Abstract
Summary
In non-osteoporotic postmenopausal women with breast cancer, aromatase inhibitors (AIs) negatively affected bone mineral density (BMD), lumbar spine trabecular bone score (TBS) as a bone microarchitecture index, and hip geometry as a bone macroarchitecture index.
Introduction
AIs increase the risk of fracture in patients with breast cancer. Therefore, we aimed to evaluate the long-term skeletal effects of AIs in postmenopausal women with primary breast cancer.
Methods
We performed a retrospective longitudinal observational study in non-osteoporotic patients with breast cancer who were treated with AIs for ≥3 years (T-score >−2.5). Patients with previous anti-osteoporosis treatment or those who were given bisphosphonate during AI treatment were excluded from the analysis. We serially assessed BMD, lumbar spine TBS, and hip geometry using dual-energy X-ray absorptiometry.
Results
BMD significantly decreased from baseline to 5 years at the lumbar spine (−6.15%), femur neck (−7.12%), and total hip (−6.35%). Lumbar spine TBS also significantly decreased from baseline to 5 years (−2.12%); this change remained significant after adjusting for lumbar spine BMD. The annual loss of lumbar spine BMD and TBS slowed after 3 and 1 year of treatment, respectively, although there was a relatively constant loss of BMD at the femur neck and total hip for up to 4 years. The cross-sectional area, cross-sectional moment of inertia, minimal neck width, femur strength index, and section modulus significantly decreased, although the buckling ratio increased over the treatment period (all P < 0.001); these changes were independent of total hip BMD.
Conclusions
Long-term adjuvant AI treatment negatively influenced bone quality in addition to BMD in patients with breast cancer. This study suggests that early monitoring and management are needed in non-osteoporotic patients with breast cancer who are starting AIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In addition to tamoxifen, aromatase inhibitors (AIs) are a standard adjuvant endocrine therapy for postmenopausal women with hormone receptor-positive breast cancer [1, 2]. AIs prevent estrogen synthesis by inhibiting the conversion of androgens to estrogens, which results in a marked depletion in circulating estrogen levels [3]. Although AI treatment has clear benefits for patients with breast cancer by increasing their disease-free survival and decreasing their risk of recurrence [4], AIs also adversely affect bone health [5–8]. AIs accelerate bone turnover and result in decreased bone mineral density (BMD) in patients with breast cancer than healthy postmenopausal women [9–11]. Furthermore, postmenopausal women with breast cancer who receive AIs have a twofold higher risk of fracture than healthy postmenopausal women do [5, 12]. Thus, it is recommended that patients with breast cancer who are initiating or already receiving AIs undergo skeletal monitoring via dual energy X-ray absorptiometry (DXA) or spine radiography [13]. In addition, anti-resorptive drugs are highly recommended for patients with a T-score of <−2.0 who are initiating or receiving AIs [14]. However, there are few studies regarding the effect of AIs on bone microarchitecture or macroarchitecture, which are also major determinants of bone strength in non-osteoporotic patients with breast cancer treated by AIs.
Trabecular bone score (TBS) is a novel texture parameter that evaluates bone microarchitecture based on lumbar spine DXA images by measuring the pixel gray-level variations [15]. Recent studies have reported that TBS values predicted osteoporotic fractures independently of BMD [16–18]. The effect of AIs on lumbar spine TBS has also been examined in two previous studies [19, 20], which both reported significantly decreased TBS after 2 years of treatment using AIs. However, those studies were limited by a small sample size and short follow-up period.
Hip geometry can be assessed as a bone macroarchitecture measure using software programs based on DXA images, such as the hip structure analysis using the Hologic system and advanced hip assessment (AHA) via the GE Lunar system. Among hip geometry parameters, hip axis length is only found to predict hip fractures independently of BMD in postmenopausal women. Although other parameters, including the cross-sectional area (CSA), section modulus (SM), buckling ratio (BR), and cross-sectional moment of inertia (CSMI), are associated with the prediction of hip fracture; these are not recommended for assessing hip fracture risk because there is insufficient evidence that this effect is independent of BMD [21]. However, hip geometry has rarely been investigated in patients with breast cancer who are receiving AIs; two small studies reported that AIs had a negative effect on hip geometry over a 2-year period [22, 23].
