source at different temperatures (20–37°C) and initial pH (pH 4–10). Then, the effect of the nature of carbon source was tested by growth under optimal pH and temperature conditions on NBRIP broth without any carbon source added or in the presence of one of the following carbon sources: glucose, galactose, glycerol, maltose, fructose, xylose, arabinose and sorbitol. The carbon source supporting maximal MPS ability was then chosen for optimization of concentration. In all experiments, cultures were conducted in 500-ml Erlenmeyer flasks containing 100 ml of culture medium and grown for 48 h in a rotary shaker (250 rpm). Supernatants fractions were used for estimating the amount of released soluble P or P2O5.
Analytical methods
Cell growth was estimated by the measurement of the absorbance at 600 nm. Concentration of the soluble P or P2O5 in the culture broth was estimated colorimetrically as described by Tandon et al. (1968) at 440 nm. For Gafsa RP, the concentration of soluble P2O5 was determined as follows: [soluble P] ×2.29. Change in pH of the culture broth was recorded by pH meter equipped with a glass electrode. Organic acids were analyzed by high performance liquid chromatography (HPLC). The culture supernatants fractions were filtered through 0.22-μm-nylon filter. The organic acids were separated using a Hyperfil RP-18 column at a flow rate of 0.5 ml/min. The operating conditions consisted of 5% acetonitrile in 0.05 M NaH2PO4 as mobile phase, and sample injection of 20 μl. The identification and estimation of the concentration of organic acids were achieved by comparing the retention times and peak areas of chromatograms with those of standard acids (gluconic acid, acetic acid, citric acid, succinic acid, oxalic acid…) added to filtrates of un-inoculated media. Retention time of each signal was recorded at a wavelength of 260 nm.
Detection of pqqA, pqqB, pqqC, pqqE and gdh by PCR
The presence of genes involved in the expression of MPS phenotype through the activation of the direct oxidation pathway of glucose (gdh gene encoding GDH and pqq genes involved in the biosynthesis of the required PQQ cofactor) was checked by PCR in the S. marcescens CTM 50650 strain. The genes pqqA and pqqB were jointly amplified using the primers OlMBF1: 5′ATGACACCTGGACTAAACC3′ and OlMBF2: 5′GGAGTGGTTGCGTCAT3′ that we designed from the available nucleotide sequence of the putative pqq genes of the S. marcescens W1 strain (GenBank accession no. DQ868536). PCR conditions were 30 cycles of 30 s at 94°C, 45 s at 60°C and 90 s at 72°C. By alignment of many accessible nucleotide sequences corresponding to putative pqqC genes from Pseudomonads, we detected highly conserved regions that have been used for designing two primers OlMBF3: 5′CCCGCGAGCAGATCCAGGGCTGGGT3′ and OlMBF4: 5′TAGGCCATGCTCATGGCGTC3′. These primers were used for PCR amplification of a fragment internal to the pqqC gene. Amplification conditions were: 30 cycles of 30 s at 94°C, 30 s at 65°C, and 30 s at 72°C. The genes gdh and pqqE were amplified using the primers pqq2/pqq4 and pqqF/pqqR2 and the cycling conditions previously reported by Pujol and Kado (1999) and Pérez et al. (2007), respectively. In preliminary experiments, fragments of the expected sizes were purified from agarose gels and sequenced. The results obtained validated the PCR protocols described above. Therefore, PCR products of the expected size were considered to correspond to the genes being amplified.
