Abstract
Summary
We evaluated the relationship of bone mineral density (BMD) by computed tomography (CT), to predict fractures in a multi-ethnic population. We demonstrated that vertebral and hip fractures were more likely in those patients with low BMD. This is one of the first studies to demonstrate that CT BMD derived from thoracic vertebrae can predict future hip and vertebral fractures.
Purpose/Introduction
Osteoporosis affects an enormous number of patients, of all races and both sexes, and its prevalence increases as the population ages. Few studies have evaluated the association between the vertebral trabecular bone mineral density(vBMD) and osteoporosis-related hip fracture in a multiethnic population, and no studies have demonstrated the predictive value of vBMD for fractures.
Method
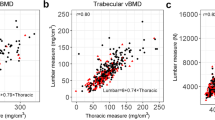
We sought to determine the predictive value of QCT-based trabecular vBMD of thoracic vertebrae derived from coronary artery calcium scan for hip fractures in the Multi-Ethnic Study of Atherosclerosis(MESA), a nationwide multicenter cohort included 6814 people from six medical centers across the USA and assess if low bone density by QCT can predict future fractures. Measures were done using trabecular bone measures, adjusted for individual patients, from three consecutive thoracic vertebrae (BDI Inc, Manhattan Beach CA, USA) from non-contrast cardiac CT scans.
Results
Six thousand eight hundred fourteen MESA baseline participants were included with a mean age of 62.2 ± 10.2 years, and 52.8% were women. The mean thoracic BMD is 162.6 ± 46.8 mg/cm3 (95% CI 161.5, 163.7), and 27.6% of participants (n = 1883) had osteoporosis (T-score 2.5 or lower). Over a median follow-up of 17.4 years, Caucasians have a higher rate of vertebral fractures (6.9%), followed by Blacks (4.4%), Hispanics (3.7%), and Chinese (3.0%). Hip fracture patients had a lower baseline vBMD as measured by QCT than the non-hip fracture group by 13.6 mg/cm3 [P < 0.001]. The same pattern was seen in the vertebral fracture population, where the mean BMD was substantially lower 18.3 mg/cm3 [P < 0.001] than in the non-vertebral fracture population. Notably, the above substantial relationship was unaffected by age, gender, race, BMI, hypertension, current smoking, medication use, or activity. Patients with low trabecular BMD of thoracic vertebrae showed a 1.57-fold greater risk of first hip fracture (HR 1.57, 95% CI 1.38–1.95) and a nearly threefold increased risk of first vertebral fracture (HR 2.93, 95% CI 1.87–4.59) compared to normal BMD patients.
Conclusion
There is significant correlation between thoracic trabecular BMD and the incidence of future hip and vertebral fracture. This study demonstrates that thoracic vertebrae BMD, as measured on cardiac CT (QCT), can predict both hip and vertebral fractures without additional radiation, scanning, or patient burden. Osteopenia and osteoporosis are markedly underdiagnosed. Finding occult disease affords the opportunity to treat the millions of people undergoing CT scans every year for other indications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bone fragility and fracture risk are increased by osteoporosis, a systemic disease marked by structural changes in bone trabeculae and a decline in bone mineral density (BMD) [1]. One of today’s significant public health concerns is osteoporotic fractures, with the hip as the primary fracture site [2], and the annual number of fractures caused by osteoporosis is expected to rise significantly. Currently, most of the attention on osteoporotic fractures is focused on hip fractures because of their apparent symptoms and severe impact on quality of life, even leading to death. Hip fractures can be caused by various reasons, including skeletal and fall-related problems [3]. Early and accurate identification of individuals at risk of hip fracture is required to prevent osteoporosis complications. The fracture can be predicted by detecting the degree of osteoporosis due to a link between the two variables [4,5,6]. The dual-energy X-ray absorptiometry (DXA) is the most commonly used non-invasive imaging tool in clinical practice for assessing fracture risk. It has produced excellent results for evaluating osteoporosis and fractures in postmenopausal women. However, more data from clinical epidemiology suggests that DXA-based bone densitometry at the hip site has significant limitations for predicting osteoporotic fractures even as the “Gold Standard” for diagnosing osteoporosis and fracture [7]. The main limitations are as follows: (1) T-scores of 2.5 SD or lower are considered osteoporosis in the DXA assessment system. More accurate assessment of bone density and fracture risk may help direct therapies better, as BMD is approximately normally distributed, and even if those with osteoporosis have the highest risk, many fractures occur in those with higher BMD; (2) DXA is of limited utility in patients with spinal abnormalities or previous spinal surgery (however, this does not apply to DXA of the hip), and (3) Two-dimensional (2D) low-resolution scans cannot differentiate between bone cortex and trabecular bone density; however, no study has demonstrated that separation of cortical and trabelucar bones provides significant advantages for hip fracture prediction. It is crucial to find approaches to better estimate fracture risk, quantify BMD with greater accuracy, and provide effective treatment strategies.
