Abstract
Summary
The association of genetic polymorphisms with low bone mineral density in elite athletes have not been considered previously. The present study found that bone mass phenotypes in elite and pre-elite dancers are related to genetic variants at the Wnt/β-catenin and ER pathways.
Introduction
Some athletes (e.g. gymnasts, dancers, swimmers) are at increased risk for low bone mineral density (BMD) which, if untreated, can lead to osteoporosis. To investigate the association of genetic polymorphisms in the oestrogen receptor (ER) and the Wnt/β-catenin signalling pathways with low BMD in elite and pre-elite dancers (impact sport athletes).
Methods
The study included three phases: (1) 151 elite and pre-elite dancers were screened for the presence of low BMD and traditional osteoporosis risk factors (low body weight, menstrual disturbances, low energy availability); (2) a genetic association study was conducted in 151 elite and pre-elite dancers and age- and sex- controls; (3) serum sclerostin was measured in 101 pre-elite dancers and age- and sex-matched controls within a 3-year period.
Results
Eighty dancers revealed low BMD: 56.3% had at least one traditional osteoporosis risk factor, whereas 28.6% did not display any risk factor (37.2% revealed traditional osteoporosis risk factors, but had normal BMD). Body weight, menstrual disturbances and energy availability did not fully predict bone mass acquisition. Instead, genetic polymorphisms in the ER and Wnt/β-catenin pathways were found to be risk factors for low BMD in elite dancers. Sclerostin was significantly increased in dancers compared to controls during the 3-year follow-up (p < 0.05).
Conclusions
Elite and pre-elite dancers demonstrate high prevalence of low BMD, which is likely related to genetic variants at the Wnt/β-catenin and ER pathways and not to factors usually associated with BMD in athletes (body weight, menstrual disturbances, energy deficiency).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ample research on animal models and human populations has shown that there is a high variability in the adaptation of bone to exercise [1, 2], which is modulated to a large extent by genetic factors [2, 3]. Therefore, identification of single-nucleotide polymorphisms (SNPs) in genes of mechanotransduction signalling pathways can contribute to a further understanding of the factors involved in low bone mass phenotypes, particularly in individuals participating in impact sports (running, basketball, gymnastics, dancing, volleyball, etc.). The recently described oestrogen receptor (ER) and the Wnt/β-catenin signalling pathways play key roles in bone responsiveness to mechanical loading [4, 5], since osteoblast lineage cells require their full activity [6, 7]. To date, our knowledge on the adaptation of bone to exercise in relation to SNPs of these pathways remains limited despite that the prevalence of low bone mineral density (BMD) remains high even in individuals participating in impact sports [8,9,10].
Physical training, in general, and impact exercise, in particular, can protect against the risk of low BMD [11, 12]. In accordance, the World Health Organisation issued exercise guidelines to promote bone health in both males and females [13]. Nevertheless, while impact exercise can prevent low BMD, the effects of organised impact sport training on bone health remain controversial [14, 15]. For instance, some elite athletes in impact sports (particularly dancing) have lower BMD values than their non-exercising counterparts [9, 10, 16]. The theory currently used to explain the low BMD in these elite athletes involves mechanisms related to the growth hormone (GH)—insulin-like growth factor-I (IGF-I) axis and to the hypothalamic–hypophyseal–gonadal (HHG) axis. It has been hypothesised that these pathways are modulated by intense impact training which, in turn, is associated with the presence of low body weight, menstrual disturbances, and/or negative energy balance [14, 17,18,19,20], leading, eventually, to impairment of bone mineralisation. This phenomenon is known as the ‘female athlete triad’ or ‘relative energy deficiency in sport’ (RED-S) [14, 21] and has been recognised by several health organisations as a condition deserving appropriate monitoring and treatment [21,22,23]. However, several reports have suggested that low BMD in female elite athletes can occur without the presence of low body weight, menstrual disturbances or energy deficiency [24,25,26,27,28]. Moreover, far less is known in relation to male athletes.
Elite dancers represent an ideal population within impact sport athletes to investigate the association of SNPs in the ER and the Wnt/β-catenin signalling pathways with low bone mass phenotypes. Given that elite dancers are exposed to long hours of impact exercise regimens and to an environment that emphasises leanness [29, 30], the study of such individuals may shed light on the variability of bone anabolic responses to both exercise and osteoporosis risk factors (e.g. low body weight and fat mass). Dance training also offers a great model of mechanical loading since it may differently affect the peripheral and axial skeleton [8]. Consequently, the aim of the present study was to investigate the association of SNPs in the ER and the Wnt/β-catenin signalling pathways with low BMD phenotypes in a group of elite and pre-elite ballet dancers.
