Abstract
Summary
The association between serum uric acid (SUA) levels and bone mineral density (BMD) is controversial. Fat accumulation is linked to SUA and BMD, thus possibly explaining the mixed results. We found that adiposity drives part of the association between SUA and BMD in women with postmenopausal osteoporosis.
Introduction
Both positive and negative associations between SUA and BMD have been reported. SUA levels and BMD increase with higher body weight and other indices of adiposity; hence, the association between SUA and BMD might be a consequence of the confounding effect of adiposity. We investigated in this cross-sectional study whether the association between SUA and BMD is independent of measures of fat accumulation and other potential confounders.
Methods
SUA levels, femur BMD, markers of bone metabolism, body mass index (BMI), fat mass (FM), waist circumference (WC), and abdominal visceral fat area were measured in 180 treatment-naive postmenopausal osteoporotic women (mean age 66.3 ± 8.5 years, age range 48–81 years).
Results
Women with higher SUA levels (third tertile) had significantly higher femur BMD and lower cross-linked C-terminal telopeptide of type I collagen (CTX) and bone alkaline phosphatase (bALP) levels. SUA levels were positively associated with all indices of adiposity. In multivariable analysis with femur BMD as dependent variable, the association between logarithmic (LG)-transformed SUA levels and BMD (beta = 0.42, p < 0.001) was lessened progressively by the different indices of adiposity, like LG-BMI (beta = 0.22, p = 0.007), LG-WC (beta = 0.21, p = 0.01), LG-FM (beta = 0.18, p = 0.01), and LG-abdominal visceral fat area (beta = 0.12, p = 0.05). The association between SUA levels and markers of bone metabolism was dependent on the effect of confounders.
Conclusion
In postmenopausal osteoporotic women, the strong univariable association between SUA levels and femur BMD is partly explained by the confounding effect of indices of adiposity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elevated serum uric acid (SUA) levels are associated with gout and renal disease [1]. SUA exists either in a crystalline state or in a soluble state, with antioxidant or pro-oxidant properties depending on plasmatic and cellular environment [2].
Reduced bone mineral density has been linked to a pro-oxidative [3] and pro-inflammatory [4] environment, and oxidative stress has been recognized as an important cofactor for estrogen deficiency-induced bone loss [5]. Recently, it has been speculated that SUA might protect from osteoporosis due to its antioxidant properties [6, 7]. Cross-sectional and longitudinal studies have shown that serum SUA levels are positively associated with bone mineral density (BMD) both in men and women [6, 8–18] irrespective of multiple confounders. Specifically, multivariable adjustment of the association between SUA and BMD for indices of adiposity like body weight [9, 10], body mass index (BMI) [6, 8, 9, 12], and fat mass (FM) [11] has been performed, with SUA levels positively associated with BMD irrespective of adiposity. However, Zhang et al. [19] did not find any association between SUA and BMD in a large cohort of men and women. Conversely, Sritara et al. [20] found a positive association between SUA levels and BMD in young and middle-aged men and an inverse association in 485 pre- and post-menopausal women. Also, Bhupathiraju et al. [21] found that SUA negatively contributed to trabecular bone mineral content in healthy postmenopausal women. Finally, Ishii et al. [12] found the robustness of the positive association between SUA and BMD was stronger when women older than 60 years were excluded, whereas Muka et al. [16] observed that the association between SUA and BMD was more prominent in older individuals. Hence, some confounding effect of aging and of additional covariates on the variability of the association between SUA and BMD in postmenopausal women is conceivable.
BMI calculation, waist circumference (WC), and assessment of FM using bioelectrical impedance are used in clinical practice as indices of either general or regional adiposity. Also, abdominal visceral fat area measured through ultrasound has been suggested as a reliable index of regional adiposity. There is overwhelming evidence supporting a strong correlation between indices of fat accumulation/distribution, on the one hand, and SUA and BMD, on the other hand [22–28]. Specifically, a positive association between either body weight, BMI or FM and BMD [22, 23] has been shown, suggesting a protective effect of fat accumulation against bone loss. However, there is also evidence of a possible detrimental impact of abdominal visceral fat on BMD [24, 25]. Hence, the influence of body fat on bone health is quite variable. Moreover, a positive association between different indices of both total and regional adiposity and SUA levels has been shown [26, 27], with visceral fat accumulation being extensively linked to elevated SUA levels [28, 29].