Results
Screening and identification of phosphate solubilizing bacterium
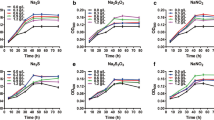
The screening strategy employed during this investigation allowed the identification of bacterial isolates with MPS ability on NBRIP agar containing hydroxyapatite as a sole inorganic phosphate source. In total, 52 isolates with clear zones of solubilization around their colonies were selected among the 279 isolates tested in this first screening step. The halo size of these 52 isolates ranged from 5 to 13 mm. This preliminary observation suggested the existence of bacterial isolates exhibiting different degrees of MPS efficiencies in the soil samples collected. Among the above selected isolates, five-ones designated US540, US468, US537, US549 and US557 exhibiting the largest clear zones (greater than 10 mm) were further screened on NBRIP broth to confirm their MPS phenotype on various inorganic phosphate sources like hydroxyapatite, Gafsa RP, CaHPO4 and Ca3(PO4)2. Inoculation of these strains into NBRIP liquid medium resulted in a gradual increase in the concentration of the released soluble P or P2O5 (in the case of RP), until about 30 h of bacterial incubation confirming the MPS ability of all these isolates on the four insoluble phosphates sources. However, obvious differences in the degrees of MPS efficiencies can be observed between the isolates (Fig. 1). In fact, the US540 strain comes out as the most efficient MPS bacterium followed by the US468 strain. The maximal soluble P2O5 concentration they produced in the presence of RP was of 754 and 623 mg/l for US540 and US468, respectively. When the CaHPO4, Ca3(PO4)2 and hydroxyapatite were used as inorganic phosphate sources, the highest soluble concentration was of 967, 500 and 595 mg/l for the US540 isolate and 847, 410 and 492 mg/l for US468, respectively.
Concentration of soluble P2O5 or P released from Gafsa RP (RP), calcium phosphate (CaHPO4), tri-calcium phosphate (Ca3(PO4)2) and hydroxyapatite, in culture supernatants of the selected US540, US537, US549, US557 and US468 isolates grown on NBRIP containing 5 g/l of phosphate sources. Each value in the panel represents mean ± SD
Based on the results of this preliminary investigation, the US540 strain was deposed in the Collection of Tunisian Microorganisms (deposit no. CTM 50650) and selected for further study and characterization. Genotypic identification of CTM 50650 was performed by analysis of its nucleotide sequences corresponding to the 16S rRNA gene (EMBL accession no. FM995217) and the 16S–23S intergenic spacer (EMBL accession no. FM995218) using BLAST research program. CTM 50650 was most closely related to S. marcescens strains. Indeed, the DNA sequence of the CTM 50650 16S rRNA gene showed 100% identity to that of Serratia sp. GW13 (GenBank accession no. EF550163), and 99% identity to 16S rDNA of several Serratia species (mainly marcescens). Regarding the CTM 50650 16S-23S intergenic region, it displayed 96% identity with the intergenic spacer from S. marcescens strain B17 (GenBank accession no. EF426100). In addition, biochemical analysis by the API 20E identification system (Table 1) revealed that the CTM 50650 strain is close to the S. marcescens with 95.9% identity. All together, theses results let us to designate this bacterium as S. marcescens CTM 50650.
Characterization of the MPS ability of the Serratia marcescens CTM 50650 strain
Serratia marcescens CTM 50650 cells inoculated in NBRIP broth containing the four previously mentioned inorganic phosphate sources started to grow exponentially after an initial lag phase of about 8 h and reached the stationary phase after 24–30 h of growth (Fig. 2). Supernatant acidification was recorded irrespective of using hydroxyapatite, Gafsa RP, CaHPO4 or Ca3(PO4)2 as sole phosphate sources (Fig. 2). As reported by Sulbarán et al. (2009), in all cases, the pH of the medium started to drop immediately after the cells were inoculated and continued to decrease for 4–8 h in the absence of any noticeable growth. Figure 2 shows that although the solubilization of all phosphate sources by S. marcescens CTM 50650 was concomitant with a drastic drop in pH, the final pH was more tardily attained with RP. This might be the consequence of its buffering capacity as suggested by Sulbarán et al. (2009). The soluble P2O5 concentration in the medium released from RP showed a gradual increase and reached 95, 726 and 751 mg/l after a growth period of 6, 24 and 48 h, respectively (Fig. 2). Similar results were recorded with CaHPO4, Ca3(PO4)2 and hydroxyapatite except that soluble P was obviously released sooner compared to RP. In effect, the concentration of soluble P reached after only 6 h of cultivation was of 479, 298, and 443 mg/l, respectively (Fig. 2).