Materials and methods
Study population
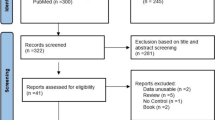
We used female and male pre-elite ballet dancers from a pre-elite dance school (offering full-time training to enter professional level; students have to audition for a place; 4–8 h of training per day) and female and male elite dancers from a professional ballet company (6–8 h of training per day). Pilot studies were administrated in a group of pre-elite dancers in order to calculate the sample size needed for prevalence estimates. In a sample of 36 dancers and 36 matched-controls, low BMD (Z-score of <− 2.0) at the lumbar spine (LS) was found in 36 and 6%, respectively. Based on this finding, we estimated that 41 participants were needed in each group to obtain 95% power, with α = 0.05.
An introductory letter briefly describing the study was sent to the executive boards of the dance school and ballet company. Following the boards’ permission, pre-elite dancers (and respective guardians) and elite dancers were presented with the purposes of the study; 126 dance students (70.0%) and 41 elite dancers (68.3%) volunteered. All volunteers completed a questionnaire concerning their ethnicity, medical history, and past/current calcium/vitamin D supplementation. Eligible criteria included participants of white European origin, with no illnesses or treatments that might affect bone metabolism, not taking medication known to affect bone metabolism and no calcium/vitamin D supplementation (two dance students and one elite dancers were excluded). Women taking oral contraceptives and hormonal therapy were also excluded (one elite dancer). Based on these criteria, the studied population consisted of 151 elite ballet dancers and pre-elite dance students.
The non-exercising participants (controls) were recruited from two local state schools and local universities. Eligibility criteria were set according to the dancers’ characteristics, i.e. controls were only considered eligible if they were of the same sex, age (defined as decimal age; 12-months difference of a dancer) and race (white European-Caucasian) as dancers. Exclusion criteria included participation in organised physical activities/sports (i.e., beyond school curriculum for students; two sessions of physical education lessons at school, 1/1.5 h per session). Control participation was also restricted to those who had received/were receiving medications known to affect bone metabolism and to who reported illnesses/treatments that might affect bone metabolism. Out of the 282 responses (105 pupils, 177 university students), 151 that fulfilled the aforementioned criteria and were further included in the study.
All participants provided signed informed consent according to the Declaration of Helsinki. The study was approved by the ethics committee of the Regional Administration of Health of Lisbon, Portugal (Proc.063/CES/INV/2012).
Study design
Study design and data collection are summarised in Fig. 1. The study consisted in three phases. The first phase included a cross-sectional study in all elite dancers and pre-elite dance students (151 participants); these dancers were screened for the presence of low BMD and traditional osteoporosis risk factors (i.e. low body weight, menstrual disturbances and low energy availability) considering current guidelines [21,22,23]. The second phase included a genetic association study in all recruited elite dancers, pre-elite dance students and aged- and sex-matched controls (151 dancers and 151 controls). Finally, the third phase included a longitudinal assessment of a subgroup of 101 pre-elite dance students (and aged- and sex-matched controls) to evaluate the associations of sclerostin with bone mass gains.
First phase (cross-sectional observations)
All 151 elite ballet dancers and pre-elite dance students were screened for the presence of low BMD. The ISCD criterion for children was used to assess pre-elite dance students (the ISCD has adopted the term “low BMD” for a Z-score less than − 2.0), and the ACSM guidelines [14] were adopted for our elite ballet dancers. The ACSM uses the term “low BMD” for a Z-score between − 1.0 and − 2.0 (along with secondary risk factors for stress fractures) and the term “osteoporotic” for a Z-score equal or less than − 2.0 (along with secondary risk factors for stress fractures). Specifically, dancers were screened for the presence of low body weight (defined as a body mass index of < 18.5 for adult participants; for children and adolescents, it was considered the body mass index expected for their age), low energy availability (< 30.0 kcal/kgFFM/day) and, in case of female participants, menstrual disturbances (primary/secondary amenorrhea, oligoamenorrhea).
Second phase (genetic associations)
Genes related to low bone mass phenotypes and involved in mechanotransduction were identified according to literature reports [4, 5]. This resulted in the identification of four genes: ESR1 and ESR2 (ER signalling pathway), as well as SOST and LRP5 (Wnt/β-catenin pathway). SNPs in or near these four genes reported to have a significant association with BMD variation and risk of osteoporosis in European populations were identified according to literature search [31]. The following SNPs were identified in SOST: rs851054, rs851056, rs10534024, rs4792909, rs9902563; LRP5: rs3736228, rs2306862, rs682429, rs491347, rs3781590, rs2508836, rs643892, rs312786; ESR1: rs2234693, rs9340799; ESR2: rs1256030, rs960070.