Overall, the effect of the balance between general and visceral adiposity on BMD is dual, whereas the positive association between fat accumulation and plasma SUA levels appears to be consistent. Therefore, the impact of indices of adiposity on the association between SUA and BMD is unpredictable and a comprehensive evaluation of the association between SUA levels and BMD after correction for multiple indices of adiposity is warranted. Moreover, inasmuch as the link between markers of bone metabolism [i.e., bone alkaline phosphatase (bALP), cross-linked C-terminal telopeptide of type I collagen (CTX), 25(OH)-vitamin D, and parathyroid hormone (PTH)] and BMD is consistent [30] and the association between some markers of bone metabolism and SUA levels has been described [31, 32], a further exploration of the association between SUA levels and markers of bone metabolism after correction for indices of adiposity would be of interest.
The aim of this study was to evaluate the relationship between SUA, femur BMD, and markers of bone metabolism (i.e., bALP, CTX) in treatment-naive postmenopausal osteoporotic women, after correction for multiple indices of adiposity.
Methods
Study design and participants
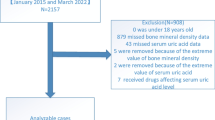
This is a cross-sectional study of 180 consecutive postmenopausal female outpatients with newly diagnosed, never-treated osteoporosis, selected among women independent in daily living activities attending our Unit of Bone and Mineral Metabolism (Perugia, Italy) for screening of postmenopausal osteoporosis (Fig. 1). Women were considered postmenopausal if they had not been menstruating for at least 1 year. Exclusion criteria included history of chronic diseases, such as renal, hepatic, cardiac, thyroid and rheumatic diseases, current or prior use of drugs that could interfere with either bone mass or uric acid levels (i.e., glucocorticoids, antiresorptive drugs, hormonal replacement therapy, calcium or vitamin D supplementation, urate-lowering therapy), history of traumatic fractures, and the absence of self-reported or radiologically documented fragility fracture in the 6 months prior to study recruitment. Also, patients under body-weight-lowering drugs or caloric restriction were not included in this study. A trained interviewer conducted a questionnaire with each participant asking questions regarding their age, age of menopause and menarche, smoking habits, family or personal fractures, medical history, comorbid diseases, and medication use. Information was also obtained by a review of medical records and laboratory data.
Clinical evaluation, bone mineral density, and visceral fat area
All the determinations were made at the medical center at 8.00 h, with a room temperature between 21 and 23 °C, after a 13-h overnight fast. Height and weight were measured to the nearest 0.1 cm and 0.1 kg, respectively, subjects were wearing hospital gowns and had bare feet. BMI was calculated as weight (kg) divided by height (m) squared. WC was also measured. Bioimpedentiometry (50 kHz, amplitude 50 mA, Tanita, Japan), using electrodes applied to the foot plantar surface, was used for the measurement of fat mass in kilograms. Abdominal ultrasound imaging was assessed for measurement of abdominal visceral fat area by the same operator, who was unaware of the results of the laboratory measurements of each patient. Imaging was performed using the MyLab 50 (Esaote, Italy) ultrasound system. Abdominal visceral fat area was measured as previously described by Hirooka et al. [33] as follows: [VFA] = −9.008 + 1.191 × [distance between the internal surface of the abdominal muscle and the splenic vein (mm)] + 0.978 [distance between the internal surface of the abdominal muscle and the posterior wall of the aorta at the umbilicus (mm)] + 3.644 × [thickness of the fat layer of the posterior right renal wall (mm)]. Ultrasonography was always performed by the same experienced operator, and the intra-operator inter-day CV was 1.8 %.
Areal BMD (g/cm2; bone mineral content relative to projection area) was measured by dual-energy X-ray absorptiometry (DXA) (Hologic Discovery W, Hologic Inc., Bedford, MA, USA) at the proximal femur. The coefficient of variation at our laboratory was 0.51 %.
