Abstract
Summary
Association between serum bone formation and resorption markers and bone mineral, structural, and strength variables derived from quantitative computed tomography (QCT) in a population-based cohort of 1745 older adults was assessed. The association was weak for lumbar spine and femoral neck areal and volumetric bone mineral density.
Introduction
The aim of this study was to examine the relationship between levels of bone turnover markers (BTMs; osteocalcin (OC), C-terminal cross-linking telopeptide of type I collagen (CTX), and procollagen type 1N propeptide (P1NP)) and quantitative computed tomography (QCT)-derived bone density, geometry, and strength indices in the lumbar spine and femoral neck (FN).
Methods
A total of 1745 older individuals (773 men and 972 women, aged 66–92 years) from the Age, Gene/Environment Susceptibility (AGES)–Reykjavik cohort were studied. QCT was performed in the lumbar spine and hip to estimate volumetric trabecular, cortical, and integral bone mineral density (BMD), areal BMD, bone geometry, and bone strength indices. Association between BTMs and QCT variables were explored using multivariable linear regression.
Results
Major findings showed that all BMD measures, FN cortical index, and compressive strength had a low negative correlation with the BTM levels in both men and women. Correlations between BTMs and bone size parameters were minimal or not significant. No associations were found between BTMs and vertebral cross-sectional area in women. BTMs alone accounted for only a relatively small percentage of the bone parameter variance (1–10 %).
Conclusion
Serum CTX, OC, and P1NP were weakly correlated with lumbar spine and FN areal and volumetric BMD and strength measures. Most of the bone size indices were not associated with BTMs; thus, the selected bone remodeling markers do not reflect periosteal bone formation. These results confirmed the limited ability of the most sensitive established BTMs to predict bone structural integrity in older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During aging, both increased endocortical bone resorption and reduced periosteal apposition cause net bone loss, often accompanied by changes in bone micro-architecture, leading to bone fragility [1]. Experimental data from animal and clinical studies clearly indicate that this loss of bone mass and deterioration of bone tissue are linked with a higher bone turnover [2, 3].
Several dual-energy X-ray absortiometry (DXA) studies have reported a negative correlation between the levels of bone turnover markers (BTMs) and areal bone mineral density (aBMD) in older men and women [4, 5]. However, the link between bone fragility and elevated turnover extends beyond bone density [6]. Some studies have suggested that BTMs may predict fracture risk, independent of BMD levels [7, 8], thus reflecting parameters of bone quality.
There has been increasing interest in studying architectural parameters of bone, including both macro- and micro-architectural parameters to investigate the physiological responses of bone to aging, disease, and treatment.
The relation between BTMs and bone quality in the elderly has been previously investigated, but the results have been conflicting, and studies of population-based samples are scarce. Some studies found that BTMs were generally, but not consistently, inversely associated with bone micro-structure at the ultradistal radius [9] and ultrasound parameters of the calcaneus [10], whereas others found no relation [11].
Only high-resolution techniques can depict micro-architecture. Micro-computed tomography (micro-CT) assesses trabecular and cortical bone morphology using ex vivo human bones or in vivo imaging of small animals (such as rodent specimens) [12]. High-resolution peripheral quantitative CT (HR-pQCT) makes possible the in vivo evaluation of micro-architectural parameters including fine cortical and trabecular structural detail at peripheral sites (distal radius and tibia) [13]. To our knowledge, no studies have investigated the relation between BTMs and QCT-derived cortical and trabecular volumetric BMD (vBMD), geometric and structural parameters, and estimated strength indices in clinically important sites among older adults. It seems reasonable to assume that if BTMs significantly correlate with bone strength indices, they can be of interest for identifying patients with higher future fracture risk.
In older adults, net periosteal bone gain has been documented [14, 15]. Although periosteal bone turnover is much slower than endosteal turnover, it has been linked to remodeling rather than modeling [15]. Thus, a dual source of BTMs could hamper the appropriate interpretation of their levels and invalidate their use to predict fracture risk, as they would reflect simultaneously bone fragility and improved bone stiffness and strength. The association between bone formation markers and bone parameters reflecting periosteal apposition has been previously investigated [16], but measurements of distal forearm and hip bone size were based on densitometric projected areas.
In the present study, we assessed whether serum bone formation and resorption markers were associated with bone mineral, structural, and strength variables derived from QCT in a population-based cohort of older adults. Based on the evidence from aBMD studies, we hypothesized that BTMs would be independently and negatively associated with trabecular and cortical vBMD and with strength indices, but the association would be modest. In addition, we also hypothesized that BTMs would be marginal associated with bone size indices, thereby not reflecting periosteal metabolism.
Methods
Study participants
Participants were drawn from the Age, Gene/Environment Susceptibility (AGES)–Reykjavik study, a single-center prospective population study of Icelandic men and women. Design and recruitment have been described in detail [17]. Data were collected between 2002 and 2006 that included both an interview and clinical examinations. Mean age of the participants at baseline AGES–Reykjavik examination was 77 years (range 66–96). The AGES–Reykjavik cohort was randomly recruited from the 11549 survivors of the Reykjavik study. An interim cohort of the first 2300 participants was formed to allow early analysis; this group also received a set of serum biochemical measurements pertinent to bone. Within this sample, 1850 had complete QCT scan and biochemical data on all bone turnover markers. Participants with kidney disease were excluded (n = 85). The final study population for analysis was 1745 participants (773 men and 972 women) aged 66 to 92 years old.
