Abstract
Summary
This study demonstrated the predictive values of radiological findings for delayed union after osteoporotic vertebral fractures (OVFs). High-signal changes on T2WI were useful findings.
Introduction
The purpose of the present study is to determine predictive radiological findings for delayed union by magnetic resonance imaging (MRI) and plain X-rays at two time points in the acute phase of OVFs.
Methods
This multicenter cohort study was performed from 2012 to 2015. A total of 218 consecutive patients with OVFs ≤2 weeks old were enrolled. MRIs and plain X-rays were performed at the time of enrollment and at 1- and 6-month follow-ups. Signal changes on T1-weighted imaging (T1WI) were classified as diffuse low-, confined low-, or no-signal change; those on T2WI were classified as high (similar to the intensity of cerebrospinal fluid), confined low-, diffuse low-, or no-signal change. The angular motion of the fractured vertebral body was measured with X-rays.
Results
A total of 153 patients completed the 6-month follow-up. A high-signal change on T2WI was most useful in predicting delayed union. Sensitivity, specificity, and positive predictive values were 53.3, 87.8, and 51.6 % at enrollment and 65.5, 84.8, and 51.4 % at the 1-month follow-up, respectively. The positive predictive value increased to 62.5 % with observation of high- or diffuse low-signal changes at both enrollment and the 1-month follow-up. The cutoff value of vertebral motion was 5 degrees. Sensitivity and specificity at enrollment were 52.4 and 74.1 %, respectively.
Conclusions
This study demonstrated the radiological factors predicting delayed union after an OVF. T2 high-signal changes showed the strongest association with delayed union. Consecutive MRIs were particularly useful as a differential tool to predict delayed union following OVFs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoporotic vertebral fractures (OVFs) in the elderly are a major public health concern. Worldwide, there are an estimated 1.4 million new clinical OVFs every year [1]. Only about 33 % of all vertebral deformities noted on radiographs come to medical attention, and less than 10 % require hospitalization [2, 3]. However, their potential long-term consequences include chronic pain, decrease in physical function and quality of life, and depression [4–7].
In the majority of OVFs, the associated pain gradually subsides as bony union and stability progress with conservative treatment. However, some patients present with intractable back pain for prolonged periods of time, while others suffer from neurological deficits that develop within a few months after the fracture. In such cases, insufficient union is noted on dynamic lateral plain radiography and/or on magnetic resonance imaging (MRI). The pain is typically aggravated by weight-bearing positions [8]. About 80 % of patients with delayed union have moderate or severe back pain, with a visual analog scale (VAS) score of more than 30 [9]. Occasionally, a vertebra with delayed union leads to delayed-onset collapse and the development of neurological symptoms [9, 10]. For delayed union, kyphoplasty, vertebroplasty, and spinal fusion surgery with instrumentation are occasionally performed [5, 11–13]. However, it can take several months to diagnose delayed union. For patients with acute OVFs, kyphoplasty and vertebroplasty are performed to reduce the acute pain and preserve vertebral height [5, 10–14]. Although early surgical intervention is effective, there are several potential problems, including the risk of subsequent OVFs, systemic cardiopulmonary side effects, and the costs of medical care [5, 13, 15]. Moreover, because bone cement has no osteoconductive or inductive properties, high polymerization temperatures can result in damage to surrounding tissues. Consequently, surgery should be limited to patients with risk factors for a poor prognosis.
Our previous report showed that diffuse low- and high-signal changes on T2 MRI predicted an increased risk of delayed union (odds ratio (OR) = 6.8 and 32.4, respectively) [16]. However, the timing of MRI examination was not unified to within 2 months of the injury after the onset of pain. In addition, we did not investigate the relationships between the change in signal intensity or the area of MRI findings at multiple time points and delayed union. Therefore, the purpose of this study was to investigate the predictive radiological findings for delayed union at a fixed time point and the predictive ability of the intensity change at two time points in the early stage after OVFs. Moreover, since no reports have provided data on the predictive ability of plain X-rays, which are usually taken in patients with OVF, the second purpose was to reveal the predictive values of radiographic findings for delayed union.