Biochemical assays
SUA was measured by a colorimetric assay (ADVIA, Siemens Healthcare Diagnostics srl, Erlangen, Germany). Serum creatinine concentration was measured by a kinetic assay (Instrumentation Laboratory spa, Richmond Hill, Canada), and glomerular filtration rate (GFR) was calculated according to the Modification of Diet in Renal Disease equation [34]. A radioimmunoassay was used to measure serum 25(OH)-vitamin D (DiaSorin Inc., MN, USA). Serum intact PTH levels were measured by an immunoenzymatic method (Access, Beckman Coulter Inc., CA, USA). Serum CTX was measured by an enzyme-linked immunosorbent assay (Pantec srl, Torino, Italy). An immunoradiometric assay was used to measure bALP (Tandem R Ostase, Pantec srl, Torino, Italy).
Statistical analysis
SPSS statistical package, release 17.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses. Values are expressed as either mean ± SD or median and interquartile range (IQR). The Shapiro-Wilk test was used to check the distribution of continuous variables. Logarithmic (LG) transformation was performed for nonnormal continuous variables. Independent-samples t test and Mann-Whitney U test were used for between-group comparisons. One-way analysis of variance (ANOVA) and Kruskal-Wallis H tests were used for comparisons between tertiles of UA levels. Correlation analyses were performed using the Pearson’s and Spearman’s coefficients of correlations (r and rho, respectively). Linear regression analysis was used to estimate the association between SUA levels and BMD, by including BMD as the dependent variable. The independent variables were those significantly associated with either SUA or femur BMD in the bivariate correlation analyses (age, GFR, smoking status, LG-SUA, LG-transformed indices of adiposity). Hence, the independent variables included in the basal model 1 included LG-SUA, age, GFR, and smoking status; BMI, WC, fat mass, and abdominal visceral fat area were added singularly to the independent variables of model 1 in order to build additional models (from model 2 to model 5). The effect of the interaction between age and SUA levels was tested in the linear regression analysis, after calculating the interaction term “age*SUA” from the centered mean values of the variables age and SUA. If the interaction term were significant at a p value ≤0.05, then analyses will be stratified by age groups. Additional multivariable analyses were performed with either LG-CTX or LG-bALP as dependent variables and the following independent variables: age, smoking status, GFR, LG-SUA, and one LG-transformed index of adiposity at a time. Standardized coefficients were calculated as a measure for the relative predictive value. A two-sided p < 0.05 was considered statistically significant.
Results
The characteristics of 180 postmenopausal osteoporotic women, grouped according to tertiles of SUA levels, are summarized in Table 1. The Shapiro-Wilk test revealed a nonnormal distribution of SUA levels, markers of bone metabolism, and indices of adiposity, thus medians and IQR or LG-transformed data have been tested and reported for these variables. The age of participants ranged from 48 to 81 years; 25 % of them were smokers; and 46, 32, and 22 % were either normal weight (BMI 18.5–24.9), overweight (BMI 25–29.9), or obese (BMI ≥30), respectively. SUA levels ranged from 2.2 to 8.0 mg/dL, with only 3 % of participants having hyperuricemia (SUA levels above 6.8 mg/dL). Comparison of biochemical and anthropometric indices among different subgroups indicated that women with higher SUA levels were older and had significantly higher indices of adiposity and femur BMD, but lower renal function, CTX, and bALP levels. Table 2 shows the correlations between indices of adiposity; a strong positive correlation between all these indices was found.
Correlates of SUA and BMD
SUA levels were significantly associated with age (rho = 0.26, p < 0.001), BMI (rho = 0.45, p < 0.001), WC (rho = 0.42, p < 0.001), FM (rho = 0.44, p < 0.001), abdominal visceral fat area (rho = 0.49, p < 0.001), GFR (rho = −0.45, p < 0.001), CTX (rho = −0.18, p = 0.013, bALP (rho = −0.17, p = 0.02), and BMD (rho = 0.37, p < 0.001).
Femur BMD was significantly associated with age (r = −0.15, p = 0.04), BMI (rho = 0.53, p < 0.001), WC (rho = 0.41, p < 0.001), FM (rho = 0.61, p < 0.001), abdominal visceral fat area (rho = 0.51, p < 0.001), 25(OH)-vitamin D (rho = 0.20, p = 0.01), CTX (rho = −0.35, p < 0.001), and bALP (rho = −0.25, p = 0.002).