Written informed consent was obtained from all participants, and the study was approved by the Icelandic National Bioethics Committee (VSN: 00–063) and the Institutional Review Board of the Intramural Research Program of the National Institute of Aging.
Measurements
Biochemical measurements
Fasting venous blood samples were collected during the first clinic visit to AGES—Reykjavik study, from September 2002 to January 2006, and samples were aliquoted and stored at −80 °C on-site in the Icelandic Heart Association laboratory until analysis.
Markers for bone formation (procollagen type 1N propeptide, P1NP; and osteocalcin, OC) and bone resorption (C-terminal cross-linking telopeptide of type I collagen, CTX) were measured using a sandwich immunoassay on an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim). All these measurements were performed at the Icelandic Heart Association (Kopavogur, Iceland) according to the manufacturers’ protocols. The interassay coefficients of variation were <3.0 % for all BTMs.
Total 25-hydroxyvitamin D (25OHD) was determined using the Liaison chemiluminescence immunoassay (DiaSorin Inc., Stillwater, Minnesota). The interassay coefficient of variation was <6.5 %, using a previously frozen serum pool as the control sample and was <12.7 % when using Liaison quality controls. Intact parathyroid hormone (PTH) was assayed using electrochemiluminescence technology from Cobas-Roche (West Sussex, UK) on a two-site immunoassay. The interassay coefficient of variation was <0.5 % when using a frozen serum pool as the control sample and was <2.8 % when using Cobas-Roche quality controls.
Serum creatinine was measured using the Roche-Hitachi 912 instrument with Roche Creatinine Jaffé compensated method, Roche Diagnostics, Mannheim, Germany. The coefficient of variation (CV) for the creatinine assay was 2.5 %.
QCT scanning and analysis
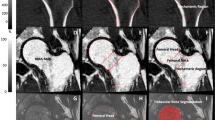
At the second examination day, lumbar spine and the left hip were scanned and analyzed using four‐row detector CT system (Sensation; Siemens Medical Systems, Erlangen, Germany), as previously described [18]. Briefly, bone mineral reference phantom (three-sample calibration phantom, Image Analysis, Columbia, KY, USA) was placed under the participants’ spine and hips and scanned simultaneously for calibration. Helical studies were done at lumbar spine (L1 and L2 vertebrae) and hip (proximal femur from 1 cm superior to acetabulum to 3–5 mm inferior to lesser trochanter) at following parameters: 120 kVp, 150 mAs for spine, and 140 mAs for hip, 1-mm slice thickness, and pitch = 1.
For each trabecular, cortical, and integral region of interest, volumetric BMD (vBMD; g/cm3), as well as a simulated areal BMD (g/cm2) obtained by dividing the BMC by the projected area of the region of interest on the coronal plane, was calculated from QCT data. The vertebral regions of interest included the vertebral centrum and another region comprising both the centrum and the spinous processes, the latter region being used to derive the simulated DXA. Cross-sectional areas (CSAs) and indices of compressive strength of the femoral neck and mid-vertebrae were estimated as previously described [18]. Femoral neck section modulus (a measure of bending strength) was computed at the femoral neck cross section as an elastic-modulus weighted polar moment of inertia divided by the neck width and normalized by nominal cortical elastic modulus. In addition, we also computed the cortical index (ratio of the femoral neck cortical volume to the total bone volume (cvol/ivol)), as a measure of the integrity of the proximal femoral cortex, as described by Lang et al. [19].
Study questionnaires and clinical data
We used questionnaire data at the time of examination for education (categorized into two levels (primary/secondary vs college/university), age at menopause, current smoking (yes/no), current alcohol use (converted into grams per week using 14 g of alcohol as a standard drink), health history (including history of osteoporosis and diabetes), and lifestyle practices, including physical activity and food intake history. Use of medications known to affect bone density was ascertained based on medications brought to the clinic at the time of the examination: estrogen replacement therapy, tibolonum, antiepileptics, systemic glucocorticosteroids, and agents for the treatment of osteoporosis (raloxifen, calcitonin, or bisphosphonates); and diuretics, including hydrochlorothiazide.
Physical activity was assessed by self-reported level of physical activity during the last 12 months, where participants were asked how frequently they engaged in both light and moderate or vigorous activity, giving examples of these levels of activity for clarification. The response categories were as follows: (1) never, (2) rarely, (3) weekly but less than 1 h per week, (4) 1–3 h per week, (5) 4–7 h per week, or (6) more than 7 h per week. In this study, a binary variable for physical activity was used as an indicator for moderate or a high frequency of participation (at least 4–7 h per week of moderate or vigorous activity or at least 1–3 h per week of moderate or vigorous activity plus at least 4–7 h per week of light-intensity activities) versus occasionally physically active at most.
Self-reported mobility disability was defined as having much difficulty or unable to walk 500 m and/or climb ten steps.
