Abstract
Summary
Osteoporosis is a major concern in patients with Duchenne muscular dystrophy. In this novel study of teriparatide treatment in 6 patients with severe osteoporosis, bone health (fractures, vertebral morphometry, and DXA) remained stable, with no adverse events. These findings will help inform future osteoporosis research in this challenging population.
Introduction
Despite standard therapy with vitamin D and bisphosphonates (BP), many patients with Duchenne muscular dystrophy (DMD) continue to sustain fragility fractures due to long-term glucocorticoid treatment and limited mobility. We aimed to evaluate the safety and efficacy of teriparatide for the treatment of severe osteoporosis in adolescent and young adult patients with DMD.
Methods
We prospectively treated 6 patients with DMD who had severe osteoporosis with teriparatide 20 mcg subcutaneously daily for 1–2 years. Inclusion criteria were long-term glucocorticoid therapy, and severe osteoporosis despite treatment with BP, or intolerance to BP. We examined long bone and vertebral fracture outcomes, including vertebral morphometry measures, bone mineral density and content, bone formation markers, safety indices, and adverse events.
Results
The mean age at teriparatide start was 17.9 years (range 13.9–22.1 years). All 6 patients were on daily glucocorticoids (mean ± SD; duration 10.9 ± 2.5 years) and 5 were non-ambulatory. Five patients had been treated with BP for 7.9 ± 4.2 years. All had vertebral and a history of long bone fragility fractures at baseline. Vertebral heights and Genant fracture grading remained stable. Long bone fracture rate appeared to decrease (from 0.84/year to 0.09/year); one patient sustained a long bone fracture at 6 months of treatment. Trajectories for change in bone mineral density and content were not different post- vs. pre-teriparatide. Procollagen type 1 amino-terminal propeptide (P1NP) increased, while laboratory safety indices remained stable and non-concerning. No adverse events were observed.
Conclusion
In six patients with DMD treated with teriparatide for severe osteoporosis, we observed stable bone health and modest increases in P1NP, without safety concerns. Further studies are needed to better understand teriparatide efficacy for treatment of osteoporosis in patients with DMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked disorder characterized by progressive muscle weakness, leading to loss of independent ambulation by age 12 years, and mortality from cardiorespiratory failure by early adulthood. The use of long-term glucocorticoid (GC) therapy prolongs ambulation, improves cardiac and pulmonary function, delays onset of scoliosis and contractures, and preserves upper extremity function [1,2,3]. However, long-term GC therapy has significant adverse effects, including osteoporosis, delayed puberty/hypogonadism, increased weight gain, and growth failure [1, 3, 4].
Osteoporosis is a common and important comorbidity in patients with DMD [4, 5]. Vertebral and long bone fractures are frequent following the introduction of GC therapy [2]. Apart from direct toxicity of GC on bone, delayed puberty/hypogonadism and disruption of the bone-muscle unit that results from muscle weakness are also detrimental to bone health in patients with DMD [2]. Unlike other groups of patients with GC-induced osteoporosis (GIO), such as those with acute leukemia or rheumatologic disorders, spontaneous recovery of vertebral fractures and vertebral reshaping are not typically observed in patients with DMD, probably due to the ongoing need for GC therapy and limited bone growth [6]. Pharmacologic treatment for GIO in this population is therefore indicated to prevent osteoporosis and its complications, which include premature loss of ambulation (a psychologically devastating milestone), pain (further impacting quality of life), or fat embolism (a potentially life-threatening event).
The off-label use of bisphosphonates (BP) has become standard practice for the treatment of GIO in patients with DMD, although there is no clear evidence on which to base consensus as to type, dose, and duration of therapy in this population [1, 7]. Both oral and intravenous BP have been reported to be associated with lower fracture frequency and increased bone mineral density (BMD) in patients with DMD [8,9,10]. However, even with the combination of vitamin D and calcium, BP therapy is frequently inadequate to prevent fractures [2]. In adult (non-DMD) patients with GIO, current treatment recommendations include supplementation with vitamin D and calcium, in addition to other therapeutic agents, including BP and parathyroid hormone (PTH) [11,12,13]. There is an urgent need to find therapeutic agents that are effective to treat or prevent osteoporosis in pediatric/adolescent patients with DMD.