Figure 2 shows the direct correlation of SUA levels with femur BMD (Fig. 2a) and LG-abdominal visceral fat area (Fig. 2b).
Independent covariates of BMD
Table 3 shows beta coefficients of the multivariable association between LG-SUA levels and femur BMD after initial adjustment for age, smoking status, and GFR (model 1), and after further adjustment for LG-transformed indices of adiposity, included in the regression models (from model 2 to model 5) one at a time. The association between LG-SUA levels and femur BMD (beta = 0.42, p < 0.001) was significant after conservative adjustment (model 1: age, GFR, smoking), and it was progressively lessened by correction for indices of adiposity (i.e., LG-BMI, LG-WC, and LG-FM) (models 2 to 4) reaching marginal significance in model 5 that included LG-abdominal visceral fat area as independent variable (beta = 0.12, p = 0.05). All indices of adiposity were able to significantly predict femur BMD irrespective of LG-SUA levels (Table 3, models 1 to 5). The centered interaction term “age*SUA” was calculated to test its influence on the association between SUA levels and BMD; in all the multivariable regression models, the association between the interaction term and BMD was not significant (range of p values 0.27–0.79) to and did not alter the degree of the association between SUA levels and BMD.
Association between SUA, indices of adiposity, and markers of bone metabolism
In the univariable association analyses, CTX levels were negatively associated with BMI (rho = −0.28, p < 0.001), WC (rho = −0.21, p = 0.012), FM (rho = −0.25, p = 0.002), and abdominal visceral fat area (rho = −0.29, p < 0.001), whereas bALP was significantly associated with abdominal visceral fat area (rho = −0.17, p = 0.03) and marginally associated with BMI (rho = −0.15, p = 0.059) and FM (rho = −0.15, p = 0.057). No significant associations were found between 25(OH)-vitamin D and markers of bone metabolism.
In the multivariable regression analysis, LG-SUA levels were not found as a significant covariate of LG-CTX levels after correction for age, smoking status, and GFR (beta = −0.12, p = 0.17). The same nonsignificant results in multivariable regression were observed with either LG-bALP, LG-25(OH)-vitamin D, or LG-PTH as dependent variables and LG-SUA (beta = −0.16, p = 0.06; beta = 0.02, p = 0.80; beta = 0.07, p = 0.38, respectively), age, smoking status, and GFR as independent variables (Table 4).
The possible confounding effect of indices of adiposity on the association between SUA and marker of bone metabolism was tested. The multivariable regression analyses with markers of bone metabolism levels as dependent variables revealed that SUA was not associated with any marker of bone metabolism when it was included as an independent variable along with singular indices of adiposity.
Discussion
Our results indicate that SUA is a potentially important correlate of femur BMD among treatment-naive postmenopausal osteoporotic women, but that total body and regional fat deposition may have a statistically relevant impact on the degree of this association. Specifically, multivariable analysis revealed that indices of adiposity (i.e., BMI, FM, WC, and abdominal visceral fat area) attenuate the association between SUA and BMD, and abdominal visceral fat area appears as the strongest confounder of this association.
The relationship between SUA and bone health has been extensively explored using experimental, cross-sectional, and longitudinal designs, and through Mendelian randomization analysis [6–21, 35–39]; however, conflicting results have emerged from these studies.
Overall, experimental studies do not seem to support a protective role of UA on bone health, with few exceptions. Despite Ahn et al. [10] showed that UA decreases osteoclastogenesis in a dose-dependent manner and reduces the production of reactive oxygen species in osteoclast precursors, most studies failed to corroborate this line of evidence. In a rat model of inducible mild hyperuricemia, there was no evidence of improvement in BMD, bone volume density, and bone biomechanical properties compared to normouricemic control animals [19]. Dalbeth et al. [36], analyzing cellular mechanisms of bone erosion in gout, found that peripheral blood mononuclear cells from patients with severe erosive gout preferentially formed osteoclast-like cells in culture with receptor activator of nuclear factor kappa-B ligand (RANKL) and monocyte colony-stimulating factor. In the same study [36], monosodium urate monohydrate crystals inhibited osteoprotegerin gene and protein expression in bone marrow stromal ST2 cells, and conditioned medium from ST2 cells cultured with monosodium urate monohydrate crystals promoted osteoclast formation from preosteoclast RAW 264.7 cells. Also, hyperuricemia suppressed 1-α-hydroxylase leading to lower 1,25(OH)2-vitamin D levels in rats [37]. Finally, inhibition of xanthine oxidase by allopurinol promoted osteoblast differentiation, leading to increased bone formation in vitro [35].
