Abstract
Summary
A study of 959 black and white women shows that the normal range for 24-h urine calcium excretion in white women is 30–300 mg and 10–285 mg in black women. Clinical laboratories use a normal range for 24-h urine calcium excretion of 100–300 mg; there is a need for them to update their age- and race-specific ranges.
Introduction
Recommendations for a normal range for 24-h urine calcium vary from a low of 50 mg to a high of 400 mg; most “laboratory normal ranges” based on older literature are incorrect. The objective of this analysis is to define a normal range for young women age 25–45 years and older women age 55–90 years, white and black, for 24-h urine calcium and calcium/creatinine ratio, and to examine the relationship between 24-h urine calcium, calcium absorption, and vitamin D metabolites.
Methods
Data from 3 studies was collected on 959 normal black and white women, ages 25–87 years, for 24-h urine calcium, creatinine, calcium intake, serum 25-hydroxyvitamin D (25OHD),1,25 dihydroxyvitamin D (1,25(OH)2D), and calcium absorption. Urine calcium and creatinine were measured on an auto-analyzer. Calcium absorption was measured by a single isotope method. Serum 25OHD and 1,25(OH)2D were measured by competitive protein binding assay or radioimmunoassay. Because 24-h urine calcium did not fit a normal distribution, non-parametric methods were used to determine the 95% reference interval (RI) and 90% non-parametric confidence intervals (CI) calculated for groups.
Results
The 95% reference intervals for 24-h urine calcium for women were as follows: black older 7–225 mg, black younger 8–285 mg; white older 37–275 mg, and white younger 23–287 mg. In older white women, 24-h urine calcium was significantly correlated with serum 1,25(OH)2 D, r = 0.23, p < 0.001, calcium intake r = 0.12, p = 0.001, and calcium absorption r = 0.18, p = 0.003, but not serum 25OHD r = 0.07, p = 0.06.
Conclusions
The normal reference interval for 24-h urine calcium for black women is lower than white women. Twenty-four-hour urine calcium was correlated with serum 1,25(OH)2 D calcium intake and calcium absorption, but not serum 25OHD. This range will be useful clinically for defining hypercalciuria and for following patients on vitamin D and calcium treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measurement of urine calcium is a common clinical test for patients with various disorders of calcium and bone metabolism, but the normal range has not been well studied. Usually, calcium excretion is based on 24-h urine collection, although some clinicians use spot urine samples. Collection of 24-h urine can be a problem for subjects, and proper instruction on the technique of collection is needed to avoid over or under collections. Urine creatinine measurements can partially correct for under and over collections; consequently, calcium/creatinine ratio is often used, but this may be a problem in ethnic groups such as black people who have higher 24-h urine creatinine excretion resulting in lower urine calcium/creatinine ratios. An appropriate reference range for 24-h urine calcium is important for a diagnosis of hypercalciuria that is found in primary hyperparathyroidism, renal stones, and in people treated with vitamin D/calcium. It is important also to look at values at the lower end on the urine calcium range where hypocalciuria may be associated with malabsorption of calcium and severe vitamin D deficiency.
Historically, the first definition of hypercalciuria was suggested by Hodkinson and Pyrah in 1958 [1]. They reported a range of 30–590 mg in women and > 300 mg/day in men, but because the frequency distribution of 24-h urine calcium showed a positive skew, they noted that 90% of women excreted less than 250 mg and suggested that values above 250 mg represented hypercalciuria. This definition has persisted since that time and our own hospital laboratory reports a normal range of 100–300 mg as do many clinical chemistry manuals without specifying a different range for age or ethnicity [2].
Since then, a number of papers have reported different means and ranges for 24-h urine calcium. Mean values in white women were reported to vary from 98 to 221 mg, and means in black women ranged from 80 to 140 mg but averaging 40–50 mg lower [1, 3,4,5,6,7,8,9,10,11,12,13]. Later data from the Medical Research Council Renal stone unit showed that hypercalciuria was > 300 mg in women and > 400 mg in men perhaps as a result of increasing dietary changes over time [5]. Another analysis showed that distribution of urine calcium in controls was not Gaussian; the upper normal 95% range for women was 340 mg day and 400 mg in men and 20% of the women and 40% of the men excreted more than 250 mg/day, and urine calcium was higher in younger people; calcium intake averaged 1025 mg/day [7]. Many studies did not have information on calcium intake that can either increase or decrease urine calcium excretion. Less interest was shown in black women, perhaps because of the lower incidence of renal stones.