To the best of our knowledge, no previous studies have simultaneously investigated the longitudinal changes in BMD, TBS, and hip geometry in non-osteoporotic patients with breast cancer receiving AI treatment. Furthermore, changes in TBS and bone geometry have not been assessed for longer than 2 years of treatment using AIs. Therefore, we aimed to investigate the simultaneous longitudinal changes in BMD, TBS, and hip geometry over a 5-year period in non-osteoporotic postmenopausal women with breast cancer who were initiating or receiving AIs.
Materials and methods
Patient population
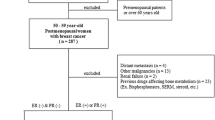
This retrospective, longitudinal, observational study included postmenopausal women with stage I–IIIA estrogen receptor- and/or progesterone receptor-positive primary breast cancer who were treated with up-front AIs for ≥3 years at the Seoul National University Hospital between January 2006 and December 2013. Eligible patients were required to have undergone complete surgical resection and/or chemotherapy and/or radiotherapy without any evidence of residual disease. All patients had a baseline T-score of >−2.5, an Eastern Cooperative Oncology Group performance status of ≤2, and had undergone at least one DXA follow-up after >3 years of AI treatment. The exclusion criteria were clinical or radiological evidence of progressive disease during AI treatment, concurrent malignancies or a history of other malignancies, renal dysfunction, previous hormonal adjuvant therapy for breast cancer (e.g., tamoxifen, raloxifene, toremifene, and goserelin), diseases that required drugs that affect bone metabolism (including thyroid disease), previous or concurrent anti-osteoporosis treatment (e.g., estrogen replacement therapy, bisphosphonate), and initiating bisphosphonate therapy while being treated by AIs. Twenty-two patients with osteopenia started to receive bisphosphonate at the time of initiation of AI therapy, and 19 and 12 patients started bisphosphonate therapy after 1 and 2 years of AI treatment, respectively. The study flow chart is shown in Fig. 1.
The study was approved by the institutional review board of Seoul National University Hospital (No. H-1601-057-734) and was conducted in accordance with the Declaration of Helsinki. Informed consent from the study participants was waived due to the study’s retrospective nature.
Anthropometric measurements
We measured height and body weight while the patient was not wearing shoes using standard methods at the same time that we performed DXA. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared.
Measurements of bone mineral density and trabecular bone score
The lumbar spine, femur neck, and total hip areal BMD values were assessed using DXA (GE Lunar Prodigy, GE Healthcare, Madison, WI, USA) and analyzed using Encore Software (version 14.10.022), according to the manufacturer’s recommendations. All patients were scanned using the same DXA machine during the study period. Given the patients’ annual visit schedule to the outpatient clinic, we regarded the 3 months before and after 1 year of treatment as an acceptable window for the follow-up BMD assessments. Lumbar spine BMD was evaluated at the L1–4 level after excluding any deformed or fractured vertebrae. Lumbar spine TBS was analyzed with TBS iNsight software (version 2.1.1.0, Med-Imaps, Pessac, France) using the anteroposterior spine DXA files for the same spine levels as used for the BMD measurement. To obtain constant BMD and TBS values over repeated measurements of the same patient, meticulous quality control was performed in our institution. The DXA device was calibrated by scanning 10 BMD measurements on the same instrument with a single anthropomorphic phantom. Additionally, a TBS calibration process was performed by scanning 10 TBS measurements using a specific TBS phantom. The precision for BMD was 0.26% with a mean BMD value of 1.263, and the precision for TBS was 0.14% with a mean TBS value of 1.462. Details regarding the precision of BMD and TBS assessed with the same instrument used in this study have been previously published [24].
Measurement of hip geometry
The Lunar AHA program incorporated into the DXA was used to evaluate hip geometry parameters as follows: CSA (cross-sectional bone surface area), CSMI (the ability of the bone to resist bending at the compressive surface), femur strength index (FSI; the ability of the bone to resist falling on the greater trochanter), minimal neck width (minimal NW; width of the narrowest femur neck), BR (the ability of the bone to resist bending at the tensile surface), SM (an indicator of bending strength), and cortical thickness of the neck (CT). The short-term precision errors for CSA, CSMI, and SM were 3.74, 9.58, and 6.37%, respectively [21]. The femur neck and total hip regions of interest were in the right femur neck, and the femur region of interest was automatically placed in the proximal part of the femur neck. Diagnoses of osteopenia were determined as −2.5< BMD T-score ≤−1.0 at any skeletal site.