Epidemiological evidence on the association between SUA and BMD has so far produced mixed results. A positive relationship between SUA and BMD has been reported in elderly subjects [6–8], in type 2 diabetes [9], premenopausal, perimenopausal, and postmenopausal women [10–12, 14, 15], young men with ankylosing spondylitis [13], primary osteoporosis [18], and in the general population [16, 17, 19, 20]. In the contrary, few studies report an inverse correlation. Thus, the association between SUA and BMD in the general population [19] and in females [20] was lost after adjustment for common confounders. Bhupathiraju et al. [21] found that SUA contributes negatively to trabecular bone mineral content in healthy postmenopausal women. Finally, Mendelian randomization analyses identified no evidence that increased SUA levels has a causal role in increasing BMD or influencing bone-related outcomes [38, 39].
In the present study, we found that part of the association between SUA and BMD is explained by indices of body fat deposition like BMI, FM, WC, and abdominal visceral fat area. The declining degree of the adjusted association between SUA and BMD until level of marginal significance can be explained from a statistical and pathophysiological perspective. A positive association between body fat and BMD has been extensively reported in the literature [22, 23] and in this study as well. In this regard, it has been hypothesized that body fat may influence bone health positively through increased skeletal loading [40]. Additionally, a positive association between fat accumulation and SUA levels has been reported here and in previous studies [26–29]. Therefore, we can conclude that the positive association between SUA and BMD could rely on the concordant impact of body fat deposition on each of the two variables. This conclusion is supported by Mendelian randomization studies that have demonstrated increased BMI to be associated with increased SUA, but not increased SUA to be associated with increased BMI [41–44] and for increased BMI to be associated with increased BMD [45]. This conclusion, although statistically logical, might hide a possible flaw when considering additional pathophysiological aspects related to the association between fat and bone. Indeed, despite our results and ample published evidence support the view that BMI and FM have a positive relationship with BMD, other studies have suggested that excess of body fat, primarily in the abdominal visceral depot, might not protect always against decreases in BMD [40].
Markers of bone metabolism may provide useful information on bone health and in the clinical investigation of osteoporosis. Hence, exploring the association between SUA and markers of bone metabolism and the possible influence of confounders of this association is of interest. We found that SUA levels were negatively associated with markers of bone metabolism like bALP and CTX, whereas SUA levels were not associated with 25(OH)-vitamin D levels. The association between markers of bone metabolism and SUA levels is already recognized [10, 46]; moreover, low SUA levels have been described in patients with higher 25(OH)-vitamin D levels [31, 47]. Additionally, the association between some markers of bone metabolism, namely 25(OH)-vitamin D [48] and CTX [49], and body fat has been observed, thus further complicating the intricate relationship between SUA, markers of bone metabolism, and indices of adiposity. In this regard, we explored the association between levels of SUA and markers of bone metabolism in the light of the putative confounding effect of additional variables (i.e., indices of adiposity). We found that SUA levels were not associated with levels of markers of bone metabolism (i.e., CTX, bALP, 25(OH)-vitamin D, and PTH) when statistical adjustment was performed in multivariable analysis for age, smoking status, GFR, and indices of adiposity. This result might suggest that SUA may not be involved directly in influencing bone turnover and that the observed univariable association between SUA and both CTX and bALP may reflect the presence of confounders. In agreement with this line of reasoning, there are the results of Mendelian randomization analyses concluding that there is no relationship between SUA levels and bone-related outcomes [38, 39].