Characteristics of each SNP were further examined using the Ensembl database, Hapmap and NCBI. Linkage disequilibrium (LD) analyses were performed using Haploview 4.1 with data retrieved from HapMap (CEU population). SNPs were then selected according to the following parameters: (a) LD (R2) within each gene < 0.8; (b) distance from the promoter and 3′UTR < 30kb and (c) minor allele frequency (MAF) < 0.2. The following eleven SNPs were selected for genotyping: SOST: rs851054, rs10534024; LRP5: rs682429, rs491347, rs2508836, rs587808, rs312786; ESR1: rs2234693, rs9340799; ESR2: rs1256030, rs960070.
Third phase (longitudinal observations)
All pre-elite dance students (n = 115) and aged- and sex-matched controls included in the cross-sectional analysis were asked to participate on a follow-up study in order to analyse sclerostin serum concentration and bone mass throughout growth. Sixty-three female and 38 male pre-elite dance students (vs. 50 and 47 age- and sex-matched controls, respectively) volunteered. Data were collected annually for three consecutive years, from January 2013 to March 2015. Details on the participants’ measurements and specific methodology appear in Fig. 1.
Anthropometry, menstrual, nutritional intake and energy availability
Chronological age (obtained as decimal age) and anthropometry measurements were collected. Height, sitting height and body weight were measured in t-shirt, shorts and bare feet using a stadiometer (Seca, Seca217 portable stadiometer, Hamburg, Germany) with accuracy of 0.1 cm and an electric scale (TANITA BC-418 MA Segmental Body Composition Analyser; Tanita Corporation, Tokyo, Japan) with an accuracy of 0.1 kg.
All female participants were presented with a questionnaire to determine age at menarche, regularity of menstrual cycles and consumption of contraceptives. Amenorrhea was defined as the absence of menses for three consecutive months, whereas oligomenorrhea was considered when menstrual cycles occurred at intervals of greater than 35 days.
Nutrient intakes were recorded via a 3-day food diary, previously validated [32]. Participants were asked to record all food and beverages consumed during two week days and one weekend day following appropriate instructions. The software Food Processor SQL Edition, version 9.8.1 was used to estimate average energy and nutrition intakes. During the week that nutrition information was collected, energy expenditure was also estimated using an accelerometer—SenseWear [33] for 7 consecutive days. Energy availability was further estimated using standard protocols (http://www.femaleathletetriad.org/calculators/); information on dietary energy intake (provided by the food diary), exercise energy expenditure (information retrieved from the accelerometer) and body fat percentage (measured by DXA) was used for the estimation of energy availability.
Hormonal analysis
Blood samples were collected in early morning after an 8-h fasting. Blood samples were submitted to centrifugation at 2500×g for 10 min; serum samples were stored at − 80 °C until they were analysed. Serum sclerostin concentrations were measured by an ELISA assay kit (Human SOST/Sclerostin Quantikine ELISA Kit, Ref DSST00), from R&D Systems, Inc. (Minneaplolis, MN 55413, USA). The intra-assay and inter-assay CV’s ranged between 1.8–2.1 and 8.2–10.8%, respectively.
Genotyping
Genomic DNA was isolated from blood using the MagNA Pure LC DNA isolation kit (Roche, Switzerland) according to product specifications. Primers were generated from the genomic sequence using Primer-BLAST and its specificity determined using BLASTn. DNA was amplified with the QIAGEN Multiplex PCR Kit (Qiagen, Germany), either in single PCR reactions (SNP rs312786) or in two sets of multiplex reactions (set 1: SNPs rs2234693, rs960070, rs682429, rs587808 and rs851054; set 2: SNPs rs9340799, rs1256030, rs491347, rs2508836 and rs10534024). PCR products were purified using Sephadex G-50 fine (Sigma-Aldrich, USA) columns on a filtration plate and genotypes determined using the Genetic Analyzer 3130 and 3130xl (Applied Biosystems).
Bone measurements
BMD at the LS, FN and forearm (1/3 distal radius) were measured using DXA. Participants were assessed in two different centres using dual-energy X-ray absorptiometry (DXA): Lunar (GE Lunar Prodigy) and Hologic (Discovery Wi). For consistency, the same certified technician performed all scans and analyses at both centres in each year. The data obtained from the two machines were homogenised by following the procedure described elsewhere [34].
Statistical analyses
First phase (cross-sectional observations)
Independent t tests were used to compare descriptive characteristics and unadjusted values of bone measurements between dancers with low BMD and dancers with normal BMD. Bone parameters were further compared between elite dancers with low BMD with elites with normal BMD after adjustment for age, sex and primary amenorrhea using analysis of covariance (ANCOVA). ANCOVA was also used to estimate bone mass values in pre-elite dance students’ bone mass values after the adjustment for sex, energy availability, fat, calcium and carbohydrates intakes.