Information on protein intake (recoded as g/day for the sum of meat, fish, and bread ingestion), coffee consumption (recoded as “yes” for consumption ≥3 cups per day; otherwise, no), cod liver oil intake (main dietary source of vitamin D; recoded as “yes” for consumption ≥3 times a week; otherwise, no), and milk intake as a teenager (recoded as “yes” for consumption ≥3 times a week; otherwise, no) was gathered using a validated food frequency questionnaire [20].
Diabetes status was based on self-report, use of glucose-lowering medication, or fasting glucose of >7 mmol/L. The questionnaire was administered in the clinic by a trained interviewer.
Height (m) and weight (kg) were measured using a Seca stadiometer and a digital scale (Marel, Reykjavik, Iceland) at the clinical assessment, and body mass index (BMI) was calculated in kg/m2.
Coronary artery calcium (CAC), as an indicator of cardiovascular risk, was assessed using the Agatston score, calculated as the sum of four major coronary artery scores to quantify the extent of CAC as previously described in detail [21, 22].
Statistical analysis
To be consistent with previous reports and because of the known differences in bone mineral and bone marker distribution, all analyses were performed separately for women and men. Mean ± SD and interquartile range, or percentages for categorical variables were used to summarize subject characteristics. Variables with non-Gaussian distribution were normal-scored transformed using Van der Waerden’s formula. The association between bone marker levels and QCT-derived bone measures was assessed visually using scatter plots, superimposing linear lines and also locally weighted scatter plot smooth (LOWESS) curves to examine potential non-linearity. Linearity was also examined with dummies by dividing bone marker levels into sex-specific quintiles (data not shown). Multivariable linear regression was used to determine the association between bone turnover markers and QCT parameters (as dependent variable). In the first stage of the hierarchical regression, we report the unadjusted model. We then adjusted for covariates including age, education level (low/high), age at menopause, history of osteoporosis or medication known to affect bone health (yes/no), and BMI (model 2). The third model added was adjusted for lifestyle factors including smoking status (yes/no), alcohol consumption (g/week), current physical activity level (low/high), mobility disability (yes/no), protein intake (g/day), coffee intake (yes/no), vitamin D intake (yes/no), and milk intake as a teenager (yes/no). History of diabetes (yes/no), CAC scoring, total 25OHD, PTH, and creatinine levels were added in the final model.
Results are expressed as standardized regression coefficient (β), p value, and the adjusted R 2 for both displayed models (unadjusted and adjusted/model 2). Results for model 3 and 4 were similar to model 2 (data not shown). Sensitivity analyses were conducted excluding BMI from the model to test the robustness of our results for the lifestyle variables.
Statistical analyses were conducted using SPSS software, version 22.0 (IBM, USA).
Results
Characteristics of the subjects are shown in Table 1. The mean age was 76 (standard deviation, 5.5) years; 56 % of participants were women. Mean BMI was in the overweight range (26.8 kg/m2). More women reported osteoporosis (13.9 %) compared with men (3.2 %), and 13 % of the subjects reported they currently smoke. Mean age at menopause was 48 (standard deviation, 4.4) years. As expected, the three BTMs were significantly intercorrelated (r = 0.8, p < 0.01).
Bone turnover markers and BMD (volumetric and areal)
All BMD measures, except for spine cortical BMD and P1NP in men, had a negative correlation with the BTM levels in both men and women (Tables 2 and 3). The association persisted after adjustment (models 2, 3, and 4), and there was no evidence of threshold effects when BTMs were categorized into quintiles (data not shown). In men, the correlation was stronger between femoral neck (FN) BMD measures and all BTMs compared to the same feature at the spine. The opposite was observed in women, although for spine cortical BMD slightly lower associations were observed, and it was only significantly associated with CTX and P1NP before adjustment for covariates (models 2, 3, and 4). Considering all bone measures, the highest simple correlation (unadjusted) was observed between each BTMs and integral BMD in both men and women. In both men and women, for all BTMs, the associations with the areal BMD were, in general, higher than those with cortical BMD, but lower compared with the other volumetric measures. The associations were stronger between BTMs and the trabecular bone than in cortical bone. Particularly in men, all models were very weak for FN cortical BMD; the final model only accounted for 1.4–2.0 % in its variability, while in women, the unadjusted model (each BTM) accounted for 1.0–2.2 %, and in the final model, only a modest improvement was observed (4.7–5.5 %). Thus, the cortical compartment displayed the lowest associations with BTMs. However, even for measures expressing trabecular bone, each BTM explained a small amount of the variation in BMD. For example, in men, serum concentrations of CTX, OC, and P1NP accounted for 9.0, 9.6, and 5.4 % of the variance in integral BMD, respectively, and in women for the same variable, the predictive values of each BTMs where less than half (CTX = 4.2 %, OC = 4.4 %, P1NP = 1.7 %).