Initial studies suggested that the trabeculae bone, rather than cortical bone, is more suited for assessing BMD, as well as for following osteoporosis and pharmaceutical treatments [8,9,10,11,12,13]. CT scans of the heart or chest are being used with increasing frequency to detect lung cancer or determine the degree of coronary artery calcification. The latest data suggest that BMD and osteoporosis risk classification can be accurately determined using non-contrast CT-based spinal BMD evaluation without incurring additional medical expenses or radiation exposure issues [14, 15]. Based on MESA data, this study investigated the predictive significance of trabecular BMD for vertebral and hip osteoporotic fractures in a community-based, multicenter, multiethnic atherosclerosis cohort study.
Methods
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a population-based prospective cohort study to identify risk factors for subclinical atherosclerosis, which includes 6814 participants, free from known CVD at baseline, comprising four race/ethnic subgroups, recruited from six clinical sites across the USA. All subjects completed cardiac CT scans for subclinical atherosclerosis evaluation at a baseline and subsequent multiple follow-up visits over 17 years.
Exposure variables
Covariates from the MESA include age, sex, race/ethnicity, systolic and diastolic blood pressure, hypertension (JNC VI definition), weight, body mass index (BMI) and waist circumference (all measured), cigarette smoking status, menopausal (year), 25 hydroxy-vitamin D, parathyroid hormone, and medication use (including corticosteroids, antacids, hormone replacement therapy, bisphosphonates, selective serotonin reuptake inhibitor, and statins).
CT scan protocol
Detailed methods were published earlier [13]. Briefly, the participants’ cardiac CT images were collected by MESA from six medical centers across the USA [14]. The specific number of images are as follows: 443 (21.8%) sets of cardiac CT images from Johns Hopkins University, 400 (19.7%) images from the University of Minnesota, 386 (19.0%) images from Northwestern University, and 369 (18.2%) images from the University of California, Los Angeles. The CT scanning parameters are uniformly set limited to 130 kVp and variable mA according to participants’ body weight with baseline tube current 630 mAs at Chicago, Los Angeles, and New York. Another three medical centers from Baltimore, Forsyth County, and St. Paul use kVp of 120 and tube current mAs of 600. The trabecular vertebral BMD was measured on the NVivo workstation (Columbia, KY). Mean doses were < 1 millisievert for this cohort.
Quantitative assessment of trabecular BMD
The measurement of three consecutive thoracic vertebral BMDs started at the section level containing the left main coronary artery caudally (Fig. 1). Only the images with at least T7-T11 vertebrae were used. The ROIs were placed in the center of each vertebral body, 2 mm from the spinal column’s cortical margin. The low-quality image regions and pathological changes, such as extraosseous calcium deposits, a crush fracture, and image regions with high noise, are automatically reconstructed or excluded (54 cases). The density of all bones was measured as trabecular bone components. Two experienced physicians evaluated the quality of BMD [16, 17]. In this study, we also analyzed the T-score, a traditional osteoporosis indicator. A T-score between − 1 and less than − 2.5 indicates osteopenia, and a T-score of − 2.5 or lower indicates osteoporosis.
The method of conversion of the Hounsfield units (HU) value to bone mineral density (BMD) was originally performed with this method by earlier work using calibration phantoms that contain known concentrations of calcium hydroxyapatite (in mg/cm3) which were scanned when the patient underwent their CT scans. From this earlier work, our methodology now entails that the mean and standard deviation of the HU values were transformed into calcium hydroxyapatite concentration (mg/cm3) using the corresponding slope and the linear regression line [18].