Methods
Patients
A total of 218 consecutive patients with symptomatic OVFs were eligible for and were willing to participate in this prospective multicenter cohort study, which was performed from January 2012 to September 2015. Eleven hospitals participated in the study. The inclusion criteria were age >65 years, diagnosis of a fresh OVF, and onset of back pain within 2 weeks prior to presentation. The exclusion criteria were pathological fractures, more than one fresh fracture, malignant disease, dementia, and high-energy injuries. Fractures were considered to be acute if the interval between the onset of symptoms and the first visit was <2 weeks and the MRI showed an abnormal signal change in the vertebral body.
The severity of pain was subjectively assessed by the patients using a VAS, which was based on the average level of back pain that the patient had felt in the previous week. The severity of pain was also reassessed using the VAS score at 1-, 3-, and 6-month follow-ups. To evaluate the patients’ activities of daily living (ADLs), we used the criteria proposed by the long-term care insurance system of the Japanese Health and Welfare Ministry for evaluation of the degree of independence of disabled elderly individuals [7]. The ADLs were ranked as follows: In rank J, although some disability is present, daily life is almost independent and patients can go outside without assistance from other individuals; in rank A, patients live independently indoors but require assistance to go out; in rank B, patients require some assistance living indoors and spend most of the day in bed but are able to sit up, and in rank C, patients spend all day in bed and require assistance with urination/defecation, getting dressed, and meals.
Imaging assessment
At the time of enrollment and at the 1- and 6-month follow-ups, the patients were examined using plain X-rays and MRIs of the spine. Sagittal images of the spine, including the fractured vertebrae, were taken with 1.5-T MRI scanners in nine hospitals and 0.5-T scanners in two hospitals. The following sequences were obtained with 1.5-T MRI scanners: T1-weighted turbo spin echo with a slice thickness of 3 mm (repetition time [TR] 400–700 ms and time to echo [TE] minimum accessible, depending on the capability of the machine), the same sequence with T2 (TR 3000–4500 ms and TE 80—120 ms), and STIR (a sequence with intrinsic fat saturation, TR 2000–4000 ms, TE 60–80 ms, and inversion time 120–170 ms). The following sequences were obtained with 0.5-T MRI scanners: T1-weighted turbo spin echo, with a slice thickness of 3 mm (TR 300–500 ms and TE minimum accessible, depending on the capability of the machine), the same sequence with T2 (TR 3000–4000 ms and TE 110—130 ms), and STIR (a sequence with intrinsic fat saturation, TR 2500–3500 ms, TE 20–40 ms, and inversion time 120–170 ms).
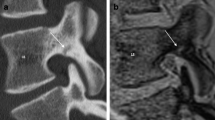
Two of the study authors (S.T. and M.H., spine surgeons with 10 and 18 years of experience in spinal MRI, respectively) assessed the plain X-ray and MRI findings using a simple classification system. The patterns of signal changes within the fractured vertebral bodies on MRI were classified based on midsagittal and bilateral parasagittal (medial aspect of pedicles) T1WI and T2WI images. The signal changes on T1WI were classified into three patterns: diffuse low-, confined low-, and no-signal change (Fig. 1). The signal changes on T2WI were classified into four patterns: high, confined low-, diffuse low-, and no-signal change. The intensity of the high-signal change on T2WI was defined as similar to that of cerebrospinal fluid. If at least two of three slices showed a low-signal change in >50 % of the vertebral body area, the finding was considered to be a diffuse low-signal change (Fig. 2). If only one of the three slices showed a low-signal change, the finding was considered to be a confined low-signal change. The two spine surgeons who analyzed the images were blinded to the outcome.