Bone turnover markers and cross-sectional area
Associations of bone size parameters with BTMs levels were weaker and less consistent. In men (but not in women), all BTMs were positively associated with vertebral CSA. However, the magnitude of association of CTX and OC was extremely small (r = 0.10), thus accounting only for 0.8 % of the variance in vertebral CSA, and after adjustment, the final model only explained 4 % of the variance. Similar in men and women, CTX and OC did not correlate with FN minimal CSA. Small positive correlations (r = 0.09 in men and r = .07 in women) were found between P1NP and FN minimal CSA, and P1NP alone accounted for 0.7 % of the variance at this site in men and 0.4 % in women. All BTMs were associated with FN maximal CSA and the associations remained significant after adjustment (models 2 to 4). Each BTM accounted for 2 % of variance at the FN maximal CSA in men and accounted for less than 1.2 % in women. In men and women, adding age, BMI, education level, history of osteoporosis, or use of any medication known to affect bone health (and age at menopause for women) only slightly increased adjusted R 2 values at the trochanter to about 3 and 4 %, respectively.
Bone turnover markers and the integrity of the proximal femoral cortex
CTX, OC, and P1NP were inversely associated with FN cortical index in both men and women. The contribution of BTMs to the cortical index variance was lower in women compared with that in men (3.5, 3.2, and 1.0 % in women vs. 8.0, 9.0, and 4.5 % in men, for CTX, OC, and P1NP, respectively). Further adjustments had no influence on the results.
Bone turnover markers and bone strength
All BTMs levels were inversely correlated with vertebra compressive strength, being slightly higher in women compared with that in men. In men, P1NP was not significantly associated with vertebra compressive strength. CTX and OC remained significantly associated with vertebra compressive strength after adjustments. All BTMs were inversely associated with FN compressive strength, and the associations were stronger in men (medium magnitude) compared with those in women. All the associations persisted after further adjustments. In contrast to BMD measures, the associations between all BTMs and compressive strength in both men and women were stronger at the FN compared with those at the spine. Similarly, CTX and OC accounted for approximately 9 and 4 % of the variance in FN compressive strength, in men and women, with values for P1NP of 4.6 and 1.3 %, respectively. In addition, FN bending/torsional strength (section modulus) was not associated with P1NP in women, but a small inverse association (r = −0.09) was found in men. Bending/torsional strength was inversely associated with CTX and OC in men and women, and with P1NP only in men; however, in women, the association between CTX and FN section modulus was not significant after adjustments (models 2 to 4). The total variance of this strength index attributed to resorption and formation markers was lower for each BTMs. For example, CTX and OC accounted for about 3 % of variance in FN section modulus (less 6 % compared with FN compressive strength).
Discussion
In this population-based study, serum concentrations of bone resorption (CTX) and formation markers (OC and P1NP) disclose an inverse association with the CT-derived volumetric BMD, estimated areal BMD, and most of the bone strength indices. In addition, a positive, weak, and less consistent association (particularly in women) was observed between BTMs and bone size parameters, suggesting that higher rates of bone remodeling are associated with impaired bone material properties but not with periosteal apposition. Interestingly the association remained significant after adjustment for several potential confounding factors such as serum PTH, 25OHD and creatinine levels, lifestyle factors, age, and BMI.
The negative and low correlation between BTMs and areal BMD at both spine and FN is consistent with data from several previous studies in older men and women using DXA [4, 5, 10, 23]. Consistent with our results, Khosla et al. [4] found that at lumbar spine, the association between OC and areal BMD were stronger in women than in men, and the opposite was observed for proximal femur.
QCT is considered to measure true volumetric BMD and enables differentiation between cortical and trabecular bone. Thus, consistent with the extensive literature regarding DXA-assessed BMD, the negative association of all BTMs with all volumetric BMD measures at both measurement sites in men and women suggests that higher rates of bone turnover are associated with endosteal (inner envelope) bone loss.
Our findings that associations with trabecular compartment were much more marked compared with the cortical compartment are consistent with the hypothesis of different inherent rates of bone turnover and distinct patterns of age-related loss [18, 24].
It has been suggested that OC is much more concentrated in the cortical than in the trabecular bone, which could be used as a possible biological marker reflecting the remodeling of a particular bone compartment [25]. In opposition, our results demonstrated that OC discloses a slightly stronger association with trabecular BMD. The reasons for these differences may include the use of QCT that separately evaluates trabecular and cortical bone compartments and bone geometry but lacks the spatial resolution to assess bone micro-structure (such as trabecular thickness or cortical porosity). Moreover, those results were based on analytical techniques for demineralization and protein extraction from bone samples.
In line with the fact that bone formation markers may also be secreted by active osteoblasts in periosteal surfaces [26], a significant association between bone formation markers and parameters of bone size could be expected. During aging, bone fragility is also explained by a reduced periosteal apposition, thus falling to compensate the endosteal bone loss [1].
However, no significant associations were found between BTMs and vertebral CSA in women, and very modest positive associations were found in men, suggesting a marginal association with the periosteal apposition. At the femoral neck, BTMs were positively associated with maximal CSA but were particularly weak among women. However, a significant positive association was observed for P1NP, by itself accounting for less than 0.7 % of the variance at minimal CSA. In the MINOS study, no associations were found between bone formation markers (i.e., OC, bone-specific alkaline phosphatase, and P1NP) and densitometric estimates of periosteal apposition [16]. The reasons for these somewhat discrepant findings between studies could include differences in population characteristics and bone measurement equipment. Thus, MIMOS study included a younger sample of older men (aged 55–85 years) and used DXA-derived indices of bone size.