Hip fracture assessment
Hip fracture diagnosis was identified upon review of International Classification of Disease (ICD) 9 and 10 codes associated with the participant’s hospital records, as well as reports from in-person examinations of hip fractures from interviewer-administered medical and surgical history forms.
Vertebral fracture assessment
Vertebral fractures were detected from all included thoracic vertebrae in each scan. Vertebral heights in the sagittal reconstructions were obtained from 2.5-mm thick CT slices. Osteoporosis-related hip fracture and vertebral fracture were classified based solely on morphometry measurements using the Genant et al. criteria [19] and the Lenchik methods [20]. Briefly, posterior heights of the T1 to L5 vertebrae were measured. The degree of vertebral deformity was derived by comparing a ratio of heights with the adjacent upper and lower vertebral body when, on visual inspection, it exhibited more than 15% height loss, and fractures were graded as mild (15–24% reduction), moderate (25–49% reduction), or severe (> 50% reduction) based on the degree of height loss (Fig. 1). The vertebral volume reduction is also referenced to the change of the adjacent vertebrae. Only moderate to severe compression vertebral fractures were recorded to avoid false-positive and ambiguity with mild borderline deformities. Changes in the degree of compression and deformation of the vertebral body were also quantitatively recorded by comparing a ratio of heights with the adjacent upper and lower vertebral body.
Prevalent fractures were considered if present on baseline CT scans at examination 1. Incident fractures were considered if newly found on subsequent scans. MESA participants underwent CT scanning approximately every 2.5 years over 10 years, so time to fracture was also assessed.
Model for prediction
A predictive model for thoracic spine osteoporosis and fracture is implemented in a two-step strategy. The initial analysis is based on univariate analysis for the initial screening of covariates in the model. In this step, the image features extraction focused on the trabecular BMD of thoracic vertebrae, which was decided by CT Hounsfield units (HU) value. The covariates in the multivariate regression model were used to quantify the relationship between thoracic BMD and fracture risk through both the unadjusted model and adjusted model based upon gender, age, race/ethnicity, body mass index (BMI), current tobacco use, use of three or more units of alcohol per day, and other causes of secondary osteoporosis. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
The demographic and clinical characters of the participants by race
The basic demographic and clinical characteristics of the participants stratified by race are shown in Table 1. There were 3601 women (52.8%) among the 6814 men and women with a mean age of 62.2 ± 10.2 years. The subgroup’s race/ethnicity was as follows: 2622 (38.5%) Caucasians, 804 (11.8%) Chinese Americans, 1892 (27.8%) African Americans, and 1496 (22.0%) Hispanics.
The mean thoracic BMD is 162.6 ± 46.8 mg/cm3 (95% CI 161.5, 163.7), and 27.6% of participants (n = 1883) had osteoporosis (T-score 2.5 or lower). Table 1 displays the values for vertebral BMD and fracture across multiple sociodemographic groups. The mean BMD of the thoracic spine was 162.6 ± 46.8 mg/cm3 (95% CI 161.5, 163.7). Over a median follow-up of 17.4 years, the mean time to first hip fracture was 11.1 ± 3.0 years, and the mean time to first vertebral fracture was 9.8 ± 3.7 years. The test for differences in fractures by race/ethnicity was performed for significant effect modification (Table 1). When stratified by race, Caucasians have a higher rate of vertebral fractures (6.9%), followed by African Americans (4.4%), Hispanics (3.7%), and Chinese (3.0%). For hip fractures, similar but slightly different fracture distribution patterns are as follows: Whites had the highest rate of hip fractures (1.9%), followed by Hispanics (0.6%), and Chinese (0.6%), and African Americans had the lowest rate of hip fractures (0.4%). The hip fracture rate was significantly higher in women than men (1.3% vs. 0.7%, P = 0.024). No significant differences in rates of vertebral fractures were seen across genders (5.1% vs. 5.0% for men and women respectively, P = 0.94). There was a high consistency between vertebral fracture and hip fracture.