Second phase (genetic associations)
Independent t tests were used to compare general characteristics between dancers and controls (stratified by bone mass phenotypes). Hardy-Weinberg equilibrium (HWE) of alleles at individual loci (level of significance set at p < 0.01) was measured at the level of the control population. Association of genotypes with study groups (defined according to bone mass status: dancers with normal BMD vs. dancers with low BMD, and controls with normal BMD vs. dancers with low BMD) and independence of SNPs were assessed by unconditional logistic regression with the “SNPassoc” package implemented in R. The minor allele of most SNPs is the ancestral allele and, thus, it has been selected as the reference allele in all analysis. Four hereditary models were considered in the analysis (codominant, dominant, recessive and log-additive) and included the variable weight. Other confounding variables such as sex and age were not included in the models because the BMD measurements were performed according to references that already included adjustment for those variables. The adjustment for multiple testing was performed by the false discovery rate (FDR) method. Haplotype frequencies were inferred using the ‘haplo.stats’ package implemented in R. Haplotype association with the study groups (OR, 95% CI and p values) was assessed for those with a minimum haplotype frequency of 0.01 and using as reference the most frequent haplotype.
Third phase (longitudinal observations)
Independent t tests were used to compare general characteristics between pre-elite dance students and aged- and sex-matched controls at each measured occasion. Bone mass values were adjusted for sex and serum sclerostin concentrations using ANCOVA. Analyses in all phases were performed with SPSS v.20.0 and statistical significance was set at p < 0.05.
Results
First phase (cross-sectional observations)
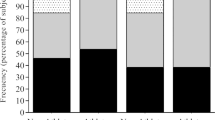
Some dancers revealed low BMD, but did not display any traditional osteoporosis risk factors (and vice-versa). Specifically, out of the 151 elite ballet dancers and pre-elite dance students, 80 were identified with low BMD (Fig. 2). Out of these 80 athletes, 56.3% had at least one traditional osteoporosis risk factor, whereas 28.6% were diagnosed with low BMD but did not display any risk factor. In contrast, 37.2% revealed one or more traditional osteoporosis risk factors, but had normal BMD (Fig. 2).
General characteristics of the 151 elite ballet dancers and pre-elite dance students are displayed in Table S1 (Supplement); briefly, all bone mass parameters were significantly attenuated in dancers with low BMD compared to the ones with normal BMD. Table S2 (Supplement) shows that following adjustments for sex, energy availability and nutrition intake (energy, fat, calcium, carbohydrates), elite dancers previously identified with low BMD continued to display a significant lower BMC and BMD values at the forearm compared with their counterparts with normal BMD (BMC: p < 0.05; BMD: p < 0.01). Similarly, pre-elite dancers with low BMD also displayed significantly lower bone mass parameters compared with pre-elite dancers with normal BMD at all anatomical sites (FN BMC/BMD, p < 0.001; LS BMC, p < 0.001; forearm BMD, p < 0.001).
Second phase (genetic associations)
Genetic variants at the ER and the Wnt/β-catenin signalling pathways were found to be risk factors for low BMD in dancers at both impact and non-impact sites. Table S3 (Supplement) shows selected characteristics of the genotyped SNPs. Three SNPs [rs682429 (LRP5), rs851054 and rs10534024 (SOST)] were significantly deviated from the Hardy-Weinberg Equilibrium (p < 0.01) and were not further considered. Table 1 shows the association of SNPs in LRP5, ESR1 and ESR2 with bone mass phenotypes comparing elite and pre-elite dancers with normal BMD (reference) and dancers with low BMD. The T allele in rs2234693 (ESR1) was associated with low BMD at the LS [OR (CI) = 1.77 (1.06–2.97), p = 0.026). However, this association was not retained after FDR correction. Considering the SNP rs9340799 (ESR1), the A allele (dominant model) was associated with low BMD in elite and pre-elite at the forearm [OR (CI) = 10.74 (1.37–83.98), p = 1.9 × 10−3]. The genotypes AG and AA were significantly associated with low BMD at the forearm with an OR of 10.63 (1.32–85.87) and 10.85 (1.35–87.36), respectively (p = 8.2 × 10−3).
Table 2 shows the distribution of genotypes in controls with normal BMD (reference) and dancers with low BMD. Considering SNP rs2508836 (LRP5), the C allele was associated with low BMD in dancers (dominant model) at the LS [OR (CI) = 6.90 (1.27–37.49), p = 1.0 × 10−3]. Considering SNP rs9340799 (ESR1), there was a significant difference in genotype frequencies between normal controls and dancers with low BMD at the forearm (p = 0.019), LS (p = 0.021) and FN (p = 0.020). The A allele (log-additive model) significantly increased the odds of low BMD in elite and pre-elite dancers at the forearm [OR (CI) = 1.95 (1.09–3.51), p = 0.020], LS [OR (CI) = 2.32 (1.24–4.32), p = 5.8 × 10−3] and FN [OR (CI) = 2.45 (1.26–4.74), p = 5.2 × 10−3]. The association of the A allele with low BMD in dancers was also observed in the dominant model at the forearm [OR (CI) = 8.37 (1.07–65.26), p = 7.0 × 10−3], and in the recessive model at the LS [OR (CI) = 2.98 (1.30–6.87), p = 9.3 × 10−3] and FN [OR (CI) = 3.08 (1.26–7.51), p = 0.012]. All aforementioned associations regarding rs9340799 SNP were retained after FDR correction. The only significant association retained after FDR correction was at LS for SNP rs9340799 in ESR1. The A allele was significantly associated with low BMD [OR (CI) = 2.10 (1.22–3.62), p = 5.4 × 10−3], an association already observed in dancers with low BMD.