There are several potential reasons for the extremely weak or not significant association between BTM concentrations and bone size estimates. Periosteal cells may not share the same phenotype from endosteal osteoblasts and produce different biochemical markers, as current bone markers reflect mainly endosteal bone remodeling [16]. Although data on biology of periosteal cells are limited and obtained mainly in animal studies, it was recently suggested that serum periostin might reflect periosteal metabolism [27]. In a prospective cohort of postmenopausal women, Rousseau et al. [28] found that increased levels of serum periostin were associated with a higher risk of all fractures independent of BMD and prior fragility fractures. Different bone turnover rates may be expected at the endosteal and periosteal surfaces. Total endosteal surface is much larger and metabolically active than periosteal surface. Although age-related subperiosteal bone formation increases the CSA of bone [1], previous studies reported modest increases when compared with endosteal bone loss [14, 18, 24]. For example, Sigurdsson et al. [18] found that CSA is slightly but similarly larger in both sexes, 4.7–6.0 % per 10 years in age in the lumbar spine, while at the same site, trabecular BMD loss was 25.1 % in women and 14.9 % in men.
In men and women, BTMs were generally negatively associated with CT-estimated cortical index and strength indices, consistent with the notion of increased bone turnover having detrimental effects in bone integrity and strength [29]. The elastic modulus and strength of cortical bone are also positively related to the degree of matrix mineralization [30]. Long-standing elevated bone turnover produces a fall in mineral content of bone tissue as new remodeling unites are initiated and the old bone is replaced by younger bone that has undergone primary, but less complete secondary, mineralization [31]. In contrast, prolonged low bone turnover will eventually increase the degree of matrix mineralization that is expected to lead to increased bone stiffness [32]. Although, to our knowledge, no previous clinical studies have addressed this issue, our results are concordant with data from studies using high-resolution peripheral QCT. Thus, in addition to being linked with micro-architectural deterioration of bone tissue (i.e., trabecular number, connectivity density, trabecular thickness, and trabecular separation) [9, 33], increased bone turnover is also associated with reduced bone strength. The estimates of bone micro-architecture were based on analysis of peripheral sites (i.e., distal radius and distal tibia), whereas our analysis included measures from central sites (i.e., lumbar spine and hip) that are highly correlated with hospitalization and mortality risk. However, the associations were not consistent in women for section modulus, and in men for vertebra compressive strength. Additional studies relating BTMs with estimates of proximal femoral strength from QCT-based finite element models will be useful in clarifying whether BTMs are causally associated with decreased bone strength.
Our study indicates that among the markers, the correlations were weaker for P1NP which is consistent with other studies using DXA [10, 23], QUS [10, 11], or 3D-pQCT [9, 33].
Although most previous observations included only men or women in their study samples, in our analysis, the associations with bone measures were, in general, stronger for men compared with those for women. However, at the spine, the associations were, most often, slightly stronger in women as observed by others [4, 9, 34]. However, in general, the sex differences observed in our study were trivial, which suggests that circulating BTMs reflect in a similar extent bone geometry (did not correlate or weak positive association), density, and strength (negative association) in older men and women.
In our study, bone turnover as assessed by serum CTX explained between 1.0 and 9.1 % and 1.3 and 5.1 % (men and women, respectively) of the variability in bone indices. The contribution of bone formation markers to bone QCT–derived parameters reflecting endeosteal bone loss variance remained low and accounted for 0.6–9.6 % in men and 0.7–4.8 % in women, suggesting that bone quantity and quality (strength) reflect biological processes different from those captured by conventional biochemical indices of overall bone turnover rate. Our findings that adding age and BMI (the most robust covariates) to the unadjusted model increased adjusted R 2 to about 20 % of the variance in QCT-derived bone parameters are consistent with the well-documented role of body weight or BMI [35] and advanced age [36] in determining BMD and bone strength as well as bone turnover. Moreover, after including lifestyle factors (such as diet, physical activity, mobility disability, and smoking) into our analysis, and afterward endocrine and metabolic factors such as total PTH, 25OHD, and creatinine concentration, diabetes, and CAC score, only a minor increase (<3 %) in adjusted R 2 beyond the effect of BMI was observed. When BMI was removed from the model in an additional analysis, the results for the lifestyle variables were unchanged. This result was unexpected, as the contribution of these variables on bone integrity has also been suggested. Among this factors, mechanical loading is considered the predominant functional osteogenic factor responsible for maintaining structurally appropriate levels of bone mass in adults [37] However, in our study, physical activity was not a significant predictor of spine, FN, and trochanter CSA (parameters reflecting periosteal apposition). Similarly, BMI had limited additive effect in predicting bone size parameters.
Thus, our findings suggest that in addition to turnover, other factors may also contribute to bone fragility. Changes in matrix composition, especially modifications of the collagen network such as accumulation of advanced glycation end products and increased cross-linking of collagen, have been proposed to be a determinant of impaired bone properties by recent in vitro and ex vivo studies [38, 39]. Osteocyte deficiency associated with aging [40] and the increased number of fatigue damage [41] have also been suggested to contribute to bone fragility.