Osteoporosis
We also analyzed the T-score, a traditional indicator of osteoporosis. The T-score for the QCT-based trabecular vBMD of thoracic vertebrae was calculated by the difference between the individual’s BMD and a reference population mean, divided by the standard deviation of the reference population, derived from the bone mass of an average healthy 30-year old adult, which we derived using CAC scans using the same method [16, 17]. In this study population, the proportion of people with normal BMD was 29.8% (T-score ≥ − 1). 42.5% of participants had low BMD but had not yet developed osteoporosis (− 2.5 < T-score < − 1), whereas 27.6% developed osteoporosis (T-score ≤ − 2.5). When stratified by race, African Americans had higher T-scores (mean − 1.0 ± 1.4), followed by Hispanics (mean T-score − 1.5) and Chinese (mean T-score − 1.5). Whites had the lowest T-score values (mean T-score − 2.1 ± 1.2), significantly lower than African Americans (P < 0.001).
We observed the presence of a clear linear relationship showing lower thoracic trabecular BMD is associated with a higher risk of incident hip fractures (Table 2). We also found that a lower trabecular thoracic BMD was associated with a higher risk of hip fracture after adjustment of age, gender, race/ethnicity, BMI, hormone levels (25OHD and PTH), and other traditional CAD risk factors. This was confirmed by the results of the multivariate regression model after adjusting the above confounders.
Compared with the non-fracture group, the hip fracture group had significantly lower trabecular BMD in the thoracic spine than the non-fracture group (BMD β (Se): − 15.34 kg/mm3, 95% CI − 24.64, − 6.04, P = 0.0012). The same trend was seen in other non-hip fracture groups (BMD β (Se): − 16.43 (2.03), 95% CI − 20.42, − 12.45, P < 0.001). In Cox proportional hazards models, a lower trabecular thoracic BMD was associated with a higher risk of hip fracture after adjustment by age, gender, race/ethnicity, BMI, hormone levels (25OH vitamin D and PTH), and other traditional CAD risk factors, as demonstrated by the multivariate regression model after adjusting the above confounders. Patients with low trabecular BMD of thoracic vertebrae showed a 1.57-fold greater risk of first hip fracture (HR 1.57, 95% CI 1.38–1.95) and a nearly threefold increased risk of first vertebral fracture (HR 2.93, 95% CI 1.87–4.59) compared to normal BMD patients.
Additional multivariable regression analysis were performed evaluating the relationship between thoracic BMD and hip fracture (Table 3). Hip fracture patients had a lower baseline BMD than the non-hip fracture group by 13.6 mg/cm3 (95% CI − 22.8, − 4.4 mg/cm3, P < 0.001). The same pattern was seen in the vertebral fracture, where the mean BMD was substantially lower at 18.3 mg/cm3 (95% CI − 22.5, − 14.0 mg/cm3, P < 0.001) than in the non-vertebral fracture population. Notably, the above substantial relationship was unaffected by age, gender, race/ethnicity, BMI, hormone levels (25OH vitamin D and PTH), and other traditional CAD risk factors demonstrated by the multivariate regression model after adjusting the above confounders.