All together, our results showed that a sharp decrease in pH was recorded during the solubilization of the different phosphate sources, which suggested secretion of organic acids by S. marcescens CTM 50650. This hypothesis was confirmed by HPLC analysis, which revealed that the solubilization of hydroxyaptite (as well as the other inorganic phosphate sources; data not shown) by this phosphate solubilizing bacterium (PSB) was mainly caused by secretion of organic acids in the culture medium. Depending on the nature of the carbon source used for growth, two different organic acids were produced by CTM 50650. Indeed, in the presence of glucose (a GDH substrate), gluconic acid (GA) was produced (Fig. 3a) while fructose (non GHD substrate) supported the production of citric acid (Fig. 3b). Estimation of the secreted acids and soluble P concentration at 48 h of growth showed that S. marcescens CTM 50650 produced approximately 45 g/l of GA and 10.85 g/l citric acid and released 595 and 106 mg/l of soluble P when glucose and fructose were used as carbon sources, respectively.
Identification by HPLC of organic acids in the culture filtrates of Serratia marcescens CTM 50650 grown at 30°C on NBRIP glucose (a) or fructose (b) supplemented with 5 g/l of hydroxyapatite for 48 h. The chromatographic separations show A260 (y axis) versus time (minutes). Gluconic acid and citric acid at 50 and 10 g/l in 0.05 M NaH2PO4 (pH = 6.5) were added to filtrates of the corresponding un-inoculated media and used as standards in (a) and (b), respectively. T = 0 h: culture filtrate taken prior to bacterial incubation. T = 48 h: filtrate from culture grown for 48 h. The retention times of gluconic acid and citric acid were of approximately 9.850 and 9.400, respectively. The peaks corresponding to gluconic acid and citric acid, were indicated by asterisks
Effect of cultural conditions on RP and hydroxyapatite solubilization by Serratia marcescens CTM 50650
Investigation of the effect of cultivation temperatures ranging from 20 to 37°C showed that the solubilization of RP and hydroxyapatite by CTM 50650 was optimal at 30°C (Fig. 4a). The concentration of the released soluble P2O5 and P was of 751 and 595 mg/l, respectively.
Effect of temperature (a), pH (b), nature of carbon source (c) and glucose concentration (d) on the solubilization of RP and hydroxyapatite by Serratia marcescens CTM 50650. Each value in the panel represents mean ± SD. (fru fructose, gal galactose, xyl xylose, glu glucose, ara arabinose, gly glycerol, mal maltose, sor sorbitol, none without carbon source)
The influence of initial pH was studied in the range of pH 4–10. The MPS efficiencies were elevated at the different pH values tested with an optimum at pH 6 (Fig. 4b). This result suggests that the S. marcescens CTM 50650 isolate is possibly an acid- and alkali-tolerant bacterium.
To investigate the effect of the nature of the carbon source on the MPS ability of CTM 50650, various carbon sources were provided at the concentration of 10 g/l. Figure 4c shows that this isolate is able to solubilize RP and hydroxyapatite with different carbon sources. Glucose and to a lesser extent glycerol, supported the highest solubilization. Our study also showed that a carbon and energy source is required for the MPS ability of S. marcescens CTM 50650. To determine the effect of glucose concentration on MPS efficacy, various concentrations of glucose ranging from 2 to 20 g/l were added to the medium. Figure 4d shows that the solubilization augments with increasing concentration of glucose up to 10 g/l.
Screening of MPS genes in the Serratia marcescens CTM 50650 strain
The fact that CTM 50650 produced GA in the presence of glucose strongly suggested the presence of genes involved in the MPS phenotype through the activation of direct oxidation pathway of glucose via the quinoprotein glucose dehydrogenase (GDH) that requires as cofactor PQQ encoded by the pqq genes. In order to confirm this hypothesis, we performed PCR amplification experiments using specific primers designed to amplify concomitantly pqqA + pqqB, pqqC, pqqE and gdh for the first time in a S. marcescens producing GA. Figure 5 shows that the results obtained confirmed that the isolate S. marcescens CTM 50650 exhibited bands of the expected size corresponding to the whole of pqqA + pqqB (1,064 bp) and fragments internal to pqqC (568 bp) and pqqE (900 bp). For gdh, we amplified a fragment of ~1.2 kb instead of ~1.4 kb as reported by Pujol and Kado (1999). Partial sequencing of these PCR products confirms that they correspond to the genes being amplified. In fact, the partial DNA sequence of the S. marcescens CTM 50650 pqqA + pqqB (871 bp), pqqC (557 bp) and pqqE (280 bp) showed a highest identity of 91, 94 and 93% with their putative homologues from the Serartia marcescens W1 strain (GenBank accession no. DQ868536). For gdh (334 bp sequenced) the highest identity (89%) was observed with the putative apoquinoprotein glucose dehydrogenase (GCD) of S. marcescens R2 (GenBank accession no. AF441442).