Statistical analysis
Data are presented as mean ± standard deviation, median (interquartile range), or n (%). Continuous variables were analyzed using the Student t test, and categorical variables were compared using the chi-square test. We used linear mixed models with a first-order autoregressive covariance structure to evaluate the percentage changes from baseline for the BMD, lumbar spine TBS, and hip geometry parameters during the treatment period. The fixed effect was time (baseline, 1, 2, 3, 4, and 5 years), and the study subjects were considered as random effects. Age and BMI were adjusted as covariates. The adjusted P value after Bonferroni correction for multiple testing was evaluated using linear mixed models, and differences with a P value of <0.05 were considered statistically significant. All statistical analyses were performed using SPSS statistics for Windows (Version 21, IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics of patients
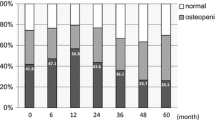
The baseline clinical and skeletal-related characteristics of study subjects are shown in Table 1. Of the 321 postmenopausal women with operable breast cancer, the mean age at initiating AI treatment was 58.8 years and the mean BMI was 25.3 kg/m2. The median duration of follow-up was 4 years. The majority of patients had experienced natural menopause, with a median menopause duration of 7 years, and had been diagnosed with stage I–II breast cancer. Two types of AI were used as an up-front AI therapy (anastrozole and letrozole), with anastrozole being more frequently prescribed than letrozole (83.8 vs. 16.2%). Of the 321 patients, 157 (48.9%) had osteopenia at baseline.
One hundred twelve patients (34.9%) underwent chemotherapy before starting treatment with AIs. Patients with previous chemotherapy were older and had experienced a longer duration of menopause (both P < 0.001). Baseline BMD, lumbar spine TBS, and hip geometry parameters were comparable between the patients with and without previous chemotherapy, except minimal NW, which was lower in patients without previous chemotherapy (P = 0.018).
Changes in bone parameters in overall patients
Patients who were treated with AIs exhibited significant decreases from their baseline BMD at the lumbar spine, femur neck, and total hip as well as a decrease in their lumbar spine TBS over the 5 years of treatment (Supplemental Fig. 1). The overall losses in lumbar spine BMD and TBS after 5 years of treatment were −6.15 and −2.12%, respectively (both P < 0.001; Supplemental Fig. 1A–B). The overall change in lumbar spine TBS remained significant after adjusting for lumbar spine BMD (P = 0.013). The estimated overall bone loss was most severe at the femur neck and total hip, with BMD decreasing by −7.12 and −6.35%, respectively (both P < 0.001; Supplemental Fig. 1C–D). The annual percentage changes in BMD and TBS are presented in Table 2. The estimated annual loss of BMD at each skeletal site and the loss of lumbar spine TBS were the most severe after 1 year of treatment (lumbar spine BMD, −3.17%; femur neck BMD, −2.40%; total hip BMD, −2.79%; lumbar spine TBS, −1.13%). However, the estimated annual loss of lumbar spine BMD and TBS slowed after 3 and 1 year of treatment, respectively, while the estimated annual BMD loss at the femur neck and total hip slowed after 4 years of treatment.
The longitudinal changes in hip geometry parameters over time are shown in Fig. 2 and Table 3. Compared with the baseline values, the linear mixed models revealed that 5 years of AI treatment was associated with significant reductions in CSA (−7.84%), CSMI (−8.01%), minimal NW (−1.35%), FSI (−7.73%), and SM (−5.95%), as well as with an increase in BR (+5.47%) (all P < 0.001) (Fig. 2 a–f). Changes in these parameters remained significant after adjusting for total hip BMD, with the exception of FSI (P = 0.205). There was no significant change in CT over the treatment period (+3.01%, P = 0.119) (Fig. 2 g).