Discussion
This study demonstrates that thoracic BMD predicts both future vertebral and hip fractures. The risk of undetected osteoporosis is too high, and can be moderated by detection of BMD on every lung and cardiac CT scan performed. This could leverage many of the 90 million CT scans done every year and allow opportunistic measure of BMD to allow for earlier detection of osteoporosis, affording patients and clinicians the ability to institute treatments, both with lifestyle and medical therapy as needed, prior to the fracture or advancement of disease. In this study, we analyzed MESA baseline CT scans and evaluated the value of thoracic vertebral BMD for incident vertebral and hip fractures (mean follow up of 12 years). Based on data from the National Health and Nutrition Examination Survey III (NHANES III), the National Osteoporosis Foundation (NOF) has estimated that approximately ten million Americans have osteoporosis and an additional 43 million have osteopenia [21]. Approximately half of Caucasian women and one in five men will experience an osteoporosis-related fracture at some point in their lifetime. Although osteoporosis is less frequent in African Americans, those who develop osteoporosis had a similar elevated fracture risk as Caucasians in this study. However, it has been African American women who sustain a fracture from osteoporosis have a one-third higher mortality than white women, highlighting the need for equity in access to osteoporosis screening [22]. The hazards of hip fractures have long been underestimated. Once a patient suffers a hip fraction, they have a 8–36% increased risk of death within 1 year, and a higher mortality in men than women [22]. Approximately 20% of hip fracture patients require long-term nursing home care, and less than half fully regain their pre-fracture level of independence [23]. Cardiac computed tomography (CT), as a 3D high-definition imaging modality has been shown to be sensitive for detecting osteoporotic vertebral fractures, and can increasing play an important role in the diagnosis and management of osteoporotic vertebral fractures. To date, tens of millions of CT scans are taken annually in the USA [24]. If utilized to assess osteoporosis and predict fracture risk, it would considerably boost diagnosis of osteoporosis and screen for people at high risk of hip fracture. An increasing body of evidence demonstrates that CT can assess bone microstructure [25] with improved resolution and accuracy as compared to other methods (ultrasound, MRI, or DXA) [26, 27]. Furthermore, the ability to detect slight changes in bone density makes it possible to intervene clinically in the early stages of osteoporosis [28]. The significant association between vertebral BMD and hip fracture shown in our work will also allow tens of millions of patients to acquire an assessment of bone health condition and hip fracture risk prediction based on chest CT images without additional radiation, scans, or patient burden. This can be introduced during or after CT acquisition, as no phantom or specific acquisition methodology is required, and it has been validated on numerous CT platforms [17].
The National Osteoporosis Foundation (NOF) recommends BMD assessment for all women age 65 years and older and men age 70 years and older, regardless of clinical risk factors, as well as younger postmenopausal women, women in the menopausal transition, and men age 50 to 69 years with clinical risk factors for fracture [23]. Furthermore, adults with a fracture after age 50 years, and conditions such as rheumatoid arthritis and medications (such as prednisone) that are associated with bone loss. Sensitive detection of BMD is a key issue to monitor and evaluate the individual’s bone mineral status [17, 25, 29,30,31] and has been shown in multiple studies to be more sensitive than DXA [31]. We have compared the 3 T method utilized here to DXA scans and demonstrated that thoracic CT BMD provides a more sensitive method for detecting bone mineral loss when compared to DXA. Quantitative computed tomography has excellent data on BMD and fracture risk assessment, and population-based measures [16, 30]. We have demonstrated that CT has the capability to assess the microarchitecture of the bone [25], with superior resolution and accuracy as compared to other measures (ultrasound, magnetic resonance, or DXA) [26, 27]. Furthermore, there are ample therapies to treat osteoporosis and osteopenia, so detection will allow for earlier intervention [28]. Knowing that this 3 T method of BMD detection can predict fractures will enable tens of millions to measure BMD without additional radiation, scanning, or patient burden. This can be added at the time of CT acquisition or retrospectively, as no phantom or specific acquisition protocols are required, and has been validated on numerous CT platforms [17].
Limitations
Limitations may affect our results. Due to the lack of available data in MESA, we did not compare QCT thoracic vertebral BMD to DXA-based hip BMD, as those variables were not available; however, we previously compared results of 3 T thoracic bone density to DXA and found high correlation [28]. While data on medications for participants in MESA is available, it is possible some participants’ osteoporosis medication records were missing, which may have led to differing BMD values and fracture risk. It is possible that site-specific assessment is superior in order to predict site-specific fracture risk (i.e., QCT or DXA of the hip to predict hip fractures). A prospective study comparing DXA of the hip to CT of the spine is warranted, but beyond the scope of this study.
We demonstrated that the OR for a hip fracture is 1.57, somewhat lower than other cohort studies evaluating QCT. The reason for this may be twofold. First, our population is healthier, and not specifically at risk of osteoporosis, unlike the other studies done in a higher risk population. We used a healthy cohort of patients undergoing cardiac CT in this study. Furthermore, it may be possible that lumbar CT is a superior method to assess BMD, but no head-to-head comparison is available. We have demonstrated previously that changes in thoracic vertebrae occur earlier than lumbar, so it is possible that thoracic is an earlier sign of bone loss, requiring longer follow-up to demonstrate incident fractures [9, 31].