Table S4 (Supplement) demonstrates an inverse association with low BMD at the FN in dancers with haplotype CG in ESR1 [OR (CI) = 0.53 (0.29–0.96), p = 0.037]. Haplotype analysis also revealed that, within the same anatomical site, the odds of low BMD were significantly increased in dancers with the haplotype GCGT in LRP5 [OR (CI) = 8.97 (1.14–70.31), p = 0.037]. Haplotype association tests considering normal controls (reference) and dancers with low BMD showed the CG haplotype in ESR1 was inversely associated with low BMD at the LS [OR (CI) = 0.43 (0.22–0.82), p = 0.001] and at the FN [OR (CI) = 0.39 (0.19–0.80), p = 0.010]. In LRP5, haplotype GCAG was significantly associated with low BMD at the forearm in dancers [OR (CI) = 6.43 (1.33–31.14) p = 0.021] and haplotype GCGT was associated with low BMD at the LS [OR (CI) = 12.7 (1.22–132.18) p = 0.033].
Third phase (longitudinal observations)
Serum sclerostin concentrations were increased throughout the 3-year study in pre-elite dance students compared to controls. Indeed, at baseline and at 1-year follow-up, female pre-elite dancers revealed significantly higher sclerostin values than their controls (p < 0.001) (Fig. 3). No significant difference was found in serum sclerostin concentrations between male pre-elite dance students and their controls. Bone mass values were further adjusted for sex and sclerostin serum concentrations; after the adjustment, no differences in BMC and BMD between groups were seen at the FN and LS (p > 0.05) (Table S5; Supplement).
Discussion
The present study used elite and pre-elite dancers as a group representing individuals participating in impact sports and showed that they are exhibiting very high prevalence (52.98 ± 7.96%) of low BMD. Moreover, we found that the low BMD in these individuals is not explained by factors usually associated with bone mass in athletes (i.e. body weight, menstrual disturbances, energy deficiency). Indeed, after adjusting for these factors, individuals with low BMD continued to display lower bone mass values at both impact and non-impact sites. More importantly, the present study shows, for the first time, that selected gene polymorphisms of the ER and the Wnt/β-catenin signalling pathways are significantly associated with low BMD in elite impact sport athletes. Specifically, the ESR1 rs9340799 A allele, LRP5 rs2508836 C allele and LRP5 GCGT/GCAG haplotypes were associated with increased odds of low BMD at both impact (LS and FN) and non-impact sites (forearm). Furthermore, we found that serum sclerostin, a protein that inhibits bone formation by blocking the action of the Wnt in osteoblasts [35], was significantly increased in dancers compared to controls. Taken together, these results suggest that the ER and Wnt/β-catenin signalling pathways may be critical in determining bone mass phenotypes in elite impact sport athletes.
Results from clinical research show that dancers are at higher risk for developing low BMD and osteoporosis compared to the general population [36,37,38], yet the mechanistic physiology to explain this phenomenon remains poorly understood [39]. Previous research highlighted that the exercise-induced response to mechanical stress by osteoblast lineage cells requires full ESR1 and LRP5 activity [6, 40]. The present study showed that dancers C homozygotes for SNP rs2508836 in LRP5, dancers ESR1 rs9340799 A-carries, and dancers LRP5 GCGT and GCAG haplotype-carriers have increased odds for developing low BMD at both impact and non-impact sites. Therefore, it can be hypothesised that the degree to which each individual responds to mechanical stress from dance training stimuli is associated with their genetic background. This is further supported by observations in mice lacking functional ESR1, which show a 70% attenuation of the osteogenic response to loading [41, 42], as well as the fact that the Lrp5 co-receptor is regulating osteoblast activity following loading [6, 7]. Based on these key roles of ESR1 and LRP5 in the regulation of mechanical loading, it can be speculated that the ESR1 rs9340799 and LRP5 rs2508836 modulated our athletes’ skeletal response to exercise.