Discussion
Our study demonstrated the presence in the Tunisian phosphate mine of Gafsa, a biotope rich in insoluble RP, of bacterial isolates (18.6% of total bacteria) able to develop clear phosphate solubilization zones greater than 5 mm on NBRIP agar containing hydroxyapatite. Similar proportions were reported in previous studies on phosphate solubilizing bacteria from phosphate mines. For instance, Hamdali et al. (2008) showed that the abundance of Actinomycetes able to solubilize RP in Moroccan phosphate mines was approximately of 18.3%. Similarly, Reyes et al. (2006) have found that phosphate solubilizing bacteria (mainly Azotobacter sp.) from the Monte-Fresco RP mine in Venezuela represent 19% of total bacteria.
Among the whole of our screened isolates, five strains designed US540, US468, US537, US549 and US557 exhibiting the greatest halo size were selected for quantification of phosphate solubilization on NBRIP broth supplemented with hydroxyapatite, Gafsa RP, CaHPO4 and Ca3(PO4)2. The results confirmed the MPS activity of all the five strains on the four sparingly soluble phosphates with some differences in the degrees of MPS efficiencies. In fact, the strain US540, which has been assigned the CTM 50650 name (deposed in the Collection of Tunisian Micoorganisms), comes out as the most efficient PSB. This strain was identified as S. marcescens CTM 50650 by biochemical and molecular approaches. The study carried out with CTM 50650 showed that the maximal soluble P concentration it produced in the presence of hydroxyapatite, CaHPO4 and Ca3(PO4)2, was of 595, 967 and 500 mg/l. When the Gafsa RP was used as phosphate source, the highest soluble P2O5 concentration released reached 754 mg/l. This result was in accordance with previous studies showing that members of the Serratia genus display high MPS activities (Chen et al. 2006; Hameeda et al. 2006; Pérez et al. 2007). Table 2 shows the excellent MPS efficiency of S. marcescens CTM 50650 among some of the phosphate solubilizing bacteria previously reported to exhibit the high MPS activity, and confirmed the good potential of our PSB especially in RP solubilization. Indeed, when using S. marcescens CTM 50650, a yield of P extraction of around 75% could be achieved in NBRIP broth containing the Gafsa RP (% of total P2O5 is of 28.11%) as unique phosphate source after only 30–48 h of incubation. Some earlier observations based on fungal strains (Bojinova et al. 2008; Reddy et al. 2002) obtained about 90% of solubilization of Morocco rock phosphate (with a P2O5 content of 31.14%) but for periods up to 15 days of cultivation.
The solubilization of inorganic phosphates by the S. marcescens CTM 50650 strain was concomitant with medium acidification. A major well-characterized mechanism of mineral phosphate solubilization in Gram-negative bacteria is GA secretion, a property shared by PSM members of the Enterobacteriaceae family (Buch et al. 2008; Hameeda et al. 2006; Han et al. 2008; Kim et al. 1997; Lin et al. 2006). This low-molecular weight organic acid results from the extracellular oxidation of glucose via the quinoprotein GDH (Goldstein 1996) that requires as cofactor PQQ, whose biosynthesis involves the pqq genes (Kim et al. 1998, 2003; Meulenberg et al. 1992). The decline in pH observed during phosphate solubilization by the S. marcescens CTM 50650 strain was inversely correlated with the concentration of released P as reported in previous studies (Chen et al. 2006; Hwangbo et al. 2003; Kim et al. 1997), suggesting organic acids secretion by this isolate. Interestingly, analysis of culture supernatants of S. marcescens CTM 50650 grown on NBRIP containing hydroxyapatite (as well as the other inorganic phosphate sources) allowed us to detect the presence of this particular organic acid in presence of glucose. Conversely, use of fructose as carbon source supported citric acid production throughout the tricarboxylic acid cycle as proposed by Reyes et al. (1999). This was in agreement with previous studies on members of the Serratia genera that have been shown to produce also citric acid (Chen et al. 2006; Hameeda et al. 2006; Pérez et al. 2007). Furthermore, the release of GA by the S. marcescens CTM 50650 strain was shown to be correlated with the simultaneous detection by PCR for the first time in a S. marcescens strain producing GA, of the gene encoding GDH (gdh), the enzyme responsible for gluconic acid production as well as the genes pqqA, B, C and E involved in the biosynthesis of the PQQ cofactor. The correspondence of PCR products to the gene being amplified was confirmed by sequencing and homology search.