Recombinant human PTH (rhPTH) (1–34), teriparatide, is an anabolic agent that promotes the formation of bone by increasing the number of osteoblasts, activating pre-existing osteoblasts, and decreasing apoptosis in osteoblasts [14]. It intuitively better addresses the underlying pathophysiology of GIO and has been shown to be superior to anti-resorptive agents such as BP to increase BMD and reduce fracture risk [15]. The predominant effects of teriparatide therapy are seen in areas of high bone turnover, mainly cancellous bone (such as the spine), which are preferentially affected by GC, with improvement in skeletal microarchitecture [16]. Several pivotal studies of teriparatide therapy in adults with osteoporosis, including GIO, have shown it to be safe and effective, with significant improvement in BMD and reduction of fracture risk [14, 17,18,19]. Teriparatide has been shown to be safe and effective when used as replacement therapy for children with hypoparathyroidism [20,21,22]. However, it has neither been used nor studied in children and adolescents for the treatment of osteoporosis because of concern for the potential for osteosarcoma based on observations in animal studies [23, 24]. There has been a single case report of teriparatide therapy for osteoporosis in a 20-year-old man with DMD, in whom 18 months of treatment was associated with decreased bone pain, increased BMD, and improved quality of life [25].
We used teriparatide to treat 6 adolescent/young adult patients with DMD and severe osteoporosis, on long-term GC, and who continued to sustain fragility fractures despite treatment with BP, or who had an intolerance to BP. The aims of this study were to evaluate the safety and efficacy of teriparatide for treatment of severe osteoporosis in patients with DMD.
Materials and methods
Study participants
Patients were considered eligible for the study by the following inclusion criteria: (i) a diagnosis of DMD, confirmed by genetic mutation analysis and/ or muscle biopsy; (ii) long-term and concurrent GC therapy of at least 1-year duration; (iii) diagnosis of osteoporosis, with a vertebral compression fracture, or height-adjusted lumbar spine (LS) BMD Z-score < − 2.0 and pathological long bone fractures [26], with progression or continuing to fracture despite being on BP therapy, or intolerance to BP; and (iv) estimated glomerular filtration rate (eGFR) > 49 mL/min, as measured by cystatin C [27]. Patients were excluded by the following criteria: (i) hypercalcemia, defined by serum calcium concentration (corrected for serum albumin) exceeding 11 mg/dL; (ii) nephrocalcinosis or nephrolithiasis (confirmed by ultrasound), with hypercalciuria, as defined by 24-h urine calcium excretion exceeding 4 mg/kg (as teriparatide treatment for osteoporosis could cause hypercalcemia/hypercalciuria); (iii) concurrent digoxin, hydrochlorothiazide, or furosemide treatment; or (iv) inability to follow up or comply with recommended teriparatide treatment.
Eligible patients were referred to Endocrinology by the Comprehensive Neuromuscular Care Center at Cincinnati Children’s Hospital Medical Center (CCHMC). Patient information, laboratory, and radiographic data were accessed by review of the electronic medical records. Informed consent and assent were obtained at study enrollment. The study was approved by the CCHMC Institutional Review Board.
Treatment protocol
Bisphosphonate therapy was discontinued at the time of teriparatide start, as the literature suggested that the simultaneous combination of BP and teriparatide may be detrimental to bone health [28]. Teriparatide was administered as a once-daily subcutaneous injection of 20 mcg, with the intent to treat for 2 years. Patients were seen at teriparatide initiation and at 6-monthly intervals over 2 years. Patients were assessed to ensure their calcium intake by diet and supplementation met required daily amounts for age, and those with known hypercalciuria discontinued or received lower supplementation to meet their needs but prevent hypercalcemia and hypercalciuria [16]. Vitamin D supplementation was continued, to maintain serum 25-hydroxyvitamin D concentrations of 30–60 ng/mL. Other therapies that could have affected bone health, including GC, and testosterone, remained constant, unless determined by clinical need.
Bone health monitoring
Fractures and vertebral morphometry
Fracture history was reviewed and confirmed radiographically during each clinic visit. Long bone fracture rates were calculated pre- and on 2 years of teriparatide therapy. The group fracture rate was assessed by the total number of long bone fractures divided by the sum of the times from the first fracture to the start of teriparatide for each patient. All patients had radiographic assessment of thoracic and lumbar vertebrae at baseline and every 6 months during teriparatide therapy, and if symptomatic. Morphometric measurement of vertebrae on lateral spine radiographs using 6-point vertebral morphometry, as previously described [29], was performed by J.C.T. and P.H. Baseline and follow-up anterior, middle, and posterior vertebral body heights were compared to document response to therapy. Vertebral height ratios were assessed by the Genant semi-quantitative method: anterior-to-posterior heights, middle-to-posterior heights, and posterior-to-upper and lower adjacent posterior vertebral heights [30]. Vertebral height ratio losses were graded as follows: grade 0 (normal), < 15%; grade 0.5 fracture (uncertain or questionable), ≥ 15–< 20%; grade 1 (mild) fracture, ≥ 20–< 25%; grade 2 (moderate) fracture, ≥ 25–< 40%; grade 3 (severe) fracture, ≥ 40%.