The main objective of this analysis is to define a normal clinical reference range for 24-h urine calcium in white and black women of all ages on an unrestricted diet and to examine potential modifiers such as dietary calcium, protein intake, sodium intake, serum 25OHD, and serum 1,25OHD on urine calcium excretion.
Subjects and methods
Study design
Data was pooled and analyzed from 3 clinical trials. We pooled the data because all studies had similar recruitment strategy, common inclusion/exclusion criteria, same tests, and visit frequency, and similar chemistry tests with the exception of measurement of vitamin D metabolites. During recruitment, we used commercial mailing lists of women for the geographical areas of Omaha, NE, and surrounding districts, and Indiana for the black women. Initial screening was by telephone followed by a letter describing the study. Exclusion criteria were as follows: chronic illness severe enough to prevent reasonable physical activity, primary hyperparathyroidism, active renal stone disease, or history of more than two kidney stones in their lifetime, chronic renal failure, liver disease, heart failure, or severe lung disease; participants currently taking bisphosphonates or had taken them for more than 3 months, no previous use of fluoride, PTH or its derivatives, corticosteroid therapy (> 10 mg/day), phenytoin, phenobarbital, high-dose thiazide therapy (> 37.5 mg/day), estrogen in the previous 6 months. Laboratory exclusions were serum calcium ≥ 10.3 mg/dl (2.575 mmol/l) or > 0.3 mg/dl more than upper limit of normal on 2 baseline tests, 24-h urine calcium > 300 mg/dl (7.5 mmol/l) on 2 baseline tests, liver function tests > one standard deviation above upper normal limit, and serum creatinine > 1.4 mg/dl.
-
Study 1 - STOP IT was 3-year double-blind, placebo controlled randomized clinical trial previously described in detail, but primarily was a study of different interventions on femoral neck BMD. Participants were community-dwelling older white postmenopausal women aged between 65 and 80 years; 489 women were randomized to placebo, estrogen, calcitriol, and both. There was no exclusion for high-serum 25OHD levels but exclusions for levels < 5 ng/ml. Full results of the primary outcome on the bone mineral density response have been published [14].
-
Study 2 - VIDOS was a 1-year double-blind, randomized clinical trial in older black and white women. The main inclusion criterion was vitamin D insufficiency defined as serum 25OHD ≤ 20 ng/ml (50 nmol/l). Subjects were at least 7 years post-menopause, ages ranged from age 56 to 87 years. There were 163 white and 110 black women (32 from Omaha and 78 from Indiana) randomized to placebo and vitamin D doses 400–4800 IU daily. Detailed results on the primary outcome relating to the dose response on serum 25OHD were published earlier [15,16,17].
-
Study 3 - ViTADAS was a 1-year double-blind, randomized clinical trial in younger black and white women. The main inclusion criterion was vitamin D insufficiency defined as serum 25OHD ≤ 20 ng/ml (50 nmol/l). We randomized 119 white women, age 25–45 years, and 79 black women age 25–45 years to placebo, vitamin D 400, 800, 1600, and 2400 IU daily. The primary outcome result for the study was the dose-response effect on serum 25OHD, published previously [18].
The IRB at Creighton University and University of Indiana approved all study protocols, and subjects were enrolled after signing an informed consent document.
Laboratory measurements
In the three randomized, prospective trials, we collected at baseline 24-h urine calcium and creatinine while on their usual home diet. A 7-day food diary was collected after instruction with the use of food models by a trained dietitian. Calcium absorption was measured while fasting using a single isotope method calibrated against a double isotope method; 100-mg elemental calcium was mixed with 5 microcuries Ca45 in 200-ml distilled water. Blood samples were collected at 1, 2, and 3 h for estimation of serum Ca45, and calcium absorption was expressed as the percentage absorbed per liter after 2 h and converted to percent absorbed using an equation derived from a comparison of single and double isotope as previously described [16]. Calcium absorption and serum 1,25(OH)2D were measured in all subjects except the older black women in Indiana.