The plain X-rays were assessed based on the lateral views between the supine and the weight-bearing positions, because the supine position is useful to elicit the intravertebral cleft sign [17]. The percent height of the anterior wall was calculated by the following formula: [2 × affected vertebral height / (lower vertebral height + upper vertebral height)] × 100 (Fig. 3). If the adjacent vertebral body, either cranial or caudal, was deformed due to an old fracture, the vertical height of the anterior wall of the fractured vertebral body was divided by the vertical height of the anterior wall of the adjacent non-deformed vertebral body. The angular motion of the fractured vertebrae was determined by the difference in the segmental angle between the supine and weight-bearing positions. Delayed union was defined as a recognizable intravertebral cleft and apparent segmental motion on dynamic plain X-rays (≥5 degrees between supine and weight-bearing positions) at the 6-month follow-up, as assessed by two spine surgeons.
Data analysis
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of each MRI finding for delayed union were calculated. The ORs of each MRI finding for delayed union were adjusted for the demographic variables of age, sex, level of OVF, and presence of a posterior wall injury, which are reportedly risk factors for delayed union [16]. The receiver operating characteristic (ROC) curve was used to investigate the relationship of the height ratio or angular motion of the fractured vertebrae with delayed union. The cutoff value was determined by two methods to estimate Youden’s index and by the distance from the top left corner of the ROC curve. To establish whether there was a significant difference in the VAS of back pain between patients with and without bony union of the OVF, a restricted maximum likelihood, mixed-model regression was used. Either the chi-square test or Fisher’s exact test was used to compare categorical variables. To assess intraobserver and interobserver reliability of the above-described MRI classification on T1WI and T2WI, weighted kappa was calculated in 30 randomly chosen vertebral bodies. The statistical test results were considered significant at p < 0.05. All p values were two-sided. All analyses were performed using the SAS software package, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
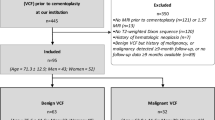
Of the 218 eligible patients, 3 died, 8 were excluded because of other diseases, and 54 were lost to follow-up. As a result, 153 patients (125 females and 28 males) completed the 6-month follow-up (a 70.2 % follow-up rate) and were effectively analyzed. Their age at the time of enrollment ranged from 65 to 93 years, with a mean age of 75.9 years. Twenty-nine OVFs were observed in the mid-thoracic spine (T7–T9), 99 in the thoracolumbar spine (T10–L2), and 25 in the lower lumbar spine (L3–L5). Overall, 47 % of the patients were hospitalized for several weeks during the acute phase. During treatment, 30 % wore tailor-made hard corsets, 62 % wore tailor-made elastic corsets, 4 % wore ready-made elastic corsets, and 4 % did not wear a corset. Each treatment was prescribed by individual physicians based on the patient’s condition. Severe back pain was reported at enrollment (average VAS score, 71.9 mm), and the pain intensity gradually improved thereafter until the 6-month follow-up (average VAS score, 21.9 mm) (Table 1). Delayed union was observed in 30 patients (19.6 %) at the 6-month follow-up.
Tables 2 and 3 show the predictive values and adjusted ORs for delayed union on MRI findings. On T1WI, the diffuse low-signal change showed a relatively high sensitivity (56.7 %), but low PPV (25.8 %) at enrollment. The OR showed no significant increase compared with that of confined low- or no-signal changes on T1WI. The findings showed very low specificity at the 1-month follow-up and no increase of the OR. In addition, all the fractured vertebrae with diffuse low-signal changes on T1WI showed high- or diffuse low-signal changes on T2WI.
On T2WI, the high-signal changes showed high specificity, PPV, and NPV at enrollment, with the sensitivity gradually increasing over time from 53.3 % at enrollment to 65.5 % at the 1-month follow-up. The ORs of T2 high-signal changes eminently increased when compared with those of confined low- and no-signal changes. At enrollment, diffuse low-signal changes on T2WI were not associated with the risk of an OVF, but there was an increased OR at the 1-month follow-up. To increase the sensitivity of T2 high-signal changes, the combination of T2 high with T2 diffuse low was established. Regarding the changes between MRIs performed at enrollment and at the 1-month follow-up, if T1-confined low changes were observed at both time points, all the fractured vertebrae were fused at the 6-month follow-up. Conversely, if T2 high or T2 diffuse low-signal changes were observed at both time points, the PPV was the highest (62.5 %).