Bone mineral density and content
All patients had dual X-ray absorptiometry (DXA) scans at baseline and at 6- to 12-month intervals to assess areal bone mineral density (aBMD) and bone mineral content (BMC) of the LS, whole body (WB), and lateral distal femur (LDF). DXA scans were performed using a Hologic densitometer that had software with the same bone detection algorithms (version 12.3) for the analysis of all scans. The coefficient of variation at our center was < 1% at LS and WB sites. For the LDF site, three regions of interest (R1, primarily trabecular bone; R2, a transitional zone; and R3, primarily cortical bone) were identified [31]. Bone mineral apparent density (BMAD) of the lumbar spine was calculated using methods previously described [32, 33]. The aBMD Z-scores were calculated from pediatric reference data [34, 35]. As accurate height measurements were not possible in the majority of these patients, aBMD Z-scores were not corrected for height.
Bone formation markers
Bone-specific alkaline phosphatase and procollagen type 1 amino-terminal propeptide (P1NP) were obtained as morning fasting samples at the start of teriparatide treatment, and at 6, 12, 18 and 24 months of therapy.
Safety monitoring
Laboratory testing was performed at the start of treatment, and at 1, 3, 6, 12, 18, and 24 months after initiation of therapy, and included intact PTH, renal panel, 25-hydroxyvitamin D, eGFR as measured by cystatin C, 24-h urine Litholink studies (Litholink Corporation, Chicago, IL, USA), urine calcium, and urine osmolality.
Statistical analysis
Data were analyzed using SAS®, version 9.4 (SAS Institute, Cary, NC). Continuous data were presented as means ± SD and ranges (minimum-maximum), and categorical data were presented as percentages. Due to the small sample size, statistical analyses were not performed on changes over time for the laboratory, vertebral morphometry, or DXA data. Inter- and intra-observer agreement of vertebral height measurements was assessed using an intraclass correlation estimated from the general linear mixed model. Landis and Koch cut-points were used to define the intraclass correlations, with values between 0.81 and 1.0 being excellent agreement [36].
Results
Baseline evaluation
Six patients were eligible and enrolled in the study (Table 1). All patients were treated with long-term GC (mean 28.3 ± 5.4 mg per day of prednisone-equivalent) and had a history of long bone and vertebral fractures, and none reported bone pain or back pain at baseline. All patients had been previously started on oral BP therapy when they had first developed early signs of bone fragility, such as mild vertebral height loss or compression deformities, in accordance with our center’s bone health protocol. All had continued to sustain fragility fractures despite treatment with vitamin D, calcium supplements, and oral BP, except patient 2 who was not on oral BP due to intolerance to treatment (swelling of lips). All patients had sufficient 25-hydroxyvitamin D concentrations (30–60 ng/mL), normal serum calcium concentrations, and renal function (Table 2). Patients 3 and 6 had hypercalciuria (urine calcium/osmolality ratio > 0.25 and/or 24-h urine calcium excretion > 4 mg/kg), but no ultrasonographic evidence of nephrocalcinosis or nephrolithiasis at baseline. Their vitamin D and calcium supplements were decreased or discontinued prior to the start of teriparatide. All patients who had delayed puberty/hypogonadism were treated with testosterone therapy, and all had appropriate serum testosterone concentrations for age during the study period.
All patients completed a 2-year course of teriparatide treatment, except for patient 4, who died following a cardiac arrest at 1 year post-initiation of treatment. The cardiac event was attributed to congestive heart failure, unrelated to teriparatide, as the patient had baseline severe cardiomyopathy, a common comorbidity of DMD.