Mean percentage changes (± SEM) in hip geometry over time for a cross-sectional area (CSA), b cross-sectional moment of inertia (CSMI), C minimal neck width (minimal NW), d femur strength index (FSI), e buckling ratio (BR), f section modulus (SM), and g cortical thickness (CT) of neck after adjusting for age and body mass index. *P < 0.05, from the baseline; † P < 0.05, between time difference after Bonferroni correction
Changes in bone parameters between patients with and without previous chemotherapy
We compared the longitudinal changes in BMD, TBS, and hip geometry between patients with and without previous chemotherapy using linear-mixed models. Two fixed effects were included: one within-subject time effect and one between-subjects group effect. Possible differences in the group during the treatment period were analyzed according to time × group interactions after adjusting for age, BMI, duration of menopause, and baseline bone parameter level. There were no significant differences in the time-group interaction for BMD at the lumbar spine, femur neck, or total hip, or for the TBS and hip geometry parameters (Supplemental Table 1).
Discussion
To our best knowledge, this is the first study to simultaneously evaluate the long-term (5-year) changes in BMD, lumbar spine TBS, and hip geometry in postmenopausal women with breast cancer who were treated with AIs. Our findings revealed significant losses in BMD at the lumbar spine, femur neck, and total hip over the 5-year period. In healthy postmenopausal women, marked decreases in their estrogen levels result in a dramatic loss in their lumbar spine BMD during the immediate postmenopausal period [25]. However, the rate of BMD loss at the lumbar spine slows during the postmenopausal period, reflecting the greater effect of estrogen deficiency on trabecular bone around the time of the menopause [26]. Meanwhile, the rate of cortical bone loss at the femur neck and total hip BMD remains stable during the menopausal period [27]. In the present study, we observed that AI therapy was associated with greater bone loss at the lumbar spine during the first 3 years of treatment, although the rate of bone loss slowed after this period. On the other hand, significant annual bone loss at the femur neck and total hip continued for up to 4 years, which was longer than that for the lumbar spine. Interestingly, prior studies have reported a similar trend in the changes for lumbar spine or total hip BMD over 5 years of AI treatment [28, 29]. This relationship may be explained by the different responses of the trabecular and cortical bones to low estrogen status, as trabecular bone loss occurs more rapidly than cortical bone loss does immediately after estrogen deprivation, although cortical bone loss continues throughout the aging process [30]. Previous observational studies reported that the annual rates of change in the lumbar spine and total hip BMD were 1.8–2.3 and 1.0–1.4% in early postmenopausal women (<10 years since menopause) who did not receive AI therapy, which thereafter decreased to 0.5–1.0% [30, 31]. Given the rates of bone loss in the lumbar spine (−3.2%) and hip BMD (−2.8%) after 1 year treatment with AI in the present study, AI seemed to exert an effect on bone loss that was approximately 2–3 times greater than that caused by aging itself.
We also observed that lumbar spine TBS significantly decreased during the treatment period and that this decrease was independent of the lumbar spine BMD. Indeed, the AI-induced decrease in TBS was smaller than that in lumbar spine BMD (−2.12 vs. −6.15%) after 5 years, which is in agreement with previous findings regarding short-term AI treatment [19, 20]. Those studies reported decreases in TBS of 2.3% after 2 years of exemestane treatment and of 2.1% after 2 years of non-steroidal AI or exemestane treatment, while lumbar spine BMD decreased by 5.3 and 5.9%, respectively. Furthermore, one of those studies reported that the annual rate of decrease in TBS was greatest at the first year (−1.7%) and subsequently slowed after the first year [19]. This finding is in line with our results, which indicated that the lumbar spine TBS significantly decreased after the first year of AI treatment, and then reached a sustained plateau. This course might be connected to the finite modifications that are possible in the trabecular bone microarchitecture [32]. For example, the marked AI-induced estrogen deprivation might preclude the generation of new trabeculae during the AI treatment, which would be reflected by a dramatic decrease in TBS during the early treatment period. On the other hand, trabecular thickness might increase over time, although trabecular thickening only has a small effect on TBS parameters, and TBS may stabilize during the late treatment period [33].