Of the 153 patients, 107 underwent dynamic plain X-rays at enrollment and at the 1-month follow-up. The ROC curve demonstrated that the area under the curve (AUC) of the vertebral height ratio showed no significant results (0.601 and 0.616 at enrollment and at the 1-month follow-up, respectively). On the other hand, the AUC of vertebral angular motion at enrollment and at the 1-month follow-up was 0.635 and 0.785, respectively (Fig. 4). The cutoff value was 5 degrees at both enrollment and at the 1-month follow-up. The sensitivity and specificity were 52.4 and 74.1 % at enrollment and 75.0 and 74.0 % at the 1-month follow-up, respectively (Table 4). Although the PPV was 33.3 % at enrollment, it was 57.1 % at the 1-month follow-up. The adjusted OR significantly increased at both enrollment and at the 1-month follow-up (3.23 and 7.40, respectively).
The receiver operating characteristic (ROC) curve was used to investigate the relationship of vertebral motion (the difference in the vertebral angle between the supine and the weight-bearing positions) with delayed union. Two methods to estimate Youden’s index (Y) and the distance from the top left corner of the receiver operating characteristic curve (D) were used to determine the cutoff value. The areas under the curve were 0.717 and 0.757, respectively (p = 0.001 and 0.003, respectively)
With regard to their ADLs before the fracture, of the 30 patients with delayed union and 123 patients with union, 23 (76.7 %) and 112 (91.1 %) patients were capable of independent living, 6 (20.0 %) and 9 (7.3 %) patients required assistance leaving home, and 1 (3.3 %) and 2 (1.6 %) patients were almost bedridden, respectively (Table 5). In terms of their ADLs at the 6-month follow-up, 14 (46.7 %) and 94 (76.4 %) patients were capable of independent living, 12 (40.0 %) and 26 (21.1 %) patients required assistance leaving home, 3 (10.0 %) and 3 (2.4 %) patients were almost bedridden, and 1 (3.3 %) and no patients were completely bedridden, among patients with delayed union and union, respectively (p = 0.002). The frequency of reduced ADLs at the time of the 6-month follow-up was 36.7 and 14.6 % for the delayed union and union groups, respectively (p = 0.006). Figure 5 shows the change in VAS scores of back pain between the delayed union and union groups. The VAS score was higher in the delayed union group throughout the study period (p < 0.001). With respect to the reliability of our MRI classification system, the weighted kappa showed excellent interrater and intrarater agreement on both T1W1 (0.844 and 0.907, respectively) and T2W1 (0.712 and 0.731, respectively).
Discussion
This study demonstrated the predictive values of radiological findings for delayed union according to the age of the OVF. The presence of an intravertebral gas cleft in an OVF, which is also known as Kümmell disease, is considered indicative of delayed union and is a rare condition [18]. However, when dynamic X-rays, CT, or MRI is used, a higher frequency (12–48 %) of delayed union or intravertebral clefts has been reported, depending on the study population and the method of diagnosis [16, 17, 19, 20]. There is no universal criterion for the diagnosis of delayed union in OVF. The current study employed the definition of a recognizable cleft with apparent segmental motion between the supine and weight-bearing positions. Dynamic X-rays are particularly important in the diagnosis of intravertebral clefts [8, 21]. Also, segmental angular motion is important in terms of the association between back pain and neurological deficits [9].