Bone health
Long bone fractures
The 6 patients had a total of 17 long bone fractures, over a 20.3 patient-year period prior to the initiation of teriparatide therapy (0.84 fractures per year). The time from the last fracture to teriparatide start ranged from 0.5 to 3.5 years (Table 1). The lower extremities were the most common sites of fractures (82%). Patient 3, who was close to losing ambulation, sustained another lower extremity long bone fracture following a fall injury at 6 months of therapy, resulting in permanent loss of ambulation. Overall, on teriparatide therapy, the long bone fracture rate appeared to decrease to 0.09 fractures per year (1 fracture over an 11.0 patient-year period).
Vertebral morphometry and fractures
The intraclass correlation coefficients for intra-observer measurements of vertebral heights were 0.88–0.93, and those for inter-observer measurements were 0.81–0.88, namely excellent. Lumbar vertebral heights were measured in all 6 patients. Due to severe thoracic kyphoscoliosis being prevalent in some of our patients, as well as severe osteoporosis, accurate measurement of some thoracic vertebral heights was not possible. There appeared to be no major changes in median lumbar vertebral heights over 2 years of teriparatide treatment (Fig. 1). Genant semi-quantitative assessment showed stable or improved fractures in most T9-L4 vertebrae during teriparatide therapy (Fig. 2). No bone or back pain was reported at baseline or during treatment.
Bone density
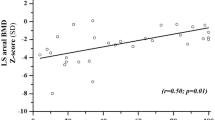
The absolute aBMD and BMC values by DXA at all skeletal sites and LS BMAD appeared to remain stable, while aBMD Z-scores appeared to continue to decline over time (Fig. 3).
Bone formation markers
While serum bone-specific alkaline phosphatase remained relatively stable, there were modest increases in P1NP concentrations at 2 years of teriparatide treatment in all patients (Table 2).
Safety indices
Mean serum PTH, calcium, phosphorus, magnesium, and cystatin C eGFR remained within normal/acceptable ranges throughout the course of treatment (Table 2). All patients had 24-h urine studies at baseline, but only patients 1 and 3 completed these at 2 years of teriparatide therapy. Mean 24-h urine calcium excretion and urine calcium/osmolality ratios remained stable in all patients. The 24-h urine oxalate and urine supersaturation-calcium oxalate was stable in patient 1 but increased over time in patient 3. Patient 6, who had hypercalciuria at baseline, developed a non-obstructive renal calculus at 22 months of teriparatide therapy.
Discontinuation of teriparatide
All except patient 4 completed a 2-year course of teriparatide therapy. Following discontinuation of teriparatide, patients 3, 5, and 6 transitioned to treatment with an oral or intravenous BP. Patient 1 switched therapy to denosumab. Patient 2 was continued on testosterone therapy only by his non-CCHMC endocrinologist.
Discussion
The key findings from our study suggest that teriparatide therapy for the treatment of GIO in adolescent and young adult patients with DMD and severe osteoporosis is associated with stable bone health and no major safety concerns. We evaluated bone health in a comprehensive manner, which included the assessment of vertebral morphometry by 6-point measurement (novel methodology in this patient population) and Genant semi-quantitative grading, in addition to long bone fracture rate and DXA indices of multiple skeletal sites. We found that vertebral morphometry and fractures were relatively stable, in keeping with lack of new vertebral fractures described in a prior case report of an adult patient with DMD treated with teriparatide [25]. Long bone fracture rate appeared to decrease over the 2 years of teriparatide treatment, and we observed a modest increase in P1NP.
We did not observe an increase in BMD while on teriparatide treatment, in contrast to gains seen in previous studies in adults with GIO [19, 37]. In our cohort, absolute BMD values at all skeletal sites appeared to remain unchanged. While there was a continuing (unchanged) trend of declining BMD Z-scores, this was not surprising as our patients with DMD were compared with standards for healthy males who undergo puberty with accelerated growth, and who continue to accrue bone mineral during adolescence. Adolescent patients with DMD are subject to GC-induced growth retardation and delayed puberty/hypogonadism, which could account for the observed decline of DXA indices that have been standardized for age and sex, but not height or pubertal stage.