The primary strength of this study is its large, community-based population, which enabled us to examine the associations between BTMs and QCT-derived bone measures at weight-bearing sites associated with high fracture risk in both elderly men and women. This analysis includes a comprehensive number of covariates (including BMI (mostly due to the fat–bone connection), physical activity and mobility disability as they are related to mechanical loading (or the lack of), history of osteoporosis or medication associated with bone metabolism (e.g., bisphosphonates and selective estrogen modulators), dietary intake (protein, calcium, caffeine, vitamin D), smoking, alcohol intake, diabetes, CAC (reflecting the anatomic presence of coronary atherosclerosis), endocrine and metabolic factors (PTH, 25OHD, creatinine), education level, and age, as the most determinant non-modifiable factor) associated with bone metabolism. To date, no other studies have considered the potential confounding effect of these factors to assess the relation between biological markers of bone turnover and bone quantity and quality. Also, we used the most sensitive and specific serum markers of bone formation and resorption measured in fasting conditions. Finally, we excluded subjects with kidney disease (and creatinine levels were added as a covariate), as renal failure may elevate bone markers due to the difference in the dialyzer membrane’s permeability [42] or due to the deleterious effect of chronic kidney disease itself [43].
There are, however, a number of limitations to be considered when interpreting the results. A potential weakness concerns the measurement of bone remodeling activity (activation frequency). Despite the use of well-established biochemical markers of bone metabolism, they have some limitations in accuracy because they are not bone-tissue-specific; they reflect mainly the enzymatic activities of osteoblasts or osteoclasts and not the activity of osteocytes; they may not be sensitive to periosteal apposition, thus reflecting essentially endosteal bone remodeling; and they are unable to distinguish the metabolic activity of the different skeletal compartments (they estimate crudely the aggregated remodeling intensity for the whole skeleton). Nevertheless, it is impossible to measure remodeling rates at individual surface locations non-invasively, and the clinical utility of novel markers such as cathepsin K, receptor activator of nuclear factor kappa-B ligand (RANKL), Dickkopf-related protein 1 (DKK1), sclerostin, or circulation microRNAs remains unclear. In addition, numerous confounding factors (example, pre-analytical conditions, assay reliability, and biological variability) may increase the variability in circulating BTM levels. In our study, blood samples were taken from 8.00 to 11.30 a.m. when fasting to minimize the influence of circadian rhythm and diet (fasting-feeding cycle). An additional limitation is the use of a single measure of BTM levels. A prospective cohort study would be the most efficient approach to help establish cause and effect relationships. Nevertheless, the present analytical cross-sectional study is useful for establishing preliminary evidence for a causal relationship and helps to clarify if measuring BTM levels at one moment in time can be useful for the positive and etiological diagnosis of bone fragility at baseline. Another concern is potential bias resulting from cohort selection (individuals of European ancestry) and due to analytical selection bias, as we included only one third of the entire original cohort that were younger and had lower presence of atherosclerosis and BMI, lower prevalence of mobility disability, and higher of high physical activity levels. Significant differences were also found for 25-hydroxyvitamin D and creatinine levels, and for most of the FN bone measures. Therefore, extrapolation beyond this group should be undertaken with caution, and the cross-sectional design limits inference on the causal nature of the observed relations. We also acknowledge that we looked to multiple outcome measures, which may increase the chance of finding at least one test statistically significant due to chance. However, if the correcting for multiple testing have been applied (Bonferroni adjustment, p < 0.003 for significance), the majority of our results would still remain significant. Finally, serum homocysteine levels were not included in the analysis. Although it has been suggested that homocysteine could influence bone strength and bone turnover and interfere with enzymatic cross-link formation [44], the association with fracture risk has been inconsistent [44, 45].
The biologic mechanisms through which bone fragility could result from elevated bone turnover might involve the age-related cellular machinery defects of the basic multicellular unit (BMU), as each BMU forms less bone and increases the volume of bone resorbed [1]. Other relevant mechanism may be qualitative abnormalities in bone precipitated by increased perforative resorption by individual BMUs causing trabecular plate perforation, and loss of connectivity [31].
In conclusion, we examined the contribution of serum markers of bone turnover to bone quantity and quality based on bone mineral and geometric analysis of clinical relevant sites and found that BTMs offered little practical information for estimating bone mass and strength in older men and women. Additional studies are needed to clarify the relationship of biological markers of bone metabolism and periosteal apposition, since our results demonstrated weak associations with trochanter CSA in men and women and with vertebral CSA only in men. These significant associations may compromise the utility of BTMs as predictors of bone loss and risk of fracture, as the periosteal expansion that occurs with age improves or at least maintains bone stiffness and strength. Future studies should address the association between bone quality and collagen maturity, including the estimation of the pyrdinoline/deoxypyridinoline and α/β CTX ratios, and the non-enzymatic cross-links such as serum or urine pentosidine levels.