Our results indicate that AI-induced estrogen deficiency also negatively influenced bone geometry. Among the AHA-derived hip geometry parameters, femur geometry (CSA and minimal NW) and bone strength parameters (CSMI, FSI, and SM) significantly decreased, while BR, which reflects the ability of the bone to resist bending forces at the tensile surface, significantly increased over 5 years. These results seem to be attributed to the accelerated endocortical resorption and decelerated periosteal apposition that is induced by severe estrogen deficiency during the early treatment period. As in healthy postmenopausal women, the acceleration of endocortical bone resorption and insufficient compensation via periosteal apposition could result in cortical thinning and a reduction in bending strength in patients with breast cancer [34]. Although we observed a dramatic first-year change in BR, which was calculated using the CT value, we did not observe a corresponding change in CT. Furthermore, it is unclear why BR rapidly increased during the first year, but that this rate of change was not maintained to the fifth year. Similar to our findings, two previous studies have reported decreases in bone strength parameters (e.g., CSA, CSMI, and SM) after 2 years of treatment using AIs, although their data regarding BR and CT at the femur neck were inconsistent with ours [22, 23]. These findings appear to indicate that adjuvant AI therapy has both short-term and long-term effects on cortical deterioration during the treatment period.
The present study has several important strengths. First, we performed a large retrospective cohort study of patients with curatively resected breast cancer from a single tertiary referral center, and we only included patients without osteoporosis who were not receiving any medical intervention for osteoporosis before or during the AI treatment. This design reduces the heterogeneity of the study sample, which may increase the reliability of our findings. Second, we simultaneously assessed overall and percent changes in BMD, TBS, and hip geometry over a 5-year period of treatment using AIs. As expected, we observed decreases in the lumbar spine, femur neck, and total hip BMD. More interestingly, we also observed that the alterations in bone microarchitecture presented as TBS and hip geometry were independent of the site-specific BMD changes during the treatment period.
This study also includes several limitations that warrant consideration. First, we only included patients with breast cancer who were treated by AIs, which precludes any comparisons to patients with breast cancer who did not receive AIs or to healthy postmenopausal women without breast cancer. Second, we excluded patients who started bisphosphonate therapy during the AI treatment period, which might have resulted in an underestimation of the magnitude of changes in BMD, lumbar spine TBS, and hip geometry, as we might have excluded some patients who experienced dramatic decreases in BMD. There have been several prospective studies that showed protective effects of bisphosphonate for long-term use of AI-induced bone loss [29, 35]. Because of issues related to health insurance coverage, bisphosphonate therapy is not widely used in clinical practice among non-osteoporotic patients with breast cancer receiving AIs in our country. Hence, we could not directly evaluate effects of bisphosphonate on BMD as well as TBS and hip geometry in the present study. Third, we did not evaluate bone turnover markers (i.e., bone-specific alkaline phosphatase and c-terminal telopeptide), 25-hydroxy vitamin D, or the daily intake of calcium and vitamin D. Given the high prevalence of vitamin D deficiency in patients with breast cancer, especially in receiving AI therapy [36], vitamin D deficiency may affect bone in those patients. Furthermore, fracture events and several clinical risk factors for osteoporosis and fracture (e.g., a history of fragility fractures, family history of hip fractures, rheumatoid arthritis, smoking, or alcohol drinking) were not assessed in the study due to its retrospective design. Additionally, we did not include cortical or trabecular BMD in our analyses, due to the absence of the related quantitative computed tomography data. Thus, further well-controlled prospective studies are needed to validate our findings.
The current guideline regarding AI-induced bone loss recommends bisphosphonate in patients with a T-score <−2.0 or with any two of the clinical risk factors [14]. The present study demonstrated that even non-osteoporotic patients experienced BMD loss at all sites. Particularly, TBS and hip geometry also decreased independently of BMD. In patients with breast cancer receiving AI therapy, TBS or hip geometry is not recommended for assessing fracture risk. However, in postmenopausal women, TBS was found to be associated with fragility fractures independently of BMD or the FRAX tool [37]. There have been insufficient data supporting the usefulness of hip geometry parameters (e.g., CSA, CSMI, SM, and BR) except the hip axis length to predict fracture risk in postmenopausal women [21]. Although we did not prove the additional role of TBS or hip geometry for evaluating fracture risk, the change in TBS and hip geometry independent of BMD in non-osteoporotic patients might capture the different bone properties and improve fracture risk assessment.
In conclusion, we performed a concomitant skeletal assessment of BMD, TBS, and hip geometry in non-osteoporotic postmenopausal women with breast cancer over a 5-year period of AI treatment. Our results indicate that adjuvant AI therapy negatively affected BMD, bone microarchitecture (lumbar spine TBS), and macroarchitecture (hip geometry) during the treatment period. These findings may improve our understanding of the deterioration in bone quality during long-term AI treatment, and may also explain the increased risk of fracture independently of BMD, even in non-osteoporotic postmenopausal women with breast cancer. Given the magnitude of the AI-associated skeletal deterioration that we observed, early intervention or preventive strategies may facilitate bone protection in patients who are initiating or receiving AI treatment.