In adults with GIO, teriparatide has been shown to be superior to BP with regard to effects on BMD, bone microarchitecture, and vertebral fracture outcomes [19, 37]. There are several possible reasons why we may not have observed similar gains. GC doses and durations reported in these studies (prednisone 7.5–8.8 mg daily for 2.3–6.4 years) were much lower compared with the treatment regimens in our patients with DMD. The adult patient with DMD (who was BP-naïve) treated with teriparatide in the previously cited case report was also on a lower GC dose (prednisone 5 mg daily) when compared with our patients [25]. Thus, differences in GC regimens and cumulative dosing could have been responsible for an attenuated response to teriparatide therapy in our patients, who remained on high doses of GC. Another possible contributing factor could have been prior long-term BP exposure that may have blunted the extent of bone remodeling from teriparatide [38, 39], although there are conflicting data on this theory. Several studies have shown the suppressive effects of BP can be overcome during teriparatide therapy, suggesting that a washout period is not needed [40,41,42], and newer studies have reported combined BP and teriparatide therapy may be beneficial [43,44,45]. Additional factors could be the severity of osteoporosis in our cohort (who represented the more severe extreme of the osteoporosis spectrum of our DMD patient population as a whole), and the presence and persistence of other risk factors (namely immobility, lack of weight-bearing ability, and severe muscle weakness due to advanced muscular dystrophy). These factors may also explain the smaller increases in bone formation markers in our cohort, in contrast to more significant increases in P1NP, osteocalcin, and bone-specific alkaline phosphatase in adults with GIO who were treated with teriparatide [19]. Lastly, teriparatide dose may also play a role in BMD response. Although the teriparatide dose of 20 mcg daily that we used is considered standard dosing, recent studies in postmenopausal women suggested that high-dose teriparatide (40 mcg daily) was associated with greater BMD gain, but without benefit for fracture outcomes [46, 47]. Further studies are needed to evaluate whether patients with high-risk osteoporosis, particularly those with DMD, would benefit from a higher dose of teriparatide.
We found that teriparatide was safe and well tolerated in adolescent and young adult patients with DMD. Hypercalciuria is a commonly reported side effect of teriparatide therapy for treatment of osteoporosis in adults and hypoparathyroidism in children, as a result of transient hypercalcemia following intermittent administration [22, 48]. We observed stable serum calcium concentrations and urinary calcium excretion (24-h urine calcium and urine calcium/osmolality ratio) during treatment with teriparatide. An increase in 24-h urine oxalate and urine supersaturation-calcium oxalate, despite stable urine calcium, was observed in one patient. This may have been due to a decrease in his dietary and supplemental calcium intake, as recommended after the finding of hypercalciuria prior to teriparatide start. A decrease in dietary calcium to bind with oxalate could lead to more free intestinal oxalate being absorbed and subsequently excreted in the urine. Finally, although one patient developed nephrolithiasis, hypercalciuria was already present at baseline despite appropriate calcium and vitamin D intake. Hypercalciuria is common in patients with DMD, conferring risk for nephrocalcinosis even without teriparatide treatment [7].
Another common concern for use of teriparatide treatment in children is the theoretical risk for the development of osteosarcoma, based on studies in rodents [49]. However, teriparatide doses and durations in these studies were much greater relative to those in humans. No patients in our cohort were known to develop osteosarcoma on short-term teriparatide therapy, including in 4 patients who had open epiphyses at baseline (8 patient-years of follow-up), in line with post-marketing surveillance studies that reported no apparent connection between osteosarcoma and teriparatide use in adults [50, 51]. Findings from long-term studies of children with hypoparathyroidism also support the safe use of teriparatide in children [22].
Strengths of our study include novel treatment and comprehensive bone health assessment, including vertebral morphometry, in this patient population. Vertebral reshaping is typically overlooked when monitoring treatment for osteoporosis in children [7]. We evaluated vertebral morphometry by using both vertebral heights and the more commonly used Genant semi-quantitative method. Vertebral height measurements allow for more in-depth comparisons of continuous variables, compared with categorical comparisons using the Genant method. However, none of these measures assesses bone quality or microarchitecture. Vertebral morphometry evaluation may also be subjective. Nevertheless, our intra- and inter-observer correlation coefficients were excellent, all above 0.81. Another point meriting consideration is the limitation of BMD use in the DMD population itself. BMD Z-scores presented in this study were not corrected for height (due to most patients being non-ambulatory) or puberty, and presence of lumbar vertebral fractures may falsely increase LS BMD. However, trajectories for absolute BMD values during treatment still serve as a useful tool for monitoring treatment response, as long as these caveats are taken into consideration when interpreting the data. Our study was also limited by the small number of patients in this cohort. Lastly, compliance with teriparatide treatment relied on patients’ reports.