It should be highlighted that BMD phenotypes are not inherited in a Mendelian manner, since low BMD onset and progression are influenced by genetic factors modulated by environmental elements [43]; the results of the present study may express this view. Comparing controls with normal BMD (not receiving exercise stimuli) to dancers with low BMD (receiving exercise stimuli), it was observed that ESR1 rs9340799 A allele and LRP5 rs2508836 C allele were associated with increased odds of low BMD in dancers, not only at non-impact sites, but at impact sites too. In turn, comparing dancers with normal BMD with dancers with low BMD (both groups receiving exercise stimuli), only the ESR1 rs9340799 A allele was associated with increased odds of low BMD at non-impact sites only. Since the associations between the risk alleles and phenotypes at impact sites are not manifested when comparing dancers with normal BMD and dancers with low BMD (both groups receiving exercise stimuli), it could be hypothesised that genetic and environmental factors were interacting closely in determining dancers’ bone mass phenotypes. However, randomised control trials are needed to confirm this hypothesis.
Both rs9340799 and rs2508836 are located in introns 1. First introns can affect gene transcription, polyadenylation, mRNA export, translational efficiency, rate of mRNA decay, and may also alter transcription factors binding sites [44,45,46]. Therefore, it seems logical to suggest that one of these processes influenced expression levels of ESR1 and LRP5 and, consequently, susceptibility for low BMD phenotypes in our study. Previous research in other population groups (e.g., postmenopausal women, elderly young adults, adolescents) also reported associations between rs9340799 and rs2508836 and bone mass phenotypes [31]. However, the mechanisms by which these SNPs may affect gene function and, ultimately, bone phenotypes remain unknown. Also, the possibility that the present associations are due to linkage disequilibrium with others, causally associated, SNPs cannot be excluded.
Osteocytes mediate the osteogenic response from mechanical loading through sclerostin (encoded by the SOST gene) [35]; SOST downregulation is associated with bone mass gains, whereas overexpression of SOST has been linked with low bone mass phenotypes [35, 47]. Intervention studies in humans showed reduced levels of serum sclerostin following exercise stimuli, resulting in bone mass gains [48, 49]. Therefore, the athletes participating in the third phase of this study (longitudinal observations) would be expected to demonstrate significantly lower sclerostin concentrations and higher bone mass values compared to controls, due to the osteogenic activity of their daily exercise training. In contrast, the athletes showed significantly higher sclerostin levels and lower BMD levels than controls. Moreover, these differences were not apparent after adjustment for sclerostin. Although the role of circulating sclerostin is not entirely clear, the aforementioned findings may indicate that this protein is inhibiting the Wnt/β-catenin pathway leading, eventually, to low BMD. However, since our population consisted of long-term elite athletes, their skeletons may have reached equilibrium and the loading impact of dancing may not be leading to a reduction in osteocyte sclerostin. Although no studies reporting the existence of an exercise stimuli threshold above which bone cells saturate were found, this hypothesis should not be excluded. As sclerostin is a key protein in Wnt/β-catenin, this pathway might be fundamental in determining bone mass phenotypes in impact sport athletes.
Researchers and clinicians should be aware that low BMD in elite and pre-elite dancers might not be associated with the factors currently proposed by health organisations (i.e. body weight, menstrual disturbances and energy deficiency). The present study shows that the underlying pathophysiology of low BMD in these athletes relates to genetic factors influencing bone mass acquisition. Nevertheless, further exploration of the genes and signalling pathways involved in dancers’ adaptive response to exercise stimuli may yield a better understanding on the pathogenesis of low BMD in dancers, and may be useful in defining new therapeutic targets for clinical interventions.
It is reasonable to assume that the present results may have been influenced by methodological limitations such as population stratification, a characteristic common in most genetic association studies. Considering the number of dancers (n = 151) and age- and sex-matched controls genotyped (n = 151 in each group), the present study has over 85% power to detect a modest genetic effect (OR of 2.0 and MAF = 0.2). However, some power is lost after stratification, which may explain the lack of associations with other SNPs. Indeed, although our participants were matched for age, body weight, and sex, the matching for age was partly lost following stratification. Furthermore, stratification of the sample size might also justify the significant associations detected under different models for the same SNP (e.g. SNP rs9340799). Another limitation is that searching for the association of several SNPs with more than one phenotype can lead to increased risk for type I error (i.e., false-positives). To avoid this limitation, a multiple test correction (FDR) was applied. The present study included in the same group of dancers adults (who might be already experiencing bone mass losses) and children (who have not yet reached their peak bone mass). Although Z-scores, instead of BMD mean values, were used in order to overcome concerns due to the employment of a mixed group of dancers, it is recommend that future studies should consider to separate adult and children dancers for better clarification. Furthermore, it would be interesting in future studies to collect data on the intensity of dance training and adjust data accordantly. Finally, it is important to note that our groups included both males and females because the distribution of Esr1 receptors in bone cells is similar among sexes [50]. Nevertheless, sex differences in the regulation of the Wnt/β-catenin signalling pathway should not be excluded; future studies should consider sexes separately to determine if one sex is influencing the genetic association or phenotypic variation more than the other.