The current study demonstrated that T2 high-signal changes were more accurate compared with T1 diffuse low and T2 diffuse low-signal changes and that the accuracy increased at the 1-month follow-up. We were able to detect 53 % of cases of delayed union at enrollment by T2 high-signal changes, with a PPV of 52 %. At the 1-month follow up, 66 % of cases of delayed union were detected, with a PPV of 51 %. To improve the low sensitivity of T2 high-signal changes, combination of T2 high-signal changes with T2 diffuse low-signal changes proved to be useful. Regarding the changes at two time points, T1-confined low changes both at enrollment and at the 1-month follow-up showed good results. T2 high or T2 diffuse low-signal changes were observed in 41 patients at enrollment. Of them, nine patients showed iso or confined signal changes at the 1-month follow-up, and most of them had healed by the 6-month follow-up. On the other hand, the fractured vertebral bodies in which T2 high or T2 diffuse low-signal changes remained at the 1-month follow-up showed the highest sensitivity, PPV, and accuracy for the prediction of OVFs. These findings suggest that sequential MRIs might facilitate prediction of the likelihood of union of OFVs; however, repeat MRIs involve a significant cost.
Previous reports have demonstrated the association between MRI findings and delayed union [16, 20, 22]. Cho et al. showed that gadolinium-DTPA enhancement was useful in predicting bony union [22] and that fractured vertebrae with a smooth pattern were likely to be indicative of union. Tsujio et al. [16] showed that diffuse low- and high-signal changes on T2 MRI predicted an increased risk of delayed union within 2 months after onset. Kanchiku et al. [20] showed that 0.5-T MRI could detect the association of delayed union with wide-type fractures showing T1 low- and T2 high-signal changes within 10 days after the fracture onset. The sensitivity and specificity were 85 and 71 %, respectively. When they divided the vertebral body into four sections, the location of the confined signal change was not associated with delayed union. Our results were similar to those of the above reports; however, these reports did not define T2 high-signal change or elucidate the level of reliability [16, 20]. In addition, these studies did not evaluate either the difference or the intensity change according to time points after injury. In the current study, we investigated the impacts using two time points in the acute phase (at the time of enrollment, which was within 2 weeks after the injury and at 1 month after the injury). We also defined T2 high-signal changes as having an intensity equivalent to that of cerebrospinal fluid, because it was often difficult to confirm a lower degree of high-signal change. This simple classification system appeared to be highly reliable.
On MRI, the presence of fluid collection has been described in rare cases of avascular necrosis of the vertebral body [23]. However, one study showed that a fluid sign was detected in 40 % of OVFs [24]. In patients with OVFs in the acute phase, histologic examination results showed that a gas-containing cleft within a collapsed vertebra is associated with signs of ischemic necrosis in both the acute and chronic phases [24, 25]. After a prolonged period of supine positioning, the clefts may become filled with fluid, resulting in a high-signal change on T2WI [26, 27]. Ischemia takes place in the anterior segment of the vertebral body, which is supplied by the anterior peripheral and metaphyseal arteries [26]. Vascular insult leads to vertebral collapse, which results in an insufficient revascularization and bone fracture healing process. Especially in elderly patients, a vertebral compression fracture seems to represent the initial event, which is followed by delayed fracture healing due to impaired vascular proliferation and subsequently by osteonecrosis [25]. Flexion and extension forces along the horizontal fracture plane may prevent union of the fragments.
In our study, T1 diffuse low-signal changes showed very low specificity at the 1-month follow-up because most of the fractured vertebrae showed T1 diffuse low-signal changes. Therefore, the signal change might be useful at the time of enrollment, but not at the 1-month follow-up. Conversely, T2 diffuse low-signal changes showed significantly increased ORs at the 1-month follow-up, but not at enrollment. Fractures have been described as having intramedullary lines of very low signal intensity extending to the inner cortical margin on T1W1 [28]. These lines are frequently surrounded by larger irregular zones of marrow abnormalities characterized by a slightly decreased signal intensity on T1WI and corresponding minimal hyperintensity on T2WI relative to the normal marrow [29–32]. Signal changes on MRI represent hematomas, inflammation, or edema adjacent to the disrupted trabeculae and cortex in the acute or subacute phase [31–33]. In the current study, the frequency of diffuse low-signal changes on T2WI was much lower than that on T1WI at enrollment and the 1-month follow-up (5.2 vs. 43.1 % and 13.8 vs. 89.7 %, respectively). Therefore, the sample size may have been too small to investigate T2 diffuse low-signal changes, especially at the time of enrollment, because of the wide OR confidence interval. However, the accuracy of T2 diffuse low-signal changes was higher than that of T1WI, which implies that T1 diffuse low-signal changes may represent bone edema rather than a trabecular fracture.