References
Seeman E (2009) Bone modeling and remodeling. Crit Rev Eukaryot Gene Expr 19(3):219–233
Bauer NB, Khassawna TE, Goldmann F, Stirn M, Ledieu D, Schlewitz G, Govindarajan P, Zahner D, Weisweiler D, Schliefke N, Bocker W, Schnettler R, Heiss C, Moritz A (2015) Characterization of bone turnover and energy metabolism in a rat model of primary and secondary osteoporosis. Exp Toxicol Pathol Off J Ges Toxikologische Pathol 67(4):287–296. doi:10.1016/j.etp.2015.01.004
Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD (1996) Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res 11(3):337–349. doi:10.1002/jbmr.5650110307
Khosla S, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL (1998) Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83(7):2266–2274
Marcus R, Holloway L, Wells B, Greendale G, James MK, Wasilauskas C, Kelaghan J (1999) The relationship of biochemical markers of bone turnover to bone density changes in postmenopausal women: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. J Bone Miner Res Off J Am Soc Bone Miner Res 14(9):1583–1595. doi:10.1359/jbmr.1999.14.9.1583
Seeman E (2008) Bone quality: the material and structural basis of bone strength. J Bone Miner Metab 26(1):1–8. doi:10.1007/s00774-007-0793-5
Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, Pettersson K, Vaananen HK, Akesson K, Obrant KJ (2004) Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res Off J Am Soc Bone Miner Res 19(3):386–393. doi:10.1359/JBMR.0301244
Ivaska KK, Gerdhem P, Vaananen HK, Akesson K, Obrant KJ (2010) Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res Off J Am Soc Bone Miner Res 25(2):393–403. doi:10.1359/jbmr.091006
Khosla S, Melton LJ 3rd, Achenbach SJ, Oberg AL, Riggs BL (2006) Hormonal and biochemical determinants of trabecular microstructure at the ultradistal radius in women and men. J Clin Endocrinol Metab 91(3):885–891. doi:10.1210/jc.2005-2065
Boonen S, Pye SR, O’Neill TW, Szulc P, Gielen E, Borghs H, Verschueren S, Claessens F, Adams JE, Ward KA, Bartfai G, Casanueva F, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Silman AJ, Tajar A, Wu FC, Vanderschueren D, Group E (2011) Influence of bone remodelling rate on quantitative ultrasound parameters at the calcaneus and DXA BMDa of the hip and spine in middle-aged and elderly European men: the European Male Ageing Study (EMAS). Eur J Endocrinol Eur Fed Endocr Soc 165(6):977–986. doi:10.1530/EJE-11-0353
Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, Herrmann M, Ebeling PR, Sanders KM (2014) Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone 64:8–12. doi:10.1016/j.bone.2014.03.008
Genant HK, Engelke K, Prevrhal S (2008) Advanced CT bone imaging in osteoporosis. Rheumatology 47(Suppl 4):iv9–iv16. doi:10.1093/rheumatology/ken180
Nishiyama KK, Shane E (2013) Clinical imaging of bone microarchitecture with HR-pQCT. Curr Osteoporos Rep 11(2):147–155. doi:10.1007/s11914-013-0142-7
Carpenter RD, Sigurdsson S, Zhao S, Lu Y, Eiriksdottir G, Sigurdsson G, Jonsson BY, Prevrhal S, Harris TB, Siggeirsdottir K, Guethnason V, Lang TF (2011) Effects of age and sex on the strength and cortical thickness of the femoral neck. Bone 48(4):741–747. doi:10.1016/j.bone.2010.12.004
Balena R, Shih MS, Parfitt AM (1992) Bone resorption and formation on the periosteal envelope of the ilium: a histomorphometric study in healthy women. J Bone Miner Res Off J Am Soc Bone Miner Res 7(12):1475–1482. doi:10.1002/jbmr.5650071216
Szulc P, Garnero P, Marchand F, Duboeuf F, Delmas PD (2005) Biochemical markers of bone formation reflect endosteal bone loss in elderly men--MINOS study. Bone 36(1):13–21. doi:10.1016/j.bone.2004.09.004
Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V (2007) Age, gene/environment susceptibility-Reykjavik study: multidisciplinary applied phenomics. Am J Epidemiol 165(9):1076–1087. doi:10.1093/aje/kwk115
Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF (2006) Increasing sex difference in bone strength in old age: the age, gene/environment susceptibility-Reykjavik study (AGES-REYKJAVIK). Bone 39(3):644–651. doi:10.1016/j.bone.2006.03.020
Lang TF, Sigurdsson S, Karlsdottir G, Oskarsdottir D, Sigmarsdottir A, Chengshi J, Kornak J, Harris TB, Sigurdsson G, Jonsson BY, Siggeirsdottir K, Eiriksdottir G, Gudnason V, Keyak JH (2012) Age-related loss of proximal femoral strength in elderly men and women: the age gene/environment susceptibility study—Reykjavik. Bone 50(3):743–748. doi:10.1016/j.bone.2011.12.001
Eysteinsdottir T, Thorsdottir I, Gunnarsdottir I, Steingrimsdottir L (2012) Assessing validity of a short food frequency questionnaire on present dietary intake of elderly Icelanders. Nutr J 11:12. doi:10.1186/1475-2891-11-12
von Bonsdorff MB, Groffen DA, Vidal JS, Rantanen T, Jonsson PV, Garcia M, Aspelund T, Eiriksdottir G, Siggeirsdottir K, Launer L, Gudnason V, Harris TB, Age GES-RSI (2013) Coronary artery calcium and physical performance as determinants of mortality in older age: the AGES-Reykjavik study. Int J Cardiol 168(3):2094–2099. doi:10.1016/j.ijcard.2013.01.067