Subjects were discontinued from any medications that could affect calcium excretion during the baseline workup. They were instructed on the collection technique for 24-h urine and were provided with a 3-l container and plastic collection hat for the toilet that was left on the toilet until the collection was completed. All serum and urine chemistries were measured in the Creighton University Clinical Chemistry Laboratory. Urine calcium was measured on a Roche auto-analyzer. The university laboratory reports a normal range for 24-h urine calcium of 100–300 mg. Calcium/creatinine ratios are often used because urine creatinine is a marker of 24-h excretion, and if part of the 24-h collection is missed, then the ratio can be used as an estimate of calcium excretion.
In STOP IT, serum 25-hydroxyvitamin D (25OHD) and serum 1,25dihydroxyvitamin D 1,25(OH)2D were measured by a competitive binding assay after chromatographic separation and purification on Sep-Pak cartridges (Waters Associates, Milford, MA). The limit of detection was 5 ng/ml, and the interassay variation was 5% [14]. In ViDOS and ViTADAS, serum 25OHD and 1,25(OH)2D were measured by radioimmunoassay (RIA) after an acetonitrile extraction in our Bone Metabolism laboratory using kits manufactured by Diasorin, Inc. (Stillwater, MN) [15, 16]. The minimum detection range of 25OHD in this assay is 5 ng/ml [14]. In STOP IT, serum PTH was measured with the intact molecule 1–84 by immunometric assay (Nichols Institute, San Juan Capistrano, CA). In VIDOS and ViTADAS, serum 25OHD was measured with the Diasorin assay and the limit of detection was 3 ng/ml, and the interassay coefficient of variation was ~ 6% [15, 16].
Food diary
Dietary intake of calcium and vitamin D was calculated by a dietician from the 7-day food diaries using the Food Processor II Plus nutrition and diet analysis system (version 5.1, ESHA Research, Salem, OR). These were done at baseline and at the end of the study. Plastic food models (NASCO, Fort Atkinson, WI) were used to help participants better estimate food quantities.
Statistical methods
Baseline characteristics are summarized using means and standard deviations (SD). T tests were used to compare 24-h urine calcium and 24-h Ca/Cr by race. Pearson correlation coefficients examined the correlations between 24-h urine calcium and diet calcium, 2-h calcium absorption, serum 25OHD, serum 1,25(OH)2D, diet protein, diet sodium, and BMI stratified by race. Baseline 24-h urine calcium and 24-h Ca/Cr in healthy women in the STOP IT, VIDOS, and ViTADAS studies were used to calculate the reference intervals (RI) for 24-h urine calcium and 24-h Ca/Cr. Neither 24-h urine calcium nor 24-h Ca/Cr data fit a normal distribution using goodness of fit tests or by examining histograms. Therefore, the 95% RI was found using non-parametric methods, and 90% non-parametric confidence intervals are provided for the endpoints of the RI [19]. Linear regression models were used to examine the effect of diet calcium on 24-h urine calcium. A group variable was created with 4 categories: older black, older white, younger black, and younger white, defining older from the VIDOS study and younger from the ViTADAS study. An interaction was used to test whether the effect of diet calcium on 24-h urine calcium differed by age/race group. SAS software version 9.4 (SAS Institute Inc., Cary, NC) was used for the statistical analysis. p values less than 0.05 are considered statistically significant.
Results
Baseline characteristics for the 3 studies are shown in Table 1. There was a total of 959 women, 770 white, and 189 black, age range 25–87 years. In the older white groups (STOP IT, VIDOS), mean dietary calcium was 659 mg and 688 mg and 756 mg in the younger white group. In the older black group (VIDOS), dietary calcium was 551 mg and 503 mg in the younger black group (Table 1).
24-h urine calcium
The 95% reference intervals for the women were as follows: older white 37–275 mg (median 138), older black 7–225 mg (median 68), younger white 23–287 mg (median 145), younger black 8–285 mg (median 98) (Table 2). The lower and upper reference points are very similar for both STOP IT and VIDOS white women (Table 2). The distributions of 24-h urine calcium for older and younger white and black women are shown in Fig. 1. Mean 24-h urine calcium is significantly lower in older black than white women (mean difference = − 57 mg, 95% CI − 71 to − 44, p < 0.001) and lower in younger black than younger white women (mean difference = −41 mg, 95% CI − 60 to − 21, p < 0.001).
24-h urine calcium/creatinine ratio
The distribution of values and reference ranges are shown in Table 1 and Fig. 2. The reference interval for 24-h calcium/creatinine ratio is lower in older black women compared with white women.