In conclusion, in our 2-year clinical experience of teriparatide treatment of 6 patients with DMD, we observed stable bone health, with modest increases in P1NP, and no safety concerns. There is a rationale for the use of anabolic agents such as teriparatide to treat GIO, and our findings will help inform the next steps in the quest to find better therapies to treat this challenging patient population. Larger intervention studies in patients with DMD not previously treated with long-term BP, studies using higher doses of teriparatide, and/or controlled trials comparing teriparatide with conventional BP treatment or newer anabolic agents, or combination therapies, are needed to better understand the efficacy of teriparatide for treatment of GIO in youth with DMD.
Abbreviations
- aBMD:
-
Areal bone mineral density
- BMAD:
-
Bone mineral apparent density
- BMD:
-
Bone mineral density
- BMC:
-
Bone mineral content
- BP:
-
Bisphosphonate(s)
- DMD:
-
Duchenne muscular dystrophy
- DXA:
-
Dual X-ray absorptiometry
- eGFR:
-
Estimated glomerular filtration rate
- GC:
-
Glucocorticoid(s)
- GIO:
-
Glucocorticoid-induced osteoporosis
- LDF:
-
Lateral distal femur
- LS:
-
Lumbar spine
- P1NP:
-
Procollagen type 1 amino-terminal propeptide
- PTH:
-
Parathyroid hormone
- rhPTH:
-
Recombinant human PTH
References
Bianchi ML, Biggar D, Bushby K, Rogol AD, Rutter MM, Tseng B (2011) Endocrine aspects of Duchenne muscular dystrophy. Neuromuscul Disord 21(4):298–303. https://doi.org/10.1016/j.nmd.2011.02.006
Mayo AL, Craven BC, McAdam LC, Biggar WD (2012) Bone health in boys with Duchenne muscular dystrophy on long-term daily deflazacort therapy. Neuromuscul Disord 22:1040–1045. https://doi.org/10.1016/j.nmd.2012.06.354
Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, Case LE, Clemens PR, Hadjiyannakis S, Pandya S, Street N, Tomezsko J, Wagner KR, Ward LM, Weber DR, Group DMDCCW (2018) Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 17(3):251–267. https://doi.org/10.1016/S1474-4422(18)30024-3
Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM, Group DMDCCW (2018) Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 17(4):347–361. https://doi.org/10.1016/S1474-4422(18)30025-5
Larson CM, Henderson RC (2000) Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 20(1):71–74
Ma J, McMillan HJ, Karaguzel G, Goodin C, Wasson J, Matzinger MA, DesClouds P, Cram D, Page M, Konji VN, Lentle B, Ward LM (2017) The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporosis Int 28(2):597–608. https://doi.org/10.1007/s00198-016-3774-5
Ward LM, Hadjiyannakis S, McMillan HJ, Noritz G, Weber DR (2018) Bone health and osteoporosis management of the patient with Duchenne muscular dystrophy. Pediatrics 142(Suppl 2):S34–S42. https://doi.org/10.1542/peds.2018-0333E
Hawker GA, Ridout R, Harris VA, Chase CC, Fielding LJ, Biggar WD (2005) Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil 86(2):284–288. https://doi.org/10.1016/j.apmr.2004.04.021
Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, Ward LM (2012) The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporosis Int 23:2703–2711. https://doi.org/10.1007/s00198-012-1911-3
Srinivasan R, Rawlings D, Wood CL, Cheetham T, Moreno AC, Mayhew A, Eagle M, Guglieri M, Straub V, Owen C, Bushby K, Sarkozy A (2016) Prophylactic oral bisphosphonate therapy in duchenne muscular dystrophy. Muscle Nerve 54(1):79–85. https://doi.org/10.1002/mus.24991
de Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW (2004) Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporosis Int 15(8):589–602. https://doi.org/10.1007/s00198-004-1614-5
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, Humphrey MB, Lane NE, Magrey M, Miller M, Morrison L, Rao M, Robinson AB, Saha S, Wolver S, Bannuru RR, Vaysbrot E, Osani M, Turgunbaev M, Miller AS, McAlindon T (2017) 2017 American College of Rheumatology Guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol 69(8):1521–1537. https://doi.org/10.1002/art.40137
Buckley L, Humphrey MB (2018) Glucocorticoid-induced osteoporosis. N Engl J Med 379(26):2547–2556. https://doi.org/10.1056/NEJMcp1800214
Brixen KT, Christensen PM, Ejersted C, Langdahl BL (2004) Teriparatide (biosynthetic human parathyroid hormone 1-34): a new paradigm in the treatment of osteoporosis. Basic Clin Pharmacol Toxicol 94(6):260–270. https://doi.org/10.1111/j.1742-7843.2004.pto940602.x
Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, Dalsky GP, Marcus R (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357(20):2028–2039. https://doi.org/10.1056/NEJMoa071408
Cipriani C, Irani D, Bilezikian JP (2012) Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res 27(12):2419–2428. https://doi.org/10.1002/jbmr.1800
Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, Kaufman JM, Clancy AD, Gaich GA (2003) The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18(1):9–17. https://doi.org/10.1359/jbmr.2003.18.1.9
Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H, Nishizawa Y, Fujita T, Shiraki M (2012) Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 97(9):3097–3106. https://doi.org/10.1210/jc.2011-3479
Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR (2009) Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60(11):3346–3355. https://doi.org/10.1002/art.24879
Winer KK, Zhang B, Shrader JA, Peterson D, Smith M, Albert PS, Cutler GB Jr (2012) Synthetic human parathyroid hormone 1-34 replacement therapy: a randomized crossover trial comparing pump versus injections in the treatment of chronic hypoparathyroidism. J Clin Endocrinol Metab 97(2):391–399. https://doi.org/10.1210/jc.2011-1908
Winer KK (2019) Advances in the treatment of hypoparathyroidism with PTH 1-34. Bone 120:535–541. https://doi.org/10.1016/j.bone.2018.09.018
Winer KK, Kelly A, Johns A, Zhang B, Dowdy K, Kim L, Reynolds JC, Albert PS, Cutler GB Jr (2018) Long-term parathyroid hormone 1-34 replacement therapy in children with hypoparathyroidism. J Pediatr 203:391–399 e391. https://doi.org/10.1016/j.jpeds.2018.08.010
Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol 32(4):426–438. https://doi.org/10.1080/01926230490462138
Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 30(3):312–321
Catalano A, Vita GL, Russo M, Vita G, Lasco A, Morabito N, Messina S (2016) Effects of teriparatide on bone mineral density and quality of life in Duchenne muscular dystrophy related osteoporosis: a case report. Osteoporosis Int 27(12):3655–3659. https://doi.org/10.1007/s00198-016-3761-x
Gordon CM, Leonard MB, Zemel BS, International Society for Clinical D (2014) 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom 17(2):219–224. https://doi.org/10.1016/j.jocd.2014.01.007
Braat E, Hoste L, De Waele L, Gheysens O, Vermeersch P, Goffin K, Pottel H, Goemans N, Levtchenko E (2015) Renal function in children and adolescents with Duchenne muscular dystrophy. Neuromuscul Disord 25(5):381–387. https://doi.org/10.1016/j.nmd.2015.01.005
Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349(13):1216–1226. https://doi.org/10.1056/NEJMoa035725
Armbrecht G, Blenk T, Chesnut CH 3rd, Gardner JC, von Ingersleben G, Mahoney P, Felsenberg D (2008) Vertebral fracture diagnosis in the multinational BONE study of oral ibandronate: quality management in radiology. J Clin Densitom 11(2):221–231. https://doi.org/10.1016/j.jocd.2007.10.002
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148. https://doi.org/10.1002/jbmr.5650080915
Henderson RC, Lark RK, Newman JE, Kecskemthy H, Fung EB, Renner JB, Harcke HT (2002) Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur. AJR Am J Roentgenol 178(2):439–443. https://doi.org/10.2214/ajr.178.2.1780439
Carter DR, Bouxsein ML, Marcus R (1992) New approaches for interpreting projected bone densitometry data. J Bone Miner Res 7(2):137–145. https://doi.org/10.1002/jbmr.5650070204
Kindler JM, Lappe JM, Gilsanz V, Oberfield S, Shepherd JA, Kelly A, Winer KK, Kalkwarf HJ, Zemel BS (2019) Lumbar spine bone mineral apparent density in children: results from the bone mineral density in childhood study. J Clin Endocrinol Metab 104(4):1283–1292. https://doi.org/10.1210/jc.2018-01693
Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK (2011) Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96(10):3160–3169. https://doi.org/10.1210/jc.2011-1111
Zemel BS, Stallings VA, Leonard MB, Paulhamus DR, Kecskemethy HH, Harcke HT, Henderson RC (2009) Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy X-ray absorptiometry. J Clin Densitom 12(2):207–218. https://doi.org/10.1016/j.jocd.2009.01.005