Conclusion
Elite and pre-elite dancers demonstrate very high prevalence of low bone mineral density, which is likely related to genetic variants at the Wnt/β-catenin and ER signalling pathways and not to factors usually associated with bone mass in athletes (i.e. body weight, menstrual disturbances, energy deficiency).
References
Jepsen KJ (2009) Systems analysis of bone. Wiley Interdiscip Rev Syst Biol Med 1:73–88
Kesavan C, Mohan S, Srivastava AK, Kapoor S, Wergedal JE, Yu H, Baylink DJ (2006) Identification of genetic loci that regulate bone adaptive response to mechanical loading in C57BL/6J and C3H/HeJ mice intercross. Bone 39:634–643
Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S (2005) Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem 96:1049–1060
Suuriniemi M, Mahonen A, Kovanen V, Alén M, Lyytikäinen A, Wang Q, Kröger H, Cheng S (2004) Association between exercise and pubertal BMD is modulated by estrogen receptor alpha genotype. J Bone Miner Res 19:1758–1765
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615
Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A (2010) Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun Elsevier Inc 394:755–759
Amorim T, Koutedakis Y, Nevill A, Wyon M, Maia J, Machado JC, Marques F, Metsios GS, Flouris AD, Adubeiro N, Nogueira L, Dimitriou L (2017) Bone mineral density in vocational and professional ballet dancers. Osteoporis Int 28:2903–2912
Scofield KL, Hecht S (2012) Bone health in endurance athletes. Curr Sports Med Rep 11:328–334
Pollock N, Grogan C, Perry M, Pedlar C, Cooke K, Morrissey D, Dimitriou L (2010) Bone-mineral density and other features of the female athlete triad in elite endurance runners: a longitudinal and cross-sectional observational study. Int J Sport Nutr Exerc Metab 20:418–426
Xu J, Lombardi G, Jiao W, Banfi G (2016) Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: an overview of systematic reviews and meta-analyses. Sports Med 46:1165–1182
Guadalupe-Grau A, Fuentes T, Guerra B, Calbet JAL (2009) Exercise and bone mass in adults. Sport Med 39:439–468
Global Recommendations on Physical Activity for Health WHO Library Cataloguing-in-Publication Data Global recommendations on physical activity for health. [cited 2016 Oct 14]; Available from: http://apps.who.int/iris/bitstream/10665/44399/1/9789241599979_eng.pdf
Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP (2007) The female athlete triad. Med Sci Sports Exerc 39:1867–1882
Gibbs JC, Williams NI, De Souza MJ (2013) Prevalence of individual and combined components of the female athlete triad. Med Sci Sports Exerc 45:985–996
Torstveit MK, Sundgot-Borgen J (2005) The female athlete triad exists in both elite athletes and controls. Med Sci Sports Exerc 37:1449–1459
Georgopoulos N a, Markou KB, Theodoropoulou A, Vagenakis G e, Mylonas P, Vagenakis AG (2004) Growth, pubertal development, skeletal maturation and bone mass acquisition in athletes. Hormones 3:233–243
Wheatley S, Khan S, Székely AD, Naughton DP, Petróczi A (2012) Expanding the female athlete triad concept to address a public health issue. Perform Enhanc Health [internet]. Elsevier Ltd 1:10–27. https://doi.org/10.1016/j.peh.2012.03.001
Bonjour JP, Chevalley T (2014) Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev 35:820–847
Di Pietro L, Stachenfeld N (2006) Refutation of the myth of the female athlete triad. Br J Sports Med 41:57–58
Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, Ljungqvist A (2014) The IOC consensus statement: beyond the female athlete triad—Relative Energy Deficiency in Sport (RED-S). Br J Sports Med 48:491–497
Kleposki RW (2002) The female athlete triad: a terrible trio implications for primary care. J Am Acad Nurse Pract 14:23–26
George C, Leonard J, Hutchinson M (2011) The female athlete triad: a current concepts review. S Afr J Sport Med 23:50–56
Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R (1995) Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J Bone Miner Res 10:26–35
Maïmoun L, Coste O, Mariano-Goulart D, Galtier F, Mura T, Philibert P, Briot K, Paris F, Sultan C (2011) In peripubertal girls, artistic gymnastics improves areal bone mineral density and femoral bone geometry without affecting serum OPG/RANKL levels. Osteoporos Int 22:3055–3066
Maïmoun L, Coste O, Philibert P, Briot K, Mura T, Galtier F et al (2013) Peripubertal female athletes in high-impact sports show improved bone mass acquisition and bone geometry. Metabolism. Elsevier Inc 62:1088–1098
Khan KM, Green RM, Saul A, Bennell KL, Crichton KJ, Hopper JL et al (1996) Retired elite female ballet dancers and nonathletic controls have similar bone mineral density at weightbearing sites. J Bone Miner Res 11:1566–1574