To examine the reason for the lower urine calcium in black women, we performed regression analysis of urine calcium on diet calcium in the VIDOS/ViTADAS, black/white, creating four groups. These results are shown in Fig. 3a and b. Examination of the slopes of urine calcium on diet calcium in the four race/age groups showed that there is no difference in slope between the four groups (interaction p = 0.93), with an overall slope of 0.05, indicating that for 1-mg increase in diet calcium, 24-h urine calcium increases on average 0.05 mg. However, the four groups are significantly different on average when looking at all studies combined (overall p < 0.001), and comparisons between the groups show intercepts of 59 mg in older black and 101 mg in older white that are different (p < 0.001) as also are the intercepts of 104 mg in younger black and 118 mg in younger white groups (p < 0.001).
If we compare the ratio of urine calcium with dietary calcium in the 4 groups, the major difference is seen in the older black group where the ratio is 0.17; i.e., they are excreting less per calcium intake than other groups. Since the younger black group has a ratio similar to the white groups, then their lower urine calcium can be explained in part by lower calcium intake (Table 1).
The data for white women from STOP IT and ViDOS studies were combined; correlations for 24-h urine calcium are as follows: serum 1,25(OH)2D r = 0.23, p = 0.004; serum 25OHD r = 0.07, p = 0.06; calcium intake r = 0.12, p = 0.001, and calcium absorption r = 0.18, p = 0.005 (Tables 3 and 4). In older black women, correlations for 24-h urine calcium with serum 25OHD were r = 0.11, p = 0.28, and calcium intake r = 0.17, p = 0.08 (Tables 3 and 4). Serum 1,25(OH)2D was not measured in black women. Twenty-four-hour urine calcium is significantly associated with GFR, r = 0.27, p < 0.0001. Younger white and black women had correlations ranging from 0.01 to 0.18, and statistical significance ranged from marginally significant to not significant in younger white women, considering that sample sizes are smaller (Tables 3 and 4).
Dietary sodium intake was measured in all white VIDOS and ViTADAS subject, mean 2413 mg and 2776 mg; in black ViTADAS and VIDOS groups, mean sodium intake was 2497 mg and 2338 mg. In all white women, there was a significant correlation between dietary sodium intake and 24-h urine calcium r = 0.12, p = 0.04. We measured urine sodium only in the STOP IT study but did not find a significant correlation with urine calcium; the mean 24-h urine sodium in STOP IT was 71 (± 30) mmol. There was no significant correlation between 24-h urine calcium with dietary protein in any of the race/age groups.
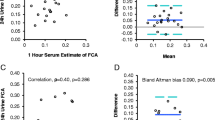
Urine calcium/creatinine was calculated on a fasting spot urine, and compared with a sample from the 24-h urine calcium, it shows that urine calcium measured on a spot urine sample does not accurately compare with the 24-h calcium value (Fig. 4).
Discussion
Analysis of 24-h urine calcium on 959 women showed that the 95% reference interval for 24-h urine calcium in older white women was 37–275 mg, 23–287 mg in young white women, 7–225 mg in older black women, and 8–286 mg in young black women. These values are very different from those recommended by clinical chemistry laboratories where the “normal range” given for 24-h urine calcium is 100–300 mg [2]. The upper range is consistent with our data, but their lower limit is too high. In our 3 groups of white women, mean 24-h urine calcium values averaged 143, 141, and 154 mg with mean diet calcium values of 659, 688, and 756 mg, respectively. These results are compared with published data where the average urine calcium in 12 studies varied from 98 to 221 mg [1, 3,4,5,6,7,8,9,10,11,12,13]. Seven of the 12 studies measured daily total calcium intake and the average varied from 699 to 1025 mg/d compared with ~ 700 mg in our studies [3, 7,8,9,10,11,12,13]. Differences in the reference ranges could depend in part on dietary intake of calcium which will be discussed later.
In our two groups of black women, the average 24-h urine calcium was 85 and 115 mg with diet calcium intakes of 551 and 503 mg, respectively, and these are similar to other published studies on black women that average 115, 110, 80, and 118 mg on diet calcium intakes of 678, 654, 790, and 1029, respectively [9,10,11,12,13]. Notably, the urine calcium excretion is much lower in black women.