Finally, measures of BMD using this technique were not performed in a completely automated fashion—this is an important deficiency for opportunistic screening, and fully automated systems are being developed to address mass screening.
Conclusions
In summary, our data demonstrate that the trabecular BMD status, as measured on three consecutive thoracic vertebrae, was an independent predictor of future hip and vertebral fractures in this population-based study. This finding has important clinical implications. Our results support our initial hypothesis that the trabecular BMD status at the thoracic vertebrae was sensitive to identify osteopenia and osteoporosis and predicted hip and vertebral fractures. It suggests that measuring BMD at the thoracic vertebrae could be a useful tool for identifying patients, who are at high risk for hip and vertebral fractures without additional burden and radiation dose exposure, and effective at screening of people at risk for fracture across multiple ethnicities, races, ages, and gender.
Data Availability
The data that support the findings of this study are available from the corresponding author, [MJB], upon reasonable request.
References
Sozen T, Ozisik L, Basaran NC (2017) An overview and management of osteoporosis. Eur J Rheumatol 4(1):46–56
van Oostwaard M (2018) Osteoporosis and the nature of fragility fracture: an overview. In: Hertz K, Santy-Tomlinson J (eds) Fragility fracture nursing: holistic care and management of the orthogeriatric patient [Internet]. Cham (CH): Springer, Chapter 1
QuickStats (2021) Percentage* of adults aged >/=50 years with osteoporosis, (dagger) by race and hispanic origin ( section sign) - United States, 2017–2018. MMWR Morb Mortal Wkly Rep. 70(19):731. https://doi.org/10.15585/mmwr.mm7019a5
Terzini M, Aldieri A, Rinaudo L, Osella G, Audenino AL, Bignardi C (2019) Improving the hip fracture risk prediction through 2D finite element models from DXA images: validation against 3D models. Front Bioeng Biotechnol 7:220
Hong N, Park H, Kim CO, Kim HC, Choi JY, Kim H et al (2021) Bone radiomics score derived from DXA hip images enhances hip fracture prediction in older women. J Bone Miner Res 36(9):1708–1716
Jazinizadeh F, Adachi JD, Quenneville CE (2020) Advanced 2D image processing technique to predict hip fracture risk in an older population based on single DXA scans. Osteoporos Int 31(10):1925–1933
Hadidi F, Khalil OA, Alhadidy A, Alqawaba R, Hammad Y, Samarah OQ et al (2021) Correlation between plain X-ray measurements and DXA scans, and its possible use as a screening tool for performing DXA scans. Int J Clin Pract 75(11):e14784
Gruenewald LD, Koch V, Martin SS, Yel I, Eichler K, Gruber-Rouh T et al (2022) Diagnostic accuracy of quantitative dual-energy CT-based volumetric bone mineral density assessment for the prediction of osteoporosis-associated fractures. Eur Radiol 32(5):3076–3084
Zhu Y, Triphuridet N, Yip R, Becker BJ, Wang Y, Yankelevitz DF et al (2021) Opportunistic CT screening of osteoporosis on thoracic and lumbar spine: a meta-analysis. Clin Imaging 80:382–390
Cheng X, Zhao K, Zha X, Du X, Li Y, Chen S et al (2021) Opportunistic screening using low-dose CT and the prevalence of osteoporosis in China: a nationwide, multicenter study. J Bone Miner Res 36(3):427–435
Keppler AM, Kraus M, Blaschke M, Thomasser N, Kammerlander C, Bocker W, et al. (2021) Reduced awareness for osteoporosis in distal radius fracture patients compared to patients with proximal femur fractures. J Clin Med 10(4). https://doi.org/10.3390/jcm10040848
Capaldi N, Kao KT, MacDonald R, Grainger KC, Joseph S, Shepherd S et al (2020) Feasibility of dual energy X-ray absorptiometry based images for measurement of height, sitting height, and leg length in children. J Clin Densitom 23(3):472–481
Shevroja E, Aubry-Rozier B, Hans G, Gonzalez- Rodriguez E, Stoll D, Lamy O et al (2019) Clinical performance of the updated trabecular bone score (TBS) algorithm, which accounts for the soft tissue thickness: the OsteoLaus study. J Bone Miner Res 34(12):2229–2237