Limitations of this study need to be acknowledged. First, the cross-sectional design allowed us to generate the hypothesis that the association between SUA and BMD is partly explained by indices of adiposity; although intriguing, this hypothesis should be validated in larger size longitudinal studies and, possibly, in clinical trials in order to draw definitive conclusions. Second, we used ultrasound abdominal visceral fat area assessment for evaluation of abdominal visceral fat accumulation; however, more reliable methods for visceral fat quantification are available, like computed tomography. Third, mechanistic explanations of the confounding effect of abdominal visceral fat area and other indices of adiposity on the association between SUA levels and BMD cannot be drawn from this exploratory association study. Among the indices of adipose tissue deposition, WC had the lowest impact on the association between SUA and BMD. Whether this result might depend on the limited reproducibility of WC measurement or even on the limited specificity of this variable for the measure of total body fat remains to be clarified. Also, the confounding effect of additional unmeasured variables (e.g., alcohol intake) on the association between SUA and BMD cannot be excluded. Similarly, we included only osteoporotic women and excluded patients with several conditions (e.g., chronic diseases, bone fractures, etc.) that might have had an impact on the association between SUA and BMD; thus, the influence of these conditions cannot be inferred from this study. Finally, a recent study by Dong et al. [50] found that elevated SUA was associated with a higher BMD and a greater muscle mass in a middle-aged and elderly Chinese population; in addition, SUA-BMD association was partly mediated by muscle mass. Unfortunately, we do not have data on muscle mass to confirm this result; however, we failed to find a significant confounding effect of free fat mass on SUA-BMD association (results not shown). Further research is needed to address all these issues in more detail.
In conclusion, our study revealed a strong univariable association between SUA levels and femur BMD; however, indices of adiposity, especially abdominal visceral fat deposition, are correlated with both SUA levels and femur BMD and appear to drive part of the association between SUA and BMD.
References
Edwards NL (2008) The role of hyperuricemia and gout in kidney and cardiovascular disease. Cleve Clin J Med 75(Suppl 5):S13–S16
Sautin YY, Johnson RJ (2008) Uric acid: the oxidant–antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 27(6):608–619
Basu S, Michaëlsson K, Olofsson H, Johansson S, Melhus H (2001) Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288(1):275–279
Hardy R, Cooper MS (2009) Bone loss in inflammatory disorders. J Endocrinol 201(3):309–320
Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ (2003) A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 112(6):915–923
Nabipour I, Sambrook PN, Blyth FM, Janu MR, Waite LM, Naganathan V, Handelsman DJ, Le Couteur DG, Cumming RG, Seibel MJ (2011) Serum uric acid is associated with bone health in older men: a cross-sectional population-based study. J Bone Miner Res 26(5):955–964
Kuyumcu ME, Yesil Y, Oztürk ZA, Cınar E, Kızılarslanoglu C, Halil M, Ulger Z, Yesil NK, Cankurtaran M, Arıoğul S (2012) The association between homocysteine (hcy) and serum natural antioxidants in elderly bone mineral densitometry (BMD. Arch Gerontol Geriatr 55(3):739–743
Lane NE, Parimi N, Lui LY, Wise BL, Yao W, Lay YA, Cawthon PM, Orwoll E, Osteoporotic Fractures in Men Study Group (2014) Association of serum uric acid and incident nonspine fractures in elderly men: the Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res 29(7):1701–1707
Zhao DD, Jiao PL, Yu JJ, Wang XJ, Zhao L, Xuan Y, Sun LH, Tao B, Wang WQ, Ning G, Liu JM, Zhao HY (2016) Higher serum uric acid is associated with higher bone mineral density in Chinese men with type 2 diabetes mellitus. Int J Endocrinol. doi:10.1155/2016/2528956
Ahn SH, Lee SH, Kim BJ, Lim KH, Bae SJ, Kim EH, Kim HK, Choe JW, Koh JM, Kim GS (2013) Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos Int 24(12):2961–2970