Although it is well known that calcium intake is correlated with dietary calcium intake, the slope is only 0.05, so a difference in calcium intake of 500 mg would account for about a 25-mg increase in 24-h urine calcium. When 24-h urine calcium is regressed on diet calcium intake for the 4 age/race groups, the results show that the lines are approximately parallel, but the average 24-h urine calcium in older black women is much lower with an intercept of 58 mg compared with 100 mg in older white women indicating that low dietary calcium is not the main cause of low urine calcium. However, in the younger black group, the ratio of urine calcium/diet calcium is similar to white women and lower urine calcium can in part be explained by lower dietary calcium intake. Another cause of low urine calcium is intestinal malabsorption of calcium but our previous studies in these groups showed that calcium absorption in black women was only 4% lower than white women which is not sufficient to explain the lower urine calcium [16, 20]. The most likely explanation for lower urine calcium in in black women is increased tubular reabsorption of calcium by the kidney due to higher serum PTH levels [21, 22]. Interestingly, there is a similar pattern in younger women with a significantly lower intercept of 103 mg in black women compared with 117 mg in white women. Why the intercept in older black women is much lower than young women is unknown at this time.
Other factors that alter urine calcium excretion are diet/urine sodium; however, we did not measure urine sodium in all our trials. We found a significant correlation between dietary sodium intake and urine calcium excretion in white, but not black women. Others found no difference in urine sodium excretion between black and white people [5, 11]. This may depend on the sodium intake but when the system is stressed, i.e., reducing sodium intake from 200 to 10 mmol, there is a marked reduction of 50% in urine calcium [23, 24]. A high dietary protein intake has been associated with increased urine calcium, but no difference between black and white women was found in our study nor in two studies [5, 11].
Urine calcium was significantly associated with calcium intake, calcium absorption, and serum 1,25(OH)2D, but not with serum 25OHD. In previous papers, we have shown that calcium absorption correlated only with serum 1,25(OH)2D and not serum 25OHD [25]; this is not all that surprising since 1,25(OH)2D is the physiological active metabolite. In normal physiology, 25OHD has no direct effect on intestinal absorption though it may have an effect at very high serum levels ~ 90 ng/ml, because at high circulating levels, it may compete with 1,25(OH)2D for the vitamin D receptor in the gut.
A long-held belief is that the normal range for 24-h urine calcium is 100–250 mg/day in women although in the original study by Hodgkinson and Pyrah, 6% excreted more than 250 mg/day [1], and in another study in the UK, 6% of women excreted more than 300 mg [6]. Our results suggest that 300 mg may be a better upper limit than 250 mg for defining hypercalciuria in white women, and in black women, hypercalciuria would be defined as > 270 mg.
In subjects treated with daily vitamin D and a calcium intake of ~ 1200 mg as recommended by many National Osteoporosis Societies, we showed in VIDOS that 24-h urine calcium values > 300 mg occurred in 30% of the subjects when tests were performed every 3 months for a year and were more likely to occur in those with baseline 24-h urine calcium > 180 mg [26]. It is noteworthy that there was a 17% increase in kidney stones in the WHI intervention group treated for 7 years with daily vitamin D 400 IU plus total calcium intake 2000 mg calcium (includes 1000 mg calcium supplement) [27]. Since there has been an increase in the number of women taking calcium and vitamin D supplements with a recent estimate in NHANES of ~ 40 percent [28], clinicians should be aware of the importance of monitoring 24-h urine calcium because of the finding that supplemental calcium rather than dietary calcium is associated with an increased risk of kidney stones [29].
At the lower end of the normal range, a 24-h urine calcium < 37 in older and < 23 mg in younger white women represents hypocalciuria. A lower reference limit of 21 mg was found by one group [12] whereas a lower limit of 73 mg in white women from the Nurses Heath Professional study seems too high and may be related to a higher calcium intake of 1297 mg in that group [11]. A lower limit of 100 mg has been used for defining hypocalciuria in many clinical laboratories [13], but clearly this value is far too high; in fact, 30% of our subjects have a 24-h urine calcium below 100 mg. In younger black women, the lower reference interval is 8 mg and 7 mg in older black women, values that seem to be extremely low. This result may be surprising to many who regard hypocalciuria < 50 mg/24 h as indicative of metabolic bone disease such as osteomalacia or malabsorption syndrome, and it may be challenging at times to separate low normal values from disease states. Usually, it will depend on history, for example, malabsorption syndromes such as celiac disease and bypass surgery, or severe nutritional deficiency, which is uncommon in North America, but is more frequent in countries where vitamin D deficiency is common.