In summary, subsequent to this study, the development of new biotechnological process using S. marcescens CTM 50650 for fertilizer production can be considered, after further investigations including the improvement of the MPS efficiency, the characterization of the nature of the products yielded by this biologic solubilization and the study of the economic feasibility of competing the traditional chemical RP processing.
References
Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria with non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204(1):57–67
Arcand M, Schneider KD (2006) Plant- and microbial-based mechanisms to improve the agronomic effectiveness of phosphate rock: a review. An Acad Bras Cienc 78:791–807
Bojinova D, Velkova R, Ivanova R (2008) Solubilization of Morocco phosphorite by Aspergillus niger. Bioresource Technol 99:7348–7353
Buch A, Archana G, Naresh KG (2008) Metabolic channelling of glucose towards gluconate in phosphate solubilizing Pseudomonas aeruginosa P4 under phosphorus deficiency. Res Microbiol 159:635–642
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41
Farhat A, Chouayekh H, Ben Farhat M, Bouchaala K, Bejar S (2008) Gene cloning and characterization of a thermostable phytase from Bacillus subtilis US417 and assessment of its potential as a feed additive in comparison with a commercial enzyme. Mol Biotechnol 40:12–135
Goldstein AH (1996) Involvement of the quinoprotein glucose dehydrogenase in the solubilization of exogenous phosphates by Gram negative bacteria. In: Torriani-Gorini A, Yagil E, Silver S (eds) Phosphate in microorganisms: cellular and molecular biology. ASM Press, Washington DC, pp 197–203
Gulati A, Rahi P, Vyas P (2008) Characterization of phosphate-solubilizing Fluorescent Pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr Microbiol 56:73–79
Gyaneshwar P, Parekh LJ, Archana G, Poole PS, Collins MD, Hutson RA, Naresh KG (1999) Involvement of a phosphate starvation inducible glucose dehydrogenase in soil phosphate solubilization by Enterobacter asburiae. FEMS Microbiol Lett 171:223–229
Gyaneshwar P, Kumar N, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93
Hamdali H, Bouizgarne B, Hafidi M, Lebrihi A, Virolle MJ, Ouhdouch Y (2008) Screening for phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Appl Soil Ecol 38:12–19
Hameeda B, Reddy Y, Rupela OP, Kumar GN, Reddy G (2006) Effect of carbon substrates on rock phosphate solubilization by bacteria from composts and macrofauna. Curr Microbiol 53:298–302
Han SH, Chul HK, Jang HL, Ju YP, Song MC, Park SK, Kim KY, Hari BK, Kim YC (2008) Inactivation of pqq genes of Enterobacter intermedium 60-2G reduces antifungal activity and induction of systemic resistance. FMS Res Lett 10:1111–1120
Hwangbo H, Park RD, Kim YW, Rim YS, Park KH, Kim TH, Suh JS, Kim KY (2003) 2-ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr Microbiol 47:87–92
Johnson SE, Loepper RH (2006) Role of organic acids in phosphate mobilization from iron oxide. Soil Sci Soc Am J 70:222–234
Kim KY, Jordan D, Krishnan HB (1997) Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilise hydroxyapatite. FEMS Microbiol Lett 153:273–277
Kim KY, Jordan D, Krishnan HB (1998) Expression of genes from Rahmella aquatilis that are necessary for mineral phosphate solubilization in Escherichia coli. FEMS Microbiol Lett 159:121–127
Kim CH, Han SH, Kim KY, Cho BH, Kim YH, Koo BS, Kim YC (2003) Cloning and expression of pyrroloquinoline quinine (PQQ) genes from a phosphate-solubilizing bacterium Enterobacter intermedium. Curr Microbiol 47:457–461
Kpomblekou AK, Tabatabai MA (1994) Effect of organic acids on release of phosphorus from phosphate rock. Soil Sci 158:442–453
Lin TF, Huang HI, Shen FT, Young CC (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Bioresource Technol 97:957–960
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 86:1–25