The current study demonstrated that motion of the fractured vertebrae at enrollment and at the 1-month follow-up was associated with delayed union. There may be two explanations for this mechanism. First, increased motion of the fractured vertebrae may be associated with a large bone deficit, which is likely to link to delayed union. Second, the increased motion may cause delayed compression.
There is no data regarding the association of plain X-ray findings with the prediction of delayed union. The sensitivity and specificity were similar to those of the MRI findings, but the PPV and ORs were lower. In addition, about 30 % of the patients did not undergo the usual flexion-extension radiographs because of back pain on extension of the spine. These results show that MRI provides more useful information than plain X-rays in predicting delayed union. However, it is important to recognize the relationship of X-ray findings with delayed union, because there are many institutions that do not have MRI scanners.
There are several limitations to this study. First, the frequency of the distribution of MRI findings might have been affected by the amount of time that the patient was in the supine position before the MRI. If patients lie in the supine position for more than an hour, the frequency of T2 high signals may increase, which may increase the sensitivity for delayed union or decrease the PPV. Second, this study used different MRI scanners, depending on the institution. Nonetheless, our simple method of MRI classification showed high reliability. Third, 53 patients (24 %) were lost to follow-up. The average age of the patients was 79.5 years, which was higher than that of patients followed up for 6 months (75.9 years old). In these patients, the average VAS scores of back pain were 70.7 and 36.1 at enrollment and the 1-month follow-up, respectively. These scores were similar to those in patients followed up for 6 months. The relatively high dropout rate could be because it is difficult for elderly patients to make repeated follow-up visits to the clinic. Finally, the ORs may have overestimated the risk ratio for delayed union, because the outcome showed high frequency [34].
In conclusion, this study demonstrated the radiological factors for prediction of delayed union after an OVF. T2 high-signal changes showed the strongest association with delayed union, for which sequential MRIs at two different time points might be more useful. Five or more degrees of angular motion on fractured vertebrae at the 1-month follow-up also increased the OR by about seven times. Because delayed union is associated with reduced ADLs and severe back pain, surgical intervention or other conservative treatments should be considered in these cases. Finally, appropriate selection of patients according to the radiological findings is important in terms of preventing surgical risk and reducing medical costs.
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Black DM, Cummings SR, Karpf DB, et al. (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet (London, England) 348:1535–1541
Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ (1992) Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res 7:221–227
Gold DT (2001) The nonskeletal consequences of osteoporotic fractures. Psychologic and social outcomes. Rheum Dis Clin N Am 27:255–262
Rao RD, Singrakhia MD (2003) Painful osteoporotic vertebral fracture. Pathogenesis, evaluation, and roles of vertebroplasty and kyphoplasty in its management. J Bone Joint Surg Am 85-A:2010–2022
Suzuki N, Ogikubo O, Hansson T (2008) The course of the acute vertebral body fragility fracture: its effect on pain, disability and quality of life during 12 months. Eur Spine J 17:1380–1390
Matsumoto T, Hoshino M, Tsujio T, et al. (2012) Prognostic factors for reduction of activities of daily living following osteoporotic vertebral fractures. Spine (Phila Pa 1976) 37:1115–1121
Hasegawa K, Homma T, Uchiyama S, Takahashi H (1998) Vertebral pseudarthrosis in the osteoporotic spine. Spine (Phila Pa 1976) 23:2201–2206
Hoshino M, Nakamura H, Terai H, et al. (2009) Factors affecting neurological deficits and intractable back pain in patients with insufficient bone union following osteoporotic vertebral fracture. Eur Spine J 18:1279–1286