The strengths of this study are the large sample size of normal white and black women from age 25–87 years and a 7-day food diary which provides a more accurate estimate than dietary recall of calcium intake over 24 h or 3 days. We avoided studying women within 5 years of the menopause because of the confounding effect of estrogen deficiency on urine calcium excretion. Not often discussed in these papers are concomitant medications such as thiazides that may affect urine calcium excretion, and in our study, urine collections were performed off concomitant medications. Our study has some limitations. The results may not be generalized to other ethnic groups such as Asians and Arabic populations and other geographical locations because of differences in nutritional calcium intake and lower vitamin D levels. These results are from white and black women who live in the Midwest area of USA, whether it applies to other women of color are not known.
Although the current definitions of abnormal 24-h urine values in clinical chemistry laboratories are widely accepted, it is unclear how thoroughly these cut points were selected, how many subjects were included, how carefully 24-h urine collections were performed, and whether the “normal” population was well characterized. These factors limit the validity and generalizability of the findings of their normal range. As we see 24-h urine calcium, values are so much lower in black compared with white women, and this finding does not appear in most clinical chemistry reference manuals.
In summary, clinical laboratories need to correct their ranges for 24-h urine calcium and change them based on reference intervals, which is the correct way to establish a normal range. Especially, they need to re-evaluate the lower range of urine calcium excretion of 100 mg in clinical laboratories, which is far too high. Patients on vitamin D and calcium supplements should be monitored for hypercalciuria by measurement of 24-h urine calcium and not by using spot urine samples which are inaccurate. Measurement of baseline 24-h urine calcium > 180 mg in patients is clinically important because this group is more likely to develop hypercalciuria on calcium/vitamin D.
References
Hodgkinson A, Pyrah LN (1958) The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg 46(195):10–18. https://doi.org/10.1002/bjs.18004619504
https://www.uclahealth.org/endocrine-center/urine-calcium-test accessed4/14/2020
Watson L, Dale NE (1966) The urinary excretion of calcium, phosphorus and creatinine in normal subjects and in patients with skin cancer. Clin Sci 31(1):77–86
Nordin BEC, Hodgkinson A, Peacock M (1967) The measurement and the meaning of urinary calcium. Clin Orthop Relat Res 52:293–322. https://doi.org/10.1097/00003086-196700520-00023
Bulusu L, Hodgkinson A, Nordin BEC, Peacock M (1970) Urinary excretion of calcium and creatinine in relation to age and body weight in normal subjects and patients with renal calculus. Clin Sci 38(5):601–612. https://doi.org/10.1042/cs0380601
Blackwood AM, Sagnella GA, Cook DG, Cappuccio FP (2001) Urinary calcium excretion, sodium intake and blood pressure in a multi-ethnic population: results of the Wandsworth Heart and Stroke Study. J Hum Hypertens 15(4):229–237. https://doi.org/10.1038/sj.jhh.1001171
Davis RH, Morgan DB, Rivlin RS (1970) The excretion of calcium in the urine and its relation to calcium intake, sex and age. Clin Sci 39(1):1–12. https://doi.org/10.1042/cs0390001
Riggs BL, O’Fallon WM, Muhs J, O’Connor MK, Kumar R, Melton LJ (1998) Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res 13(2):168–174. https://doi.org/10.1359/jbmr.1998.13.2.168
Curhan GC, Willett WC, Speizer FE, Stampfer MJ (2001) Twenty-four–hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 59(6):2290–2298. https://doi.org/10.1046/j.1523-1755.2001.00746.x
Meier DE, Luckey MM, Wallenstein S, Clemens TL, Orwoll ES, Waslien CI (1991) Calcium, vitamin D, and parathyroid hormone status in young white and black women: association with racial differences in bone mass*. J Clin Endocrinol Metab 72(3):703–710. https://doi.org/10.1210/jcem-72-3-703
Meier DE, Luckey MM, Wallenstein S, Lapinski RH, Catherwood B (1992) Racial differences in pre- and postmenopausal bone homeostasis: association with bone density. J Bone Miner Res 7(10):1181–1189. https://doi.org/10.1002/jbmr.5650071010