Makovey J, Macara M, Chen JS, Hayward CS, March L, Seibel MJ, Sambrook PN (2013) Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: a longitudinal study. Bone 52(1):400–406
Ishii S, Miyao M, Mizuno Y, Tanaka-Ishikawa M, Akishita M, Ouchi Y (2014) Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos Int 25(3):1099–1105
Kang KY, Hong YS, Park SH, JH J (2015) Low levels of serum uric acid increase the risk of low bone mineral density in young male patients with ankylosing spondylitis. J Rheumatol 42(6):968–974
Kim S, Jung J, Jung JH, Kim SK, Kim RB, Hahm JR (2015) Risk factors of bone mass loss at the lumbar spine: a longitudinal study in healthy Korean pre- and perimenopausal women older than 40 years. PLoS One 10(8):e0136283. doi:10.1371/journal.pone.0136283
Lee YJ, Hong JY, Kim SC, Joo JK, Na YJ, Lee KS (2015) The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet Gynecol Sci 58(1):46–52
Muka T, de Jonge EA, de Jong JC, Uitterlinden AG, Hofman A, Dehghan A, Zillikens MC, Franco OH, Rivadeneira F (2016) The influence of serum uric acid on bone mineral density, hip geometry, and fracture risk: the Rotterdam Study. J Clin Endocrinol Metab 101(3):1113–1122
Lin X, Zhao C, Qin A, Hong D, Liu W, Huang K, Mo J, Yu H, Wu S, Fan S (2015) Association between serum uric acid and bone health in general population: a large and multicentre study. Oncotarget 6(34):35395–35403
Chen L, Peng Y, Fang F, Chen J, Pan L, You L (2015) Correlation of serum uric acid with bone mineral density and fragility fracture in patients with primary osteoporosis: a single-center retrospective study of 253 cases. Int J Clin Exp Med 8(4):6291–6294
Zhang D, Bobulescu IA, Maalouf NM, Adams-Huet B, Poindexter J, Park S, Wei F, Chen C, Moe OW, Sakhaee K (2015) Relationship between serum uric acid and bone mineral density in the general population and in rats with experimental hyperuricemia. J Bone Miner Res 30(6):992–999
Sritara C, Ongphiphadhanakul B, Chailurkit L, Yamwong S, Ratanachaiwong W, Sritara P (2013) Serum uric acid levels in relation to bone-related phenotypes in men and women. J Clin Densitom 16(3):336–340
Bhupathiraju SN, Alekel DL, Stewart JW, Hanson LN, Shedd KM, Reddy MB, Hanson KB, Van Loan MD, Genschel U, Koehler KJ (2007) Relationship of circulating total homocysteine and C-reactive protein to trabecular bone in postmenopausal women. J Clin Densitom 10(4):395–403
Morin S, Tsang JF, Leslie WD (2009) Weight and body mass index predict bone mineral density and fractures in women aged 40 to 59 years. Osteoporos Int 20(3):363–370
Dytfeld J, Ignaszak-Szczepaniak M, Gowin E, Michalak M, Horst-Sikorska W (2011) Influence of lean and fat mass on bone mineral density (BMD) in postmenopausal women with osteoporosis. Arch Gerontol Geriatr 53(2):e237–e242
Kim KC, Shin DH, Lee SY, Im JA, Lee DC (2010) Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J 51(6):857–863
Schorr M, Dichtel LE, Gerweck AV, Torriani M, Miller KK, Bredella MA (2016) Body composition predictors of skeletal integrity in obesity. Skelet Radiol 45(6):813–819
Hikita M, Ohno I, Mori Y, Ichida K, Yokose T, Hosoya T (2007) Relationship between hyperuricemia and body fat distribution. Intern Med 46(17):1353–1358
Wang H, Wang L, Xie R, Dai W, Gao C, Shen P, Huang X, Zhang F, Yang X, Ji G (2014) Association of serum uric acid with body mass index: a cross-sectional study from Jiangsu province, China. Iran J Public Health 43(11):1503–1509
Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Yamakita J, Higashino K (1997) Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism 46(10):1162–1165
Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, Matsuzawa Y (1998) Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 47(8):929–933
Henriksen K, Christiansen C, Karsdal MA (2015) Role of biochemical markers in the management of osteoporosis. Climacteric 18(Suppl 2):10–18
Peng H, Li H, Li C, Chao X, Zhang Q, Zhang Y (2013) Association between vitamin D insufficiency and elevated serum uric acid among middle-aged and elderly Chinese Han women. PLoS One 8(4):e61159. doi:10.1371/journal.pone.0061159