References
Bauer M, Bryce J, Hadji P (2012) Aromatase inhibitor-associated bone loss and its management with bisphosphonates in patients with breast cancer. Breast cancer (Dove Medical Press) 4:91–101
Hadji P (2010) Guidelines for Osteoprotection in breast cancer patients on an aromatase inhibitor. Breast care (Basel) 5:290–296
Becker T, Lipscombe L, Narod S, Simmons C, Anderson GM, Rochon PA (2012) Systematic review of bone health in older women treated with aromatase inhibitors for early-stage breast cancer. J Am Geriatr Soc 60:1761–1767
Senkus E, Kyriakides S, Penault-Llorca F, Poortmans P, Thompson A, Zackrisson S, Cardoso F (2013) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi7–v23
Hadji P (2009) Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol 69:73–82
Servitja S, Nogues X, Prieto-Alhambra D, Martinez-Garcia M, Garrigos L, Pena MJ, de Ramon M, Diez-Perez A, Albanell J, Tusquets I (2012) Bone health in a prospective cohort of postmenopausal women receiving aromatase inhibitors for early breast cancer. Breast 21:95–101
Melton LJ 3rd, Hartmann LC, Achenbach SJ, Atkinson EJ, Therneau TM, Khosla S (2012) Fracture risk in women with breast cancer: a population-based study. J Bone Miner Res 27:1196–1205
Goss PE, Hershman DL, Cheung AM, Ingle JN, Khosla S, Stearns V, Chalchal H, Rowland K, Muss HB, Linden HM, Scher J, Pritchard KI, Elliott CR, Badovinac-Crnjevic T, St Louis J, Chapman JA, Shepherd LE (2014) Effects of adjuvant exemestane versus anastrozole on bone mineral density for women with early breast cancer (MA.27B): a companion analysis of a randomised controlled trial. Lancet Oncol 15:474–482
Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE (2006) Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the anastrozole, tamoxifen, alone or in combination (ATAC) trial (18233230). J Bone Miner Res 21:1215–1223
Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, Barlow D, Makris A, Eastell R (2010) Prevention of aromatase inhibitor-induced bone loss using risedronate: the SABRE trial. J Clin Oncol 28:967–975
Zaman K, Thurlimann B, Huober J, Schonenberger A, Pagani O, Luthi J, Simcock M, Giobbie-Hurder A, Berthod G, Genton C, Brauchli P, Aebi S (2012) Bone mineral density in breast cancer patients treated with adjuvant letrozole, tamoxifen, or sequences of letrozole and tamoxifen in the BIG 1-98 study (SAKK 21/07). Ann Oncol 23:1474–1481
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (Arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60–62
Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE, Gnant M, Guise T, Lipton A (2011) Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 22:2546–2555
Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J (2014) Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol 25(Suppl 3):iii124–iii137
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N, McCloskey EV, Kanis JA, Bilezikian JP (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24:77–85
Iki M, Tamaki J, Kadowaki E, Sato Y, Dongmei N, Winzenrieth R, Kagamimori S, Kagawa Y, Yoneshima H (2014) Trabecular bone score (TBS) predicts vertebral fractures in Japanese women over 10 years independently of bone density and prevalent vertebral deformity: the Japanese population-based osteoporosis (JPOS) cohort study. J Bone Miner Res 29:399–407
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 2: trabecular bone score. J Clin Densitom 18:309–330
Kalder M, Hans D, Kyvernitakis I, Lamy O, Bauer M, Hadji P (2014) Effects of exemestane and tamoxifen treatment on bone texture analysis assessed by TBS in comparison with bone mineral density assessed by DXA in women with breast cancer. J Clin Densitom 17:66–71
Pedrazzoni M, Casola A, Verzicco I, Abbate B, Vescovini R, Sansoni P (2014) Longitudinal changes of trabecular bone score after estrogen deprivation: effect of menopause and aromatase inhibition. J Endocrinol Investig 37:871–874
Broy SB, Cauley JA, Lewiecki ME, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 1: hip geometry. J Clin Densitom 18:287–308
Lee SJ, Kim KM, Brown JK, Brett A, Roh YH, Kang DR, Park BW, Rhee Y (2015) Negative impact of aromatase inhibitors on proximal femoral bone mass and geometry in postmenopausal women with breast cancer. Calcif Tissue Int 97:551–559