Taylor EN, Curhan GC (2007) Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18(2):654–659. https://doi.org/10.1681/ASN.2006080854
Aloia JF, Shieh A, Mikhail M, Islam S (2015) Urinary calcium excretion in postmenopausal African American women. Clin Nephrol 84(09):130–137. https://doi.org/10.5414/CN108548
Gallagher JC, Fowler SE, Detter JR, Sherman SS (2001) Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab 86(8):3618–3628. https://doi.org/10.1210/jcem.86.8.7703
Gallagher JC, Sai A, Templin T, Smith L (2012) Dose response to vitamin D supplementation in postmenopausal women. Ann Intern Med 156(6):425. https://doi.org/10.7326/0003-4819-156-6-201203200-00005
Gallagher JC, Yalamanchili V, Smith LM (2012) The effect of vitamin D on calcium absorption in older women. J Clin Endocrinol Metab 97(10):3550–3556. https://doi.org/10.1210/jc.2012-2020
Gallagher JC, Peacock M, Yalamanchili V, Smith LM (2013) Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab 98(3):1137–1146. https://doi.org/10.1210/jc.2012-3106
Gallagher JC, Jindal PS, Smith LM (2014) Vitamin D supplementation in young white and African American women. J Bone Miner Res 29(1):173–181. https://doi.org/10.1002/jbmr.2010
Geffré A, Friedrichs K, Harr K, Concordet D, Trumel C, Braun J-P (2009) Reference values: a review. Vet Clin Pathol 38(3):288–298. https://doi.org/10.1111/j.1939-165X.2009.00179.x
Gallagher JC, Jindal PS, Smith LM (2014) Vitamin D does not increase calcium absorption in young women: a randomized clinical trial. J Bone Miner Res 29(5):1081–1087. https://doi.org/10.1002/jbmr.2121
Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, Lindsay R, Parisien M (1997) Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res 12(6):958–966. https://doi.org/10.1359/jbmr.1997.12.6.958
Smith LM, Gallagher JC (2019) Effect of vitamin D supplementation on total and free 25 hydroxyvitamin D and parathyroid hormone. An analysis of two randomized controlled trials. J Intern Med 286(6):651–659. https://doi.org/10.1111/joim.12950
Seeger H, Kaelin FPM, Weber D et al (2017) Changes in urinary risk profile after short-term low sodium and low calcium diet in recurrent Swiss kidney stone formers. BMC Nephrol 18(1):349, 2-9. https://doi.org/10.1186/s12882-017-0755-7
Bayomy O, Zaheer S, Williams JS, Curhan G, Vaidya A (2020) Disentangling the relationships between the renin-angiotensin-aldosterone system, calcium physiology, and risk for kidney stones. J Clin Endocrinol Metab 105(6):1937–1946. https://doi.org/10.1210/clinem/dgaa123
Sai AJ, Walters RW, Fang X, Gallagher JC (2011) Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab 96(3):E436–E446. https://doi.org/10.1210/jc.2010-1886
Gallagher JC, Smith LM, Yalamanchili V (2014) Incidence of hypercalciuria and hypercalcemia during vitamin D and calcium supplementation in older women. Menopause 21(11):1173–1180. https://doi.org/10.1097/GME.0000000000000270
Jackson RD, LaCroix AZ, Gass M et al (2006) Women’s health initiative investigators. Calcium plus vitamin D and the risk of fractures. N Engl J Med 354:669–683
Bailey RL, Dodd KW, Goldman JA, Gahche JJ, Dwyer JT, Moshfegh AJ, Sempos CT, Picciano MF (2010) Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 140(4):817–822. https://doi.org/10.3945/jn.109.118539
Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ (1997) Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 126(7):497–504. https://doi.org/10.7326/0003-4819-126-7-199704010-00001
Acknowledgments
Dr Munro Peacock for valuable help in recruiting black women in Indiana for the study. This data was first presented as a poster in the ASBMR meeting in 2013.
Funding
This work was supported by grants from National Institute of Health grants: UO1-AG10373, RO1-AG10358, AG28168, and Department of Defense (DOD) W81XWH-07-1-201. Clinical trial registration: NCT00472823.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Smith, L.M., Gallagher, J.C. Reference range for 24-h urine calcium, calcium/creatinine ratio, and correlations with calcium absorption and serum vitamin D metabolites in normal women. Osteoporos Int 32, 539–547 (2021). https://doi.org/10.1007/s00198-020-05615-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05615-6