Macias FA, Marin D, Oliveros-Bastidas A, Varela RM, Simonet AM, Carrera C, Molinillo JMG (2003) Allelopathy as a new strategy for sustainable ecosystems development. Biol Sci Space 17:18–23
Meulenberg JJM, Sellink E, Riegman NH, Postma W (1992) Nucleotide sequence and structure of the Klebsiella neumoniae pqq operon. Mol Gen Genet 232:284–294
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Patel DK, Archana G, Kumar GN (2008) Variation in the nature of organic acid secretion and mineral phosphate solubilization by Citrobacter sp. DHRSS in the presence of different sugars. Curr Microbiol 56:168–174
Pérez E, Sulbaran M, Ball M, Yarzabal LA (2007) Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem 39:2905–2914
Pujol C, Kado C (1999) gdhB, a gene encoding a second quinoprotein glucose dehydrogenase in Pantoea citrea, is responsible for pink disease of pineapple. Microbiology 145:1217–1226
Rajan SSS, Watkinson JH, Sinclair AG (1996) Phosphate rocks for direct application to soils. Adv Agron 57:77–159
Reddy MS, Kumar S, Babita K, Reddy MS (2002) Biosolubilization of poorly soluble rock phosphates by Aspergillus tubingensis and Aspergillus niger. Bioresource Technol 84:187–189
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312
Reyes I, Bernier L, Simard R, Antoun H (1999) Effect of nitrogen source on solubilization of different inorganic phosphates by an isolate of Pencillium rugulosum and two UV-induced mutants. FEMS Microbiol Ecol 28:281–290
Reyes I, Valery A, Valduz Z (2006) Phosphate solubilizing microorganisms isolated from rhizospheric and bulk soils of colonizer plants at an abandoned rock phosphate mine. Plant Soil 287:69–75
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotech Adv 17:319–339
Sambrook JE, Fritsh EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Son HJ, Park GT, Cha MS, Heo MS (2006) Solubilization of insoluble inorganic phosphates by a novel salt and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresource Technol 97:204–210
Sulbarán M, Pérez E, Ball MM, Bahsas A, Yarzábal LA (2009) Characterization of the mineral phosphate-solubilizing activity of Pantoea agglomerans MMB051 isolated from an iron-rich soil in southeastern Venezuela (Bolívar State). Curr Microbiol 58(4):378–383
Tandon HLS, Cescas MP, Tyner EH (1968) An acid-free vanadate-molybdate reagent for the determination of total phosphorus in soils. Soil Sci Soc Am Proc 32:48–51
Vance CP (2001) Symbiotic nitrogen fixation and phosphorus acquisition plant nutrition in a world of declining renewable resources. Plant Physiol 127:390–397
Vassilev N, Vassileva M, Nikolaeva I (2006) Simultaneous P solubilizing and biocontrol activity of microorganisms: potentials and future trends. Appl Microbiol Biotechnol 71:137–144
Whitelaw MA (2000) Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv Agron 69:99–151
Zou K, Binkley D, Doxtader KG (1992) New method for estimating gross P mineralization and mobilization rates in soils. Plant Soil 147:243–250
Acknowledgments
This research was funded by the Tunisian Government “Contrat Programme CBS-LEMP” and the local Company “Groupe Chimique Tunisien” through a PhD Fellowship to Miss Mounira Ben Farhat. The authors are grateful to Dr. Sofiane Ghorbel for his contribution in soil sampling and to Mr Kamel Walha and Ms Najla Fourati for their help in monitoring HPLC apparatus. Many thanks also to Dr. Abdelhafidh Dhouib, Mr Aboulhassen Charfi, Mr Foued Khmiri and Ms Najoua Ammar for their collaboration.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Shuang-Jiang Liu.
Rights and permissions
About this article
Cite this article
Ben Farhat, M., Farhat, A., Bejar, W. et al. Characterization of the mineral phosphate solubilizing activity of Serratia marcescens CTM 50650 isolated from the phosphate mine of Gafsa. Arch Microbiol 191, 815–824 (2009). https://doi.org/10.1007/s00203-009-0513-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-009-0513-8