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Gluer CC, Marin F, Ringe JD, Hawkins F, Moricke R, Papaioannu N, Farahmand P, Minisola S, Martinez G, Nolla JM, Niedhart C, Guanabens N, Nuti R, Martin-Mola E, Thomasius F, Kapetanos G, Pena J, Graeff C, Petto H, Sanz B, Reisinger A, Zysset PK (2013) Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res 28(6):1355–1368. https://doi.org/10.1002/jbmr.1870
Ettinger B, San Martin J, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19(5):745–751. https://doi.org/10.1359/JBMR.040117
Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, Gibson K, Neer RM (2006) Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 91(8):2882–2887. https://doi.org/10.1210/jc.2006-0190
Blumsohn A, Marin F, Nickelsen T, Brixen K, Sigurdsson G, Gonzalez de la Vera J, Boonen S, Liu-Leage S, Barker C, Eastell R, Group ES (2011) Early changes in biochemical markers of bone turnover and their relationship with bone mineral density changes after 24 months of treatment with teriparatide. Osteoporosis Int 22(6):1935–1946. https://doi.org/10.1007/s00198-010-1379-y
Keel C, Kraenzlin ME, Kraenzlin CA, Muller B, Meier C (2010) Impact of bisphosphonate wash-out prior to teriparatide therapy in clinical practice. J Bone Miner Metab 28(1):68–76. https://doi.org/10.1007/s00774-009-0101-7
Stepan JJ, Burr DB, Li J, Ma YL, Petto H, Sipos A, Dobnig H, Fahrleitner-Pammer A, Michalska D, Pavo I (2010) Histomorphometric changes by teriparatide in alendronate-pretreated women with osteoporosis. Osteoporosis Int 21(12):2027–2036. https://doi.org/10.1007/s00198-009-1168-7
Gamsjaeger S, Buchinger B, Zoehrer R, Phipps R, Klaushofer K, Paschalis EP (2011) Effects of one year daily teriparatide treatment on trabecular bone material properties in postmenopausal osteoporotic women previously treated with alendronate or risedronate. Bone 49(6):1160–1165. https://doi.org/10.1016/j.bone.2011.08.015
Fahrleitner-Pammer A, Burr D, Dobnig H, Stepan JJ, Petto H, Li J, Krege JH, Pavo I (2016) Improvement of cancellous bone microstructure in patients on teriparatide following alendronate pretreatment. Bone 89:16–24. https://doi.org/10.1016/j.bone.2016.05.004
Lou S, Lv H, Li Z, Zhang L, Tang P (2018) Combination therapy of anabolic agents and bisphosphonates on bone mineral density in patients with osteoporosis: a meta-analysis of randomised controlled trials. BMJ Open 8(3):e015187. https://doi.org/10.1136/bmjopen-2016-015187
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344(19):1434–1441. https://doi.org/10.1056/NEJM200105103441904
Tsai JN, Lee H, David NL, Eastell R, Leder BZ (2019) Combination denosumab and high dose teriparatide for postmenopausal osteoporosis (DATA-HD): a randomised, controlled phase 4 trial. Lancet Diabetes Endocrinol 7(10):767–775. https://doi.org/10.1016/S2213-8587(19)30255-4
Miller PD, Bilezikian JP, Diaz-Curiel M, Chen P, Marin F, Krege JH, Wong M, Marcus R (2007) Occurrence of hypercalciuria in patients with osteoporosis treated with teriparatide. J Clin Endocrinol Metab 92(9):3535–3541. https://doi.org/10.1210/jc.2006-2439
Tashjian AH Jr, Goltzman D (2008) On the interpretation of rat carcinogenicity studies for human PTH(1-34) and human PTH(1-84). J Bone Miner Res 23(6):803–811. https://doi.org/10.1359/jbmr.080208
Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH, Masica D (2012) The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res 27(12):2429–2437. https://doi.org/10.1002/jbmr.1768
Gilsenan A, Harding A, Kellier-Steele N, Harris D, Midkiff K, Andrews E (2018) The Forteo Patient Registry linkage to multiple state cancer registries: study design and results from the first 8 years. Osteoporosis Int 29(10):2335–2343. https://doi.org/10.1007/s00198-018-4604-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics approval
The study was approved by the Cincinnati Children’s Hospital Institutional Review Board.
Consent to participate
Informed consent and assent were obtained at study enrollment.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasomyont, N., Keefe, C., Tian, C. et al. Safety and efficacy of teriparatide treatment for severe osteoporosis in patients with Duchenne muscular dystrophy. Osteoporos Int 31, 2449–2459 (2020). https://doi.org/10.1007/s00198-020-05549-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05549-z