van Londen GJ, Perera S, Vujevich KT, Sereika SM, Bhattacharya R, Greenspan SL (2010) The effect of risedronate on hip structural geometry in chemotherapy-induced postmenopausal women with or without use of aromatase inhibitors: a 2-year trial. Bone 46:655–659
Riggs BL, Khosla S, Melton LJ 3rd (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302
Khosla S, Melton LJ 3rd, Riggs BL (2011) The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J Bone Miner Res 26:441–451
Seeman E (2001) Clinical review 137: sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab 86:4576–4584
Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G (2008) Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 26:1051–1057
Brufsky AM, Harker WG, Beck JT, Bosserman L, Vogel C, Seidler C, Jin L, Warsi G, Argonza-Aviles E, Hohneker J, Ericson SG, Perez EA (2012) Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer 118:1192–1201
Manolagas SC, O’Brien CA, Almeida M (2013) The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9:699–712
Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM (2008) Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 93:861–868
Warming L, Hassager C, Christiansen C (2002) Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int 13:105–112
Di Gregorio S, Del Rio L, Rodriguez-Tolra J, Bonel E, Garcia M, Winzenrieth R (2015) Comparison between different bone treatments on areal bone mineral density (aBMD) and bone microarchitectural texture as assessed by the trabecular bone score (TBS). Bone 75:138–143
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14:302–312
Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD (2006) Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21:1856–1863
Coleman R, de Boer R, Eidtmann H, Llombart A, Davidson N, Neven P, von Minckwitz G, Sleeboom HP, Forbes J, Barrios C, Frassoldati A, Campbell I, Paija O, Martin N, Modi A, Bundred N (2013) Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol 24:398–405
Rose AA, Elser C, Ennis M, Goodwin PJ (2013) Blood levels of vitamin D and early stage breast cancer prognosis: a systematic review and meta-analysis. Breast Cancer Res Treat 141:331–339
McCloskey EV, Oden A, Harvey NC, Leslie WD, Hans D, Johansson H, Barkmann R, Boutroy S, Brown J, Chapurlat R, Elders PJ, Fujita Y, Glüer CC, Goltzman D, Iki M, Karlsson M, Kindmark A, Kotowicz M, Kurumatani N, Kwok T, Lamy O, Leung J, Lippuner K, Ljunggren Ö, Lorentzon M, Mellström D, Merlijn T, Oei L, Ohlsson C, Pasco JA, Rivadeneira F, Rosengren B, Sornay-Rendu E, Szulc P, Tamaki J, Kanis JA (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31:940–948
Acknowledgments
We appreciate the Medical Research Collaborating Center (MRCC) of Seoul National University Hospital for statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted in accordance with the Declaration of Helsinki and complies with the current laws of the countries in which it was performed. An independent ethics committee or institutional review board for each study site approved the study protocol. An informed consent was waived due to a retrospective study.
Conflicts of interest
None.
Funding
This study was supported by a grant from Seoul National University Hospital (No. 0420140760 (2014–1293)).
The study was approved by the institutional review board of Seoul National University Hospital (No. H-1601-057-734) and was conducted in accordance with the Declaration of Helsinki. Informed consent from the study participants was waived due to the study’s retrospective nature.
Electronic supplementary material
Supplemental Figure 1
Mean percentage changes (± SEM) in bone mineral density (BMD) over time for (A) lumbar spine, (B) femur neck, and (C) total hip and (D) lumbar spine trabecular bone score (TBS) after adjusting for age and body mass index. * P < 0.05, from the baseline; † P < 0.05, between time difference after Bonferroni correction. (GIF 95 kb)
Supplemental Table 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Hong, A., Kim, J.H., Lee, K.H. et al. Long-term effect of aromatase inhibitors on bone microarchitecture and macroarchitecture in non-osteoporotic postmenopausal women with breast cancer. Osteoporos Int 28, 1413–1422 (2017). https://doi.org/10.1007/s00198-016-3899-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3899-6