Ito Y, Hasegawa Y, Toda K, Nakahara S Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J 2:101–106
Garfin SR, Yuan HA, Reiley MA (2001) New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976) 26:1511–1515
Diamond TH, Champion B, Clark WA (2003) Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med 114:257–265
Klazen CA, Lohle PN, de Vries J, et al. (2010) Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 376:1085–1092
Watts NB, Harris ST, Genant HK (2001) Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int 12:429–437
Chandra RV, Yoo AJ, Hirsch JA (2013) Vertebral augmentation: update on safety, efficacy, cost effectiveness and increased survival? Pain Physician J 16:309–320
Tsujio T, Nakamura H, Terai H, et al. (2011) Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine (Phila Pa 1976) 36:1229–1235
McKiernan F, Faciszewski T (2003) Intravertebral clefts in osteoporotic vertebral compression fractures. Arthritis Rheum 48:1414–1419
Maldague BE, Noel HM, Malghem JJ (1978) The intravertebral vacuum cleft: a sign of ischemic vertebral collapse. Radiology 129:23–29
Fang X, Yu F, Fu S, Song H (2015) Intravertebral clefts in osteoporotic compression fractures of the spine: incidence, characteristics, and therapeutic efficacy. Int J Clin Exp Med 8:16960–16968
Kanchiku T, Imajo Y, Suzuki H, et al. (2013) Usefulness of an early MRI-based classification system for predicting vertebral collapse and pseudoarthrosis after osteoporotic vertebral fractures. J Spinal Disord Tech 27:61–65
McKiernan F, Jensen R, Faciszewski T (2003) The dynamic mobility of vertebral compression fractures. J Bone Miner Res 18:24–29
Cho T, Matsuda M, Sakurai M (1996) MRI findings on healing process of vertebral fracture in osteoporosis. J Orthop Sci 1:16–33
Dupuy DE, Palmer WE, Rosenthal DI (1996) Vertebral fluid collection associated with vertebral collapse. AJR Am J Roentgenol 167:1535–1538
Baur A, Stabler A, Arbogast S, et al. (2002) Acute osteoporotic and neoplastic vertebral compression fractures: fluid sign at MR imaging. Radiology 225:730–735. doi:10.1148/radiol.2253011413
Libicher M, Appelt A, Berger I, et al. (2007) The intravertebral vacuum phenomen as specific sign of osteonecrosis in vertebral compression fractures: results from a radiological and histological study. Eur Radiol 17:2248–2252
Sarli M, Pérez Manghi FC, Gallo R, Zanchetta JR (2005) The vacuum cleft sign: an uncommon radiological sign. Osteoporos Int 16:1210–1214
Malghem J, Maldague B, Labaisse MA, et al. (1993) Intravertebral vacuum cleft: changes in content after supine positioning. Radiology 187:483–487
Meyers SP, Wiener SN (1991) Magnetic resonance imaging features of fractures using the short tau inversion recovery (STIR) sequence: correlation with radiographic findings. Skelet Radiol 20:499–507
Yao L, Lee JK (1988) Occult intraosseous fracture: detection with MR imaging. Radiology 167:749–751
Deutsch AL, Mink JH (1989) Magnetic resonance imaging of musculoskeletal injuries. Radiol Clin N Am 27:983–1002
Vogler JB, Murphy WA (1988) Bone marrow imaging. Radiology 168:679–693
Smith SR, Williams CE, Davies JM, Edwards RH (1989) Bone marrow disorders: characterization with quantitative MR imaging. Radiology 172:805–810
Kanchiku T, Taguchi T, Kawai S (2003) Magnetic resonance imaging diagnosis and new classification of the osteoporotic vertebral fracture. J Orthop 8:463–466
Zhang J, Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690–1691
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Funding
This study was funded by the Japan Orthopedics and Traumatology Research Foundation, Inc. (Grant no. 270).
Ethical approval
This study was approved by the Ethics Committee of Osaka City University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Takahashi, S., Hoshino, M., Takayama, K. et al. Predicting delayed union in osteoporotic vertebral fractures with consecutive magnetic resonance imaging in the acute phase: a multicenter cohort study. Osteoporos Int 27, 3567–3575 (2016). https://doi.org/10.1007/s00198-016-3687-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3687-3