Hui JY, Choi JW, Mount DB, Zhu Y, Zhang Y, Choi HK (2012) The independent association between parathyroid hormone levels and hyperuricemia: a national population study. Arthritis Res Ther 14(2):R56
Hirooka M, Kumagi T, Kurose K, Nakanishi S, Michitaka K, Matsuura B, Horiike N, Onji M (2005) A technique for the measurement of visceral fat by ultrasonography: comparison of measurements by ultrasonography and computed tomography. Intern Med 44(8):794–799
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130(6):461–470
Orriss IR, Arnett TR, George J, Witham MD (2016) Allopurinol and oxypurinol promote osteoblast differentiation and increase bone formation. Exp Cell Res 342(2):166–174
Dalbeth N, Smith T, Nicolson B, Clark B, Callon K, Naot D, Haskard DO, McQueen FM, Reid IR, Cornish J (2008) Enhanced osteoclastogenesis in patients with tophaceous gout: urate crystals promote osteoclast development through interactions with stromal cells. Arthritis Rheum 58(6):1854–1865
Chen W, Roncal-Jimenez C, Lanaspa M, Gerard S, Chonchol M, Johnson RJ, Jalal D (2014) Uric acid suppresses 1 alpha hydroxylase in vitro and in vivo. Metabolism 63(1):150–160
Dalbeth N, Topless R, Flynn T, Cadzow M, Bolland MJ, Merriman TR (2015) Mendelian randomization analysis to examine for a causal effect of urate on bone mineral density. J Bone Miner Res 30(6):985–991
Xiong A, Yao Q, He J, Fu W, Yu J, Zhang Z (2016) No causal effect of serum urate on bone-related outcomes among a population of postmenopausal women and elderly men of Chinese Han ethnicity—a Mendelian randomization study. Osteoporos Int 27(3):1031–1039
Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW (2008) Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 23(1):17–29
Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber G, Vollenweider P, Bochud M (2012) Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One 7(6):e39321. doi:10.1371/journal.pone.0039321
Burgess S, Daniel RM, Butterworth AS, Thompson SG, EPIC-InterAct Consortium (2015) Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol 44(2):484–495
Oikonen M, Wendelin-Saarenhovi M, Lyytikäinen LP, Siitonen N, Loo BM, Jula A, Seppälä I, Saarikoski L, Lehtimäki T, Hutri-Kähönen N, Juonala M, Kähönen M, Huupponen R, Viikari JS, Raitakari OT (2012) Associations between serum uric acid and markers of subclinical atherosclerosis in young adults. The cardiovascular risk in Young Finns study. Atherosclerosis 223(2):497–503
Palmer TM, Nordestgaard BG, Benn M, Tybjærg-Hansen A, Davey Smith G, Lawlor DA, Timpson NJ (2013) Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ 347:f4262. doi:10.1136/bmj.f4262
Warodomwichit D, Sritara C, Thakkinstian A, Chailurkit LO, Yamwong S, Ratanachaiwong W, Ongphiphadhanakul B, Sritara P (2013) Causal inference of the effect of adiposity on bone mineral density in adults. Clin Endocrinol 78(5):694–699
Hernández JL, Nan D, Martínez J, Pariente E, Sierra I, González-Macías J, Olmos JM (2015) Serum uric acid is associated with quantitative ultrasound parameters in men: data from the Camargo cohort. Osteoporos Int 26(7):1989–1995
Hordon LD, Peacock M (1987) Vitamin D metabolism in women with femoral neck fracture. Bone Miner 2(5):413–426
Mutt SJ, Hyppönen E, Saarnio J, Järvelin MR, Herzig KH (2014) Vitamin D and adipose tissue-more than storage. Front Physiol 24(5):228
Thomas SD, Morris HA, Nordin BE (2015) Acute effect of a supplemented milk drink on bone metabolism in healthy postmenopausal women is influenced by the metabolic syndrome. Nutr J 14:99
Dong XW, Tian HY, He J, Wang C, Qiu R, Chen YM (2016) Elevated serum uric acid is associated with greater bone mineral density and skeletal muscle mass in middle-aged and older adults. PLoS One 11(5):e0154692. doi:10.1371/journal.pone.0154692
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the local Ethics Committee, and all participants gave their informed consent.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Pirro, M., Mannarino, M.R., Bianconi, V. et al. Uric acid and bone mineral density in postmenopausal osteoporotic women: the link lies within the fat. Osteoporos Int 28, 973–981 (2017). https://doi.org/10.1007/s00198-016-3792-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3792-3