Vidal JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Thorgeirsson G, Kjartansson O, Garcia ME, van Buchem MA, Harris TB, Gudnason V, Launer LJ (2010) Coronary artery calcium, brain function and structure: the AGES-Reykjavik study. Stroke J Cereb Circ 41(5):891–897. doi:10.1161/STROKEAHA.110.579581
Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD (2001) Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res Off J Am Soc Bone Miner Res 16(9):1642–1650. doi:10.1359/jbmr.2001.16.9.1642
Riggs BL, Melton Iii LJ, Melton Iii LJ 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S (2004) Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res Off J Am Soc Bone Miner Res 19(12):1945–1954. doi:10.1359/JBMR.040916
Ninomiya JT, Tracy RP, Calore JD, Gendreau MA, Kelm RJ, Mann KG (1990) Heterogeneity of human bone. J Bone Miner Res Off J Am Soc Bone Miner Res 5(9):933–938. doi:10.1002/jbmr.5650050906
Gruber R, Mayer C, Bobacz K, Krauth MT, Graninger W, Luyten FP, Erlacher L (2001) Effects of cartilage-derived morphogenetic proteins and osteogenic protein-1 on osteochondrogenic differentiation of periosteum-derived cells. Endocrinology 142(5):2087–2094. doi:10.1210/endo.142.5.8163
Garnero P (2014) New developments in biological markers of bone metabolism in osteoporosis. Bone 66:46–55. doi:10.1016/j.bone.2014.05.016
Rousseau JC, Sornay-Rendu E, Bertholon C, Chapurlat R, Garnero P (2014) Serum periostin is associated with fracture risk in postmenopausal women: a 7-year prospective analysis of the OFELY study. J Clin Endocrinol Metab 99(7):2533–2539. doi:10.1210/jc.2013-3893
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteopor Int J Established Result Coop Eur Found Osteopor Natl Osteopor Found USA 21(2):195–214. doi:10.1007/s00198-009-1066-z
Currey JD (1999) What determines the bending strength of compact bone? J Exp Biol 202(Pt 18):2495–2503
Seeman E, Delmas PD (2006) Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med 354(21):2250–2261. doi:10.1056/NEJMra053077
Roschger P, Misof B, Paschalis E, Fratzl P, Klaushofer K (2014) Changes in the degree of mineralization with osteoporosis and its treatment. Curr Osteoporos Rep 12(3):338–350. doi:10.1007/s11914-014-0218-z
Chaitou A, Boutroy S, Vilayphiou N, Munoz F, Delmas PD, Chapurlat R, Szulc P (2010) Association between bone turnover rate and bone microarchitecture in men: the STRAMBO study. J Bone Miner Res Off J Am Soc Bone Miner Res 25(11):2313–2323. doi:10.1002/jbmr.124
Bauer DC, Garnero P, Bilezikian JP, Greenspan SL, Ensrud KE, Rosen CJ, Palermo L, Black DM (2006) Short-term changes in bone turnover markers and bone mineral density response to parathyroid hormone in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 91(4):1370–1375. doi:10.1210/jc.2005-1712
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31(5):547–555
Kung AW, Huang QY (2007) Genetic and environmental determinants of osteoporosis. J Musculoskelet Neuronal Interact 7(1):26–32
Skerry TM (2008) The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys 473(2):117–123. doi:10.1016/j.abb.2008.02.028
Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, Christiansen C, Delmas PD (2006) Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38(3):300–309. doi:10.1016/j.bone.2005.09.014
Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R, Gamsjaeger S, Buchinger B, Roschger A, Phipps R, Boskey AL, Dall’Ara E, Varga P, Zysset P, Klaushofer K, Roschger P (2011) Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone 49(6):1232–1241. doi:10.1016/j.bone.2011.08.027
Qiu S, Rao DS, Palnitkar S, Parfitt AM (2002) Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone 31(2):313–318
Norman TL, Wang Z (1997) Microdamage of human cortical bone: incidence and morphology in long bones. Bone 20(4):375–379
Ferreira A, Ghazali A, Galvao J, Souberbielle JC, Jehle PM, Mohan S, Descamps-Latscha B, Oprisiu R, Fournier A, Drueke TB (2001) Effect of type of dialysis membrane on bone in haemodialysis patients. Nephrol Dial Trans Off Publ Eur Dial Transpl Assoc Eur Ren Assoc 16(6):1230–1238
Malluche HH, Porter DS, Pienkowski D (2013) Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol 9(11):671–680. doi:10.1038/nrneph.2013.198
Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA (2005) Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res Off J Am Soc Bone Miner Res 20(6):921–929. doi:10.1359/JBMR.050202
Gerdhem P, Ivaska KK, Isaksson A, Pettersson K, Vaananen HK, Obrant KJ, Akesson K (2007) Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. J Bone Miner Res Off J Am Soc Bone Miner Res 22(1):127–134. doi:10.1359/jbmr.061003
Acknowledgments
This study was funded by National Institutes of Health contract N01-AG-012100, the National Institute on Aging Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). EAM and TBH were supported in part by and the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Marques, E.A., Gudnason, V., Sigurdsson, G. et al. Are bone turnover markers associated with volumetric bone density, size, and strength in older men and women? The AGES–Reykjavik study. Osteoporos Int 27, 1765–1776 (2016). https://doi.org/10.1007/s00198-015-3442-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3442-1