Kirchner EM, Lewis RD, O’Connor PJ (1994) Bone mineral density and dietary intake of female college gymnasts. Med Sci Sport Exerc 27:543–549
Twitchett EA, Koutedakis Y, Wyon MA (2009) Physiological fitness and professional classical ballet performance: a brief review. J Strength Cond Res 23:2732–2740
Twitchett E, Ph D, Brodrick A, Sc M, Nevill AM, Koutedakis Y et al (2010) Does physical fitness affect injury occurrence and time loss due to injury in elite vocational ballet students? J Dance Med Sci 14:26–31
Li WF, Hou SX, Yu B, Li MM, Férec C, Chen JM (2010) Genetics of osteoporosis: accelerating pace in gene identification and validation. Hum Genet 127:249–285
Crawford PB, Obarzanek E, Morrison J, Sabry ZI (1994) Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. J Am Diet Assoc 94:626–630
Brazeau AS, Beaudoin N, Bélisle V, Messier V, Karelis AD, Rabasa-Lhoret R (2016) Validation and reliability of two activity monitors for energy expenditure assessment. J Sci Med Sport 19:46–50
Amorim T (2017) Bone mass of female dance students prior to professional dance training: a cross-sectional study. PLoS One 12:1–11
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D et al (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone Elsevier BV 50:209–217
Doyle-Lucas AF, Akers JD, Davy BM (2010) Energetic efficiency, menstrual irregularity, and bone mineral density in elite professional female ballet dancers. J Dance Med Sci 14:146–154
Burckhardt P, Wynn E, Krieg M-A, Bagutti C, Faouzi M (2011) The effects of nutrition, puberty and dancing on bone density in adolescent ballet dancers. J Dance Med Sci 15:51–60
Valentino R, Savastano S, Tommaselli AP, D’Amore G, Dorato M, Lombardi G (2001) The influence of intense ballet training on trabecular bone mass, hormone status, and gonadotropin structure in young women. J Clin Endocrinol Metab 86:4674–4678
Amorim T, Wyon M, Machado JC, Metsios GS, Flouris D, Koutedakis Y (2015) Prevalence of low bone mineral density in female dancers. Sport Med 45:257–268
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ (2012) Update on Wnt signaling in bone cell biology and bone disease. Gene. Elsevier B.V. 492:1–18
Tobias JH (2003) At the crossroads of skeletal responses to estrogen and exercise. Trends Endocrinol Metab 14:441–443
Lee K, Lanyon L (2004) Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev 32:64–68
Delgado-Calle J (2012) Do epigenetic marks govern bone mass and homeostasis? Curr Genomics 13:252–263
Li H, Chen D, Zhang J (2012) Analysis of intron sequence features associated with transcriptional regulation in human genes. PLoS One 7:1–9
Majewski J, Majewski J, Ott J, Ott J (2002) Distribution and characterization of regulatory elements in the human genome. Genome Res 12:1827–1836
Nott A, Meislin SH, Moore MJ (2003) A quantitative analysis of intron effects on mammalian gene expression a quantitative analysis of intron effects on mammalian gene expression. RNA 9:607–617
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Hinton PS, Nigh P, Thyfault J (2016) Serum sclerostin decreases following 12months of resistance- or jump-training in men with low bone mass. Bone 96:85–90. https://doi.org/10.1016/j.bone.2016.10.011
Ardawi M-SM, Rouzi AA, Qari MH (2012) Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab 97:3691–3699
Braidman IP, Hainey L, Batra G, Selby PL, Saunders PT, J a H (2001) Localization of estrogen receptor beta protein expression in adult human bone. J Bone Miner Res 16:214–220
Acknowledgements
The authors thank the Portuguese Foundation for Science and Technology for supporting Tânia Amorim (PhD grant ref.: SFRH/BD/88585/2012) and Cecília Durães (postdoctoral grant ref.: SFRH/BPD/62974/2009). Thanks are also expressed to the Ipatimup Diagnostics Service.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All participants provided signed informed consent according to the Declaration of Helsinki. The study was approved by the ethics committee of the Regional Administration of Health of Lisbon, Portugal (Proc.063/CES/INV/2012).
Conflicts of interest
None
Electronic supplementary material
ESM 1
(DOCX 104 kb)
Rights and permissions
About this article
Cite this article
Amorim, T., Durães, C., Machado, J.C. et al. Genetic variation in Wnt/β-catenin and ER signalling pathways in female and male elite dancers and its associations with low bone mineral density: a cross-section and longitudinal study. Osteoporos Int 29, 2261–2274 (2018). https://doi.org/10.1007/s00198-018-4610-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4610-x