Boutin RD, Hernandez AM, Lenchik L, Seibert JA, Gress DA, Boone JM (2021) CT phantom evaluation of 67,392 American College of Radiology Accreditation Examinations: implications for opportunistic screening of osteoporosis using CT. AJR Am J Roentgenol 216(2):447–452
Aggarwal V, Maslen C, Abel RL, Bhattacharya P, Bromiley PA, Clark EM et al (2021) Opportunistic diagnosis of osteoporosis, fragile bone strength and vertebral fractures from routine CT scans: a review of approved technology systems and pathways to implementation. Ther Adv Musculoskelet Dis 13:1759720X211024029
Li D, Mao SS, Khazai B, Hyder JA, Allison M, McClelland R et al (2013) Noncontrast cardiac computed tomography image-based vertebral bone mineral density: the Multi-Ethnic Study of Atherosclerosis (MESA). Acad Radiol 20(5):621–627
Budoff MJ, Malpeso JM, Zeb I, Gao YL, Li D, Choi TY et al (2013) Measurement of phantomless thoracic bone mineral density on coronary artery calcium CT scans acquired with various CT scanner models. Radiology 267(3):830–836
Budoff MJ, Khairallah W, Li D, Gao YL, Ismaeel H, Flores F, Child J, Carson S, Mao SS (2012Feb) Trabecular bone mineral density measurement using thoracic and lumbar quantitative computed tomography. Acad Radiol 19(2):179–183
Genant HK, Wu CY, van Kuijk C et al (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148
Lenchik L, Rogers LF, Delmas PD et al (2004) Diagnosis of osteoporotic vertebral fractures: importance of recognition and description by radiologists. AJR Am J Roentgenol 183(4):949–958
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S et al (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29(11):2520–2526
Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C (2009) Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 20(10):1633–1650
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S et al (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25(10):2359–2381
Budoff MJ, Achenbach S, Berman DS, Fayad ZA, Poon M, Taylor AJ et al (2008) Task force 13: training in advanced cardiovascular imaging (computed tomography) endorsed by the American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 51(3):409–414
Mao SS, Li D, Luo Y, Syed YS, Budoff MJ (2016) Application of quantitative computed tomography for assessment of trabecular bone mineral density, microarchitecture and mechanical property. Clin Imaging 40(2):330–338
Mao S, Lu B, Oudiz RJ, Bakhsheshi H, Liu SC, Budoff MJ (2000) Coronary artery motion in electron beam tomography. J Comput Assist Tomogr 24(2):253–258
Budoff MJ, Cohen MC, Garcia MJ, Hodgson JM, Hundley WG, Lima JA et al (2005) ACCF/AHA clinical competence statement on cardiac imaging with computed tomography and magnetic resonance: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol 46(2):383–402
Bansal N, Katz R, de Boer IH, Kestenbaum B, Siscovick DS, Hoofnagle AN et al (2013) Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab 98(12):4890–4898
Ahmadi N, Mao SS, Hajsadeghi F, Arnold B, Kiramijyan S, Gao Y et al (2018) The relation of low levels of bone mineral density with coronary artery calcium and mortality. Osteoporos Int 29(7):1609–1616
Budoff MJ, Hamirani YS, Gao YL, Ismaeel H, Flores FR, Child J et al (2010) Measurement of thoracic bone mineral density with quantitative CT. Radiology 257(2):434–440
Mao SS, Li D, Syed YS, Gao Y, Luo Y, Flores F et al (2017) Thoracic quantitative computed tomography (QCT) can sensitively monitor bone mineral metabolism: comparison of thoracic QCT vs lumbar QCT and dual-energy X-ray absorptiometry in detection of age-relative change in bone mineral density. Acad Radiol 24(12):1582–1587
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
This work was funded by NIH Grant 2R42AR070713. This research was supported by R01HL146666, and all bone density measures were supported by 42AR070713. MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, D., Mao, S.S. & Budoff, M.J. Trabecular bone mineral density as measured by thoracic vertebrae predicts incident hip and vertebral fractures: the multi-ethnic study of atherosclerosis. Osteoporos Int 35, 1061–1068 (2024). https://doi.org/10.1007/s00198-024-07040-